Back to Journals » OncoTargets and Therapy » Volume 9
Effects of exercise intervention in breast cancer survivors: a meta-analysis of 33 randomized controlled trails
Authors Zhu G, Zhang X, Wang Y, Xiong H, Zhao Y , Sun F
Received 8 October 2015
Accepted for publication 30 November 2015
Published 13 April 2016 Volume 2016:9 Pages 2153—2168
DOI https://doi.org/10.2147/OTT.S97864
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Daniele Santini
Guoqing Zhu,1 Xiao Zhang,1 Yulan Wang,1 Huizi Xiong,2 Yinghui Zhao,1 Fenyong Sun1
1Department of Clinical Laboratory Medicine, 2Department of Dermatology, Shanghai Tenth People’s Hospital of Tongji University, Shanghai, People’s Republic of China
Background: Exercise is associated with favorable outcomes in cancer survivors. The purpose of this meta-analysis is to comprehensively summarize the effects of exercise intervention in breast cancer survivors.
Methods: A systematic search of PubMed, Elsevier, and Google scholar was conducted up to March 2015. References from relevant meta-analyses and reviews were also checked.
Results: Thirty-three randomized controlled trials were included in this meta-analysis, including 2,659 breast cancer survivors. Compared with the control group, quality of life was significantly improved in exercise intervention group, especially in mental health and general health subscales of short form 36 questionnaire, as well as emotion well-being and social well-being subscales of the Functional Assessment of Cancer Therapy. Besides, exercise alleviated the symptoms of depression and anxiety in the exercise group. Furthermore, exercise was also associated with positive outcomes in body mass index, lean mass, and muscle strength. In addition, the serum concentration of insulin, insulin-like growth factor-II, and insulin-like growth factor binding protein-1 was significantly reduced in exercise intervention group. However, based on the current data of this meta-analysis, there were no significant differences in sleep dysfunction or fatigue between groups.
Conclusion: Our study suggested that exercise intervention was beneficial to breast cancer survivors. Therefore, exercise should be recommended to this patient group.
Keywords: exercise, quality of life, depression, BMI, insulin
Introduction
Breast cancer is one of the main causes of cancer deaths in women,1 which was responsible for 23% of total cancer cases and 14% of cancer deaths.2 With the improvements in early detection and treatment, the number of cancer survivors continued to increase, in which women with breast cancer accounted for 22% of total cancer survivors in 2012.3 However, the problems related to breast cancer and cancer treatment, such as cardiac toxicity of adjuvant systemic therapy,4,5 arm or shoulder problems, body image,6 change in social life, fear,7 and poorer quality of life8 were negatively associated with the overall well-being of breast cancer survivors.
A growing body of evidence indicated that exercise intervention results in beneficial outcomes in cancer patients. Some studies had suggested that exercise increased cardiorespiratory fitness,9 physical performance,10 and reduced overall mortality.11 There were also studies demonstrating that exercise was associated with improvements in the symptom of depression,12 body image, self-esteem,13 and quality of life,14–16 though some conclusions were not inconsistent in terms of fatigue.17
Previously, these effects of exercise intervention in breast cancer patients had been assessed in several meta-analyses and systematic reviews.18–22 However, some of them only summarized some of effects related to intervention,18,19 or compared the effects of group exercise with individual exercise.20 Others either only focused on one special symptom,21 or evaluated the efficacy of Tai Chi Chuan alone.22 Moreover, new evidences in recent years have not been included. Thus, we aim to comprehensively summarize the effects of exercise intervention on breast cancer patients based on the available data from randomized controlled trials.
Methods
Literature search
We searched PubMed, Elsevier, and Google Scholar up to March 2015. The reference lists of relevant systematic reviews and meta-analyses were also examined to identify additional studies. The search terms used in this meta-analysis were related to breast cancer (breast neoplasm, cancer, tumour, tumor, carcinoma) and exercise (exercise, physical activity, sport, weight training).
Inclusion criteria
Studies were considered eligible if they met the following criteria: 1) were written in English; 2) adopted a randomized controlled trial design, comparing exercise intervention group with control group (usual care, maintain current activity level, or waitlist); 3) included adults diagnosed with breast cancer; and 4) evaluated the effects of exercise in breast cancer patients.
Studies were excluded if: 1) included mixed cancer populations, including other types of cancer patients; 2) included other types of intervention (exercise intervention combined with diet); and 3) exercise merely focused on upper limb or arm.
Data extraction
Relevant data were independently extracted by GQ Zhu and X Zhang with a standard excel template, including 1) characteristics of the study and participants (first author, year of publication, mean age, sample size); 2) content of exercise intervention: exercise type, timing (before, during, or after treatment), and the frequency, intensity, and duration of intervention; 3) outcomes of intervention (quality of life, depression, anxiety, fatigue, muscle strength, body composition, physiological markers); and 4) assessment methods. Any disagreements were checked and discussed until a consensus was reached.
Methodological quality assessment
The methodological quality of the studies were independently assessed by two reviewers (GQ Zhu and YL Wang) using the Delphi criteria list,23 which is a set of nine criteria for quality assessment of randomized controlled trials. It was hard to blind the participants and providers in the interventional study. Therefore, participants blinding and provider blinding were not rated, and we only assessed the blinding of the outcome assessors. Each item was scored as yes (+) or no (−).
Statistical analysis
The outcomes were assessed if the data were available in at least two studies. For continuous outcomes, standardized mean differences with 95% confidence intervals (CIs) were calculated, with P<0.05 considered statistically significant. Statistical heterogeneity among studies was measured by I2 test, in which values above 25% and 50% were considered as the indicative of moderate and high heterogeneity, respectively.24 A fixed-effect model was adopted when I2<50%; otherwise, a random-effect model was used.
In the presence of heterogeneity, subgroup analysis was performed based on the measurement methods or the type of exercise. Besides, sensitivity analysis was carried out to evaluate the influence of a single study to the overall estimate. Publication bias was estimated through Begg’s test and Egger’s linear regression, with P<0.10 consumed as an indication of publication bias.25 All analyses were conducted using Review Manager Version 5.3 (Cochrane Collaboration, Copenhagen, Denmark) and Stata 12.0 (College Station, TX, USA).
Results
Study selection
A total of 3,429 records were identified from the database (Figure 1). After screening the titles and abstracts, the full texts of 161 articles were further reviewed for eligibility. Finally, 33 articles26–58 were included and assessed for methodological quality, with 128 articles excluded in which the aim, intervention type, or design of the study failed to meet the inclusion criteria.
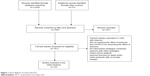  | Figure 1 Flow diagram of study selection. |
Characteristics of the exercise interventions
There were 2,659 breast cancer patients, with the mean age of 54 (46.3–60.6) years (Table 1). The main types of exercise interventions reported in this meta-analysis were aerobic, resistance, and stretching exercises. Besides, there were also six studies on yoga intervention,28,39,42,4,50,52 two studies on tai chi chuan,35,55 and one on dancing.46 Twenty Five studies performed intervention after treatment,26,28,29,31,33–36,38,40,41,44,46–58 seven studies during treatment,20,27,32,37,43,45,51 and the remaining two studies before treatment.39,42 The duration of intervention lasted from 6 weeks to 12 months, with the frequency of intervention varying from two times a week to every day. The intensity of exercise also varied from low to vigorous in different situations, among which the moderate intensity was most frequently reported.
Methodological quality of included studies
We assessed 33 articles according to the Delphi criteria list, and seven criteria were examined in each of the study. In all, 14 studies met five criteria,26,30,35,38–41,51–54,56–58 12 studies more than five criteria,29,31,33,34,36,37,42,44,46,48–50 and the remaining 7 studies less than five criteria.27,28,32,43,45,47,55 Of these, 19 studies failed to conceal the allocation,26–28,32–34,38–40,43,45,47,52–58 and 22 studies did not blind the outcome assessor.27–28,30–32,35–36,38–41,43,45–47,51–55,57,58 Besides, 14 studies were not intention-to-treat analysis (Table 2).26–28,30,32,35,37,41,43,45,47,49,55,56
  | Table 2 Methodological quality assessment of 33 randomized controlled trials |
Pooled effect estimates for outcome measures
In this meta-analysis, we examined the effects of exercise intervention on quality of life, psychological outcomes, body composition, physical function and symptom, and physiological markers of breast cancer survivors. A total of 53 outcomes were evaluated, which were reported in at least two studies (Table 3).
Results of quality of life
The quality of life was reported as an outcome in 18 studies,26,30,31,36,37,41,42,45,46,51–58 among which 10 studies used the Functional Assessment of Cancer Therapy-Breast (FACT-B) and the Functional Assessment of Cancer Therapy–General (FACT-G) questionnaire,31,36,37,41,45,46,51,53–55 4 studies the Medical Outcomes Study Short Form health survey (SF-36) questionnaire,26,46,51,57 and two studies Treatment of Cancer-Quality of Life questionnaire (EORTCQoLC30).42,58 The other 4 studies used SF-12 health survey (SF-12),52 the Functional Assessment of Cancer Therapy–Anemia,30 the World Health Organization Quality of Life (WHOQOL-BREF),45 and the Cancer Rehabilitation Evaluation System-Short Form,56 respectively. We only pooled the outcomes that data could be extracted in at least two studies. Therefore, the data of 12 studies, involving 15 quality life domains, were included in this meta-analysis.26,31,36,37,41,45,46,51,53–55,57
Measured by SF-36 or MOS SF-36, the exercise intervention significantly improved the mental health (I2=0%, P=0.006, 95% CI: 0.11, 0.62, Figure 2) and general health (I2=95%, P=0.02, 95% CI: 0.70, 8.48, Figure 2) compared with the control groups. Besides, exercise was associated with a significant increase in emotion well-being (I2=2%, P=0.0006, 95% CI: 0.12, 0.43, Figure 2) and social well-being subscales (I2=0%, P=0.01, 95% CI: 0.19, 1.69, Figure 2) of the Functional Assessment of Cancer Therapy. The pooled results of five studies showed a significant increase in breast cancer subscale of the Functional Assessment of Cancer Therapy from exercise (I2=15%, P=0.000001, 95% CI: 1.85, 4.04) (Table 3).36,37,44,54,57
However, substantial heterogeneity was observed for some outcomes. There was no evidence of publication bias except for SF-36 role-emotion (P=0.062, Table 3).
Results of psychological outcomes
Pooled data from three studies indicated that the self-esteem score was higher in the intervention group (I2=0%, P=0.02, 95% CI: 0.18, 2.22), measured by the Rosenberg Self-Esteem Scale (Table 3).29,44,51 Meanwhile, exercise intervention reduced depression (I2=2%, P=0.001, 95% CI: −3.36, −0.80, Figure 3) and anxiety score (I2=0%, P<0.0001, 95% CI: −4.76, −1.58, Figure 3), which were of clinical importance. However, the pooled results showed no difference in happiness and stress between intervention and control groups, assessed by the 2-item Fordyce Happiness Measure and the Perceived Stress Scale (Table 3).
Furthermore, exercise led to improvement in the positive and negative attitudes in breast cancer survivors, measured by the Positive and Negative Affect Schedule (PANAS), of which the PANAS negative score decreased by 3.51 points (I2=76%, P=0.02, 95% CI: −9.92, −0.71) and the PANAS positive score increased by 4.46 points (I2=0, P<0.00001, 95% CI: 2.48, 6.44) (Table 3). In addition, measured by the Functional Assessment of Chronic Illness Therapy–Spiritual, exercise was associated with positive effect in spirit compared with control groups (I2=0%, P=0.02, 95% CI: 0.76, 7.13) (Table 3).
No publication bias were detected in any of the results, except the Pittsburgh Sleep Quality Index (P=0.082). High heterogeneity was only found for PANAS negative (Table 3).
Results of body compositions
Seven parameters were included in this meta–analysis (Table 3). Body mass index (BMI) was examined in nine studies, and the pooled results indicated it reduced significantly with exercise (I2=0%, P<0.00001, 95% CI: −1.09, −0.47, Figure 4).34,35,37,38,40,41,44,49,54 Besides, the pooled results of four studies showed that exercise was associated with significant increase in lean mass compared with control groups (I2=57%, P=0.04, 95% CI: 0.08, 2.25) (Table 3).
  | Figure 4 The association between exercise intervention and body mass index in breast cancer survivors. |
Similarly, body fat percentage (I2=57%, P=0.02, 95% CI: −3.33, −0.35) and fat mass (I2=0%, P=0.05, 95% CI: −4.83, −0.04) were significantly reduced in the exercise intervention groups (Table 3). However, there were no significant differences on waist circumference, hip circumference, and waist-to-hip ratio between intervention and control groups.
No publication bias was observed, with only moderate heterogeneity for lean mass and body fat (Table 3).
Results of physical function and symptom
Muscle strength was reported in five studies, which indicated significant improvement (I2=48%, P=0.0009, 95% CI: 1.76, 6.78, Figure 5) in exercise intervention group. Besides, no significant improvement was showed on peak oxygen consumption, based on the data from two studies (Table 3).27,30,34,40,41
  | Figure 5 The association between exercise intervention and muscle strength in breast cancer survivors. |
Fatigue was assessed in 12 studies, the pooled results of which indicated that there was no difference on fatigue between the intervention and control groups (Figure 6).29,30,36,37,40,47,50,52–54,58 In the present of high heterogeneity, we performed the subgroup analysis stratified by the measurement method and the type of exercise intervention (Figure 6). However, the effect of exercise on the symptom of fatigue still remained insignificant in both of the subgroups, except small reduction in Fatigue Symptom Inventory (I2=68%, P=0.04, 95% CI: −1.68, −0.02). No evidence of publication bias was detected in any of the results (Table 3).
Results of physiological markers
Eight physiological markers were examined in this meta-analysis (Table 3). When the data of postintervention were used, only insulin (I2=95%, P=0.05, 95% CI: −13.64, 0.06) and insulin-like growth factor binding protein (IGFBP)-1 (I2=46%, P<0.00001, 95% CI: −4.40, −1.91) were significantly reduced after exercise intervention. However, based on the changed serum concentration of physiological markers after intervention (postintervention minus baseline), exercise significantly reduced the serum concentration of insulin (I2=97%, P<0.00001, 95% CI: −9.26, −0.33, Figure 7), IGFBP-1 (I2=0%, P<0.00001, 95% CI: −3.93, −1.93, Figure 7), and insulin-like growth factor (IGF)-II (I2=0%, P<0.00001, 95% CI: −61.41, −47.00). Significant increases were shown in interleukin (IL)-6 (I2=69%, P=0.02, 95% CI: 0.27, 2.65) and glucose (I2=99%, P<0.00001, 95% CI: 0.27, 2.65). There were substantial heterogeneity in some of the physiological markers, and evident publication bias was only observed in IL-6 (P=0.046) (Table 3).
Discussion
In this meta-analysis, we summarized the effects of exercise intervention on breast cancer survivors, including 53 outcomes reported from 33 articles. Results showed that exercise was associated with significant improvements in quality of life, self-esteem, and the response attitude toward life. Besides, it alleviated the symptoms of depression and anxiety in breast cancer survivors. In addition to the beneficial outcome in body composition, exercise also increased muscle strength in the intervention groups. Furthermore, the serum concentration of some physiological markers, such as insulin, IGF-II, and IGFBP-1, was significantly reduced after exercise intervention.
In line with previous meta-analyses,19,59,60 the pooled results supported the evidences that exercise improved the quality of life in cancer patients. However, a significant improvement was shown in general health subscale of SF-36 in our meta-analysis, but not in general health scale of the Functional Assessment of Cancer Therapy.
Similarly, a statistically significant increase was only detected in the social function and emotion function scales of the Functional Assessment of Cancer Therapy, but not in the role emotion and social function subscales of SF-36. It was the same as the evidence of Fong who discovered that exercise improved SF-36 scores in physical function, social function, and mental health with mixed type of cancer survivors.61 Thus, despite the fact that exercise was proved to improve quality of life, there were slight differences in the domains of quality life, owing to the differences in the features of patients and exercise, as well as measurement methods.
According to the sensitivity analysis of the Functional Assessment of Cancer Therapy, the study by Mustian was identified as an outlier: the timing of intervention was during treatment in the study by Milne, whereas, the other studies were posttreatment.36 When excluding this outliers from analysis, the heterogeneity in the Functional Assessment of Cancer Therapy subscales (social well-being, function well-being, emotion well-being, physical well-being) decreased evidently (I2=0%, 27%, 20%, 0%, 8%, respectively).
The study by Basen-Engquist was also identified as an outlier, based on the sensitivity analysis of SF-36 subscales: in this study, the exercise intervention was lifestyle intervention, which encouraged participants to integrate activity into daily routine and perform activities they choose.26 When this study was excluded, the heterogeneity was evidently decreased in vitality (I2=0%), body pain of SF-36 (I2=0%), and general health (I2=86%).
In our current meta-analysis, we only calculated the effect size of outcomes reported in at least two studies. Therefore, the data of life quality measured by the Functional Assessment of Cancer Therapy–Anemia,30 the European Organization for the Research and Treatment of Cancer-Quality of Life (EORTCQoLC30 questionnaire),42,58 the Cancer Rehabilitation Evaluation System-Short Form,56 and the WHOQOL-BREF were not pooled.45 Even though all the five studies favored exercise intervention, only three of them reported a clinical significant improvement in quality of life on breast cancer survivors,42,45,56 and the results in other two studies failed to reach statistical significance.30,58
We observed a significant improvement in depression, anxiety, and self-esteem in breast cancer patients, which were frequently reported in pervious meta-analyses and systematic reviews with mixed cancer patients.59,61–64 We also discovered that the attitude toward life in intervention group was more positive than control group. The positive attitude played a critical role in the emotion well-being, which might have some correlation with improved quality of life in breast cancer survivors.
There was no clinical significant change on the symptom of fatigue between groups, based on the pooled results in our meta-analysis, which was consistent with a previous meta-analysis.61 However, physical activity was reported to be associated with improvement on the symptom of fatigue in several meta-analyses, both breast and other cancer survivors.19,59,62,65,66
In the subgroup analysis based on the measurement methods, a significant decrease of fatigue was only observed in the Fatigue Symptom Inventory. We then stratified the results by the types of intervention, the results of which still remained insignificant. Even yoga, a “mind–body” exercise, had no significant effect on fatigue, which had been suggested to be associated with a moderate reduction of fatigue in a previous study.67 We further performed sensitivity analysis, the results of which indicated the studies by Bower and Milne exerted substantial influence to the overall estimate.28,36 However, when excluding the two studies, the fatigue level was increased in exercise intervention groups (I2=50%, P=0.05, 95% CI: 0.02, 2.19), which had not been reported in previous meta-analysis. Given the current inconsistent conclusions, more researches are needed to further examine this effect.
Several system reviews and meta-analyses had suggested positive effects of exercise on peak oxygen consumption.18,19,61,66 However, the pooled results of two studies observed no statistical significance change of peak oxygen consumption between groups, which might be attributed to the small size in our meta-analysis. Furthermore, owing to the lack of sufficient data, the outcomes, such as the 3-minute step test52 and the figure-8 running test,38 were not included in our meta-analysis, which also showed improvements compared with control groups.
Results indicated that exercise led to a statistically significant reduction in BMI and insulin. Each 5 kg weight gain might increase the breast cancer-specific mortality by 13% and all-caused mortality by 12%.68 Besides, research showed that insulin was associated with BMI, and the increase of insulin was related to a twofold increased risk of breast cancer recurrence.69 Thus, the decreased BMI and insulin from exercise might potentially contribute to a reduced risk of mortality and recurrence on breast cancer survivors.
Additionally, one study suggested that IGFBP-1 and IGFBP-5 as IGF-I antagonists might block mammary gland development.70 However, pooled results showed that IGFBP-1 was significantly decreased in exercise intervention group, while the change of IGF-1 was insignificant. Contrary to previous results, two meta-analyses reported only IGF-I was reduced significantly, and no evidences of significant change were found in insulin, IGFBP-1, and glucose in both breast cancer patients or mixed cancer patients.61,62 Therefore, given this inconsistency, we should treat the association between exercise and the change of physiological markers with caution, and more researches are needed before making a confirmed conclusion.
The sensitivity analysis of physiological markers identified two outliers: the study by Schmitz and Melinda, in which the duration of intervention lasted for 6 to 12 months, while the durations were 12 to 15 weeks in other studies.34,39 Therefore, it is likely that the duration of exercise intervention is the source of heterogeneity among these results.
Limitations
In our meta-analysis, we only included published randomized control trails in two databases, though we further searched the relevant reference lists for potential articles, which may increase the risk of publication bias. Besides, there was a lack of consistency in terms of the outcomes reported and measurement methods among the studies. The outcomes, such as erythrocyte Levels,32 salivary cortisol,29 were reported in only one study respectively, and we, therefore, failed to calculate their effect sizes in present meta-analysis.
Similarly, the quality of life was measured by different methods, which made it difficult to combine the diverse outcomes. Therefore, we only pooled the outcomes of quality life components measured by the Functional Assessment of Cancer Therapy and SF-36, respectively, which were used in most of the studies.
Furthermore, some data could not be extracted in several studies, and we did not try to contact the authors for detailed information. In addition, we used the mean and standard deviation of postintervention to calculate the effect sizes for most of the data, rather than the changes after the intervention, the results of which may be influenced by the differences at baselines.
Implications for future research and practice
The differences of intervention type, intensity, and duration might account for some variations in the effects of exercise, and we could have performed subgroup analysis based on these differences. However, it would lead to insufficient data to calculate the effect sizes of some outcomes. Therefore, future research should further explore the correlation between intervention effects and the exercise type, intensity, and duration.
Additionally, the survival outcomes in breast cancer survivors are likely to have some correlation with exercise intervention.71,72 However, the studies included in our current meta-analysis failed to examine this association. Therefore, it is recommended that future studies examine the effects of exercise intervention on survival outcomes and determine whether exercise will provide benefit to the survival outcomes.
Taken together, the present evidences support the idea that exercise intervention is beneficial to breast cancer survivors, although it fails to identify the optimal type, timing, and intensity of exercise intervention. In addition, previous studies demonstrated that it was feasible and safe for various cancer patients to exercise during treatment,73 without increasing the risk or exacerbating the symptom of lymphedema.74 Nevertheless, some prospective longitudinal studies showed that the physical activity decreased during treatment.75,76 The frequency of exercise was also lower off-treatment than prediagnosis in breast cancer patients.77 Therefore, exercise intervention should be prescribed to breast cancer survivors, encouraging them to continue their established exercise habits or adopt a right type of exercise.
Conclusion
Though with some limitations, there are evidences that exercise was associated with beneficial outcomes in breast cancer survivors. Based on the results from 33 studies, exercise improved the quality of life and alleviated the symptoms of depression and anxiety in breast cancer survivors. There were also benefits on muscle strength and body composition. Besides, exercise intervention was associated with reduced serum concentration of insulin, IGF-II, and IGFBP-1. Therefore, on the basis of our current evidences, exercise should be recommended to breast cancer survivors.
Acknowledgments
This work was supported by the China National 973 Project (2012CB966904 and 20110402), the National Natural Science Foundation of China (81301689 and 81202958), the Yangfan Project of Shanghai Science and Technology Commission (14YF1412300), the Outstanding Youth Training Program of Tongji University (1501219080), and the Shanghai Tenth People’s Hospital Climbing Training Program (04.01.13024).
Disclosure
The authors report no conflicts of interest in this work.
References
Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64(1):9–29. | ||
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90. | ||
de Moor JS, Mariotto AB, Parry C, et al. Cancer survivors in the United States: prevalence across the survivorship trajectory and implications for care. Cancer Epidemiol Biomarkers Prev. 2013;22(4):561–570. | ||
Short CE, James EL, Girgis A, Mcelduff P, Plotnikoff RC. Move more for life: the protocol for a randomised efficacy trial of a tailored-print physical activity intervention for post-treatment breast cancersurvivors. BMC Cancer. 2012;12(1):1–10. | ||
Bird BR, Swain SM. Cardiac toxicity in breast cancer survivors: review of potential cardiac problems. Clin Cancer Res. 2008;14(1):14–24. | ||
Montazeri A, Vahdaninia M, Harirchi I, Ebrahimi M, Khaleghi F, Jarvandi S. Quality of life in patients with breast cancer before and after diagnosis: an eighteen months follow-up study. BMC Cancer. 2008;8:330. | ||
Roundtree AK, Giordano SH, Price A, Suarez-Almazor ME. Problems in transition and quality of care: perspectives of breast cancer survivors. Support Care Cancer. 2011;19(12):1921–1929. | ||
Nesvold IL, Reinertsen KV, Fossa SD, Dahl AA. The relation between arm/shoulder problems and quality of life in breast cancer survivors: a cross-sectional and longitudinal study. J Cancer Surviv. 2011;5(1):62–72. | ||
Thorsen L, Skovlund E, Stromme SB, Hornslien K, Dahl AA, Fossa SD. Effectiveness of physical activity on cardiorespiratory fitness and health-related quality of life in young and middle-aged cancer patients shortly after chemotherapy. J Clin Oncol. 2005;23(10):2378–2388. | ||
Ligibel JA, Meyerhardt J, Pierce JP, et al. Impact of a telephone-based physical activity intervention upon exercise behaviors and fitness in cancer survivors enrolled in a cooperative group setting. Breast Cancer Res. 2012;132(1):205–213. | ||
Holick CN, Newcomb PA, Trentham-Dietz A, et al. Physical activity and survival after diagnosis of invasive breast cancer. Cancer Epidemiol Biomarkers Prev. 2008;17(2):379–386. | ||
Basen-Engquist K, Hughes D, Perkins H, Shinn E, Taylor CC. Dimensions of physical activity and their relationship to physical and emotional symptoms in breast cancer survivors. J Cancer Surviv. 2008;2(4):253–261. | ||
Ogunleye AA, Holmes MD. Physical activity and breast cancer survival. Breast Cancer Res. 2009;11(5):106. | ||
Mandelblatt JS, Luta G, Kwan ML, et al. Associations of physical activity with quality of life and functional ability in breast cancer patients during active adjuvant treatment: the pathways study. Breast Cancer Res Treat. 2011;129(2):521–529. | ||
Paxton RJ, Phillips KL, Jones LA, et al. Associations among physical activity, body mass index, and health-related quality of life by race/ethnicity in a diverse sample of breast cancer survivors. Cancer. 2012;118(16):4024–4031. | ||
Vallance JK, Courneya KS, Plotnikoff RC, Yasui Y, Mackey JR. Randomized controlled trial of the effects of print materials and step pedometers on physical activity and quality of life in breast cancer survivors. J Clin Oncol. 2007;25(17):2352–2359. | ||
Dimeo FC, Thomas F, Raabe-Menssen C, Propper F, Mathias M. Effect of aerobic exercise and relaxation training on fatigue and physical performance of cancer patients after surgery: a randomised controlled trial. Support Care Cancer. 2004;12(11):774–779. | ||
Kim CJ. A meta-analysis of aerobic exercise interventions for women with breast cancer. West J Nurs Res. 2009;31(4):437–461. | ||
McNeely ML, Campbell KL, Rowe BH, Klassen TP, Mackey JR, Courneya KS. Effects of exercise on breast cancer patients and survivors: a systematic review and meta-analysis. CMAJ. 2006;175(1):34–41. | ||
Floyd A, Moyer A. Group vs individual exercise interventions for women with breast cancer: a meta-analysis. Health Psychol Rev. 2009;4(1):22–41. | ||
Meneses-Echavez JF, Gonzalez-Jimenez E, Ramirez-Velez R. Effects of supervised exercise on cancer-related fatigue in breast cancer survivors: a systematic review and meta-analysis. BMC Cancer. 2015;15:77. | ||
Pan Y, Yang K, Shi X, Liang H, Zhang F, Lv Q. Tai chi chuan exercise for patients with breast cancer: a systematic review and meta-analysis. Evid Based Complement Alternat Med. 2015;2015:535237. | ||
Verhagen AP, de Vet HC, de Bie RA, et al. The Delphi list: a criteria list for quality assessment of randomized clinical trials for conducting systematic reviews developed by Delphi consensus. J Clin Epidemiol. 1998;51(12):1235–1241. | ||
Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. | ||
Hayashino J, Noguchi Y, Fukui T. Systematic evaluation and comparison of statistical tests for publication bias. J Epidemiol. 2005;15(6):235–243. | ||
Basen-Engquist K, Taylor CL, Rosenblum C, et al. Randomized pilot test of a lifestyle physical activity intervention for breast cancer survivors. Patient Educ Couns. 2006;64(1–3):225–234. | ||
Battaglini C. The effects of an individualized exercise intervention on body composition in breast cancer patients undergoing treatment. Sao Paulo Med J. 2007;125(1):22–28. | ||
Bower JE, Greendale G, Crosswell AD, et al. Yoga reduces inflammatory signaling in fatigued breast cancer survivors: a randomized controlled trial. Psychoneuroendocrinology. 2014;43:20–29. | ||
Cantarero-Villanueva I, Fernández-Lao C, Díaz-Rodriguez L, Fernández-de-las-Peñas C, del Moral-Avila R, Arroyo-Morales M. A multimodal exercise program and multimedia support reduce cancer-related fatigue in breast cancer survivors: a randomised controlled clinical trial. Eur J Integr Med. 2011;3(3):e189–e200. | ||
Courneya KS, Segal RJ, Mackey JR, et al. Effects of aerobic and resistance exercise in breast cancer patients receiving adjuvant chemotherapy: a multicenter randomized controlled trial. J Clin Oncol. 2007;25(28):4396–4404. | ||
Daley AJ, Crank H, Saxton JM, Mutrie N, Coleman R, Roalfe A. Randomized trial of exercise therapy in women treated for breast cancer. J Clin Oncol. 2007;25(13):1713–1721. | ||
Drouin JS, Young TJ, Beeler J, et al. Random control clinical trial on the effects of aerobic exercise training on erythrocyte levels during radiation treatment for breast cancer. Cancer. 2006;107(10):2490–2495. | ||
Irwin ML, Alvarez-Reeves M, Cadmus L, et al. Exercise improves body fat, lean mass, and bone mass in breast cancer survivors. Obesity. 2009;17(8):1534–1541. | ||
Irwin ML, Varma K, Alvarez-Reeves M, et al. Randomized controlled trial of aerobic exercise on insulin and insulin-like growth factors in breast cancer survivors: the Yale Exercise and Survivorship study. Cancer Epidemiol Biomarkers Prev. 2009;18(1):306–313. | ||
Janelsins MC, Davis PG, Wideman L, et al. Effects of Tai Chi Chuan on insulin and cytokine levels in a randomized controlled pilot study on breast cancer survivors. Clin Breast Cancer. 2011;11(3):161–170. | ||
Milne HM, Wallman KE, Gordon S, Courneya KS. Effects of a combined aerobic and resistance exercise program in breast cancer survivors: a randomized controlled trial. Breast Cancer Res Treat. 2008;108(2):279–288. | ||
Mutrieet N, Campbell AM, Whyte F, et al. Benefits of supervised group exercise programme for women being treated for early stage breast cancer: pragmatic randomised controlled trial. BMJ. 2007;334(7592):517. | ||
Nikander R, Sievanen H, Ojala K, Oivanen T, Kellokumpu-Lehtinen PL, Saarto T. Effect of a vigorous aerobic regimen on physical performance in breast cancer patients – a randomized controlled pilot trial. Acta Oncol. 2007;46(2):181–186. | ||
Rao MR, Raghuram N, Nagendra HR, et al. Anxiolytic effects of a yoga program in early breast cancer patients undergoing conventional treatment: a randomized controlled trial. Complement Ther Med. 2009;17(1):1–8. | ||
Rogers LQ, Fogleman A, Trammell R, et al. Effects of a physical activity behavior change intervention on inflammation and related health outcomes in breast cancer survivors: pilot randomized trial. Integr Cancer Ther. 2013;12(4):323–335. | ||
Rogers LQ, Hopkins-Price P, Vicari S, et al. A randomized trial to increase physical activity in breast cancer survivors. Med Sci Sports Exerc. 2009;41(4):935–946. | ||
Vadiraja HS, Rao MR, Nagarathna R, et al. Effects of yoga program on quality of life and affect in early breast cancer patients undergoing adjuvant radiotherapy: a randomized controlled trial. Complement Ther Med. 2009;17(5–6):274–280. | ||
Battaglini CL. Effect of exercise on the caloric intake of breast cancer patients undergoing treatment. Braz J Med Biol Res. 2008;41:709–715. | ||
Courneya KS, Mackey JR, Bell GJ, Jones LW, Field CJ, Fairey AS. Randomized controlled trial of exercise training in postmenopausal breast cancer survivors: cardiopulmonary and quality of life outcomes. J Clin Oncol. 2003;21(9):1660–1668. | ||
Hwang JH, Chang HJ, Shim YH, et al. Effects of supervised exercise therapy in patients receiving radiotherapy for breast cancer. Yonsei Med J. 2008;49(3):443–450. | ||
Sandel SL, Judge JO, Landry N, Faria L, Ouellette R, Majczak M. Dance and movement program improves quality-of-life measures in breast cancer survivors. Cancer Nurs. 2005;28:301–309. | ||
Moadel AB, Shah C, Wylie-Rosett J, et al. Randomized controlled trial of yoga among a multiethnic sample of breast cancer patients: effects on quality of life. J Clin Oncol. 2007;25(28):4387–4395. | ||
Fairey AS, Courneya KS, Field CJ, Bell GJ, Jones LW, Mackey JR. Effects of exercise training on fasting insulin, insulin resistance, insulin-like growth factors, and insulin-like growth factor binding proteins in postmenopausal breast cancer survivors: a randomized controlled trial. Cancer Epidemiol Biomarkers Prev. 2003;12:721–727. | ||
Schmitz KH, Ahmed RL, Hannan PJ, Yee D. Safety and efficacy of weight training in recent breast cancer survivors to alter body composition, insulin, and insulin-like growth factor axis protein. Cancer Epidemiol Biomarkers Prev. 2005;14:1672–1680. | ||
Bower JE, Garet D, Sternlieb B, et al. Yoga for persistent fatigue in breast cancer survivors: a randomized controlled trial. Cancer. 2012;118(15):3766–3775. | ||
Cadmus LA, Salovey P, Yu H, Chung G, Kasl S, Irwin ML. Exercise and quality of life during and after treatment for breast cancer: results of two randomized controlled trials. Psychooncology. 2009;18(4):343–352. | ||
Danhauer SC, Mihalko SL, Russell GB, et al. Restorative yoga for women with breast cancer: findings from a randomized pilot study. Psychooncology. 2009;18(4):360–368. | ||
Hayes SC, Rye S, Disipio T, et al. Exercise for health: a randomized, controlled trial evaluating the impact of a pragmatic, translational exercise intervention on the quality of life, function and treatment-related side effects following breast cancer. Breast Cancer Res Treat. 2013;137(1):175–186. | ||
Littman AJ, Bertram LC, Ceballos R, et al. Randomized controlled pilot trial of yoga in overweight and obese breast cancer survivors: effects on quality of life and anthropometric measures. Support Care Cancer. 2012;20(2):267–277. | ||
Mustian KM, Katula JA, Gill DL, Roscoe JA, Lang D, Murphy K. Tai Chi Chuan, health-related quality of life and self-esteem: a randomized trial with breast cancer survivors. Support Care Cancer. 2004;12(12):871–876. | ||
Ohira T, Schmitz KH, Ahmed RL, Yee D. Effects of weight training on quality of life in recent breast cancer survivors: the Weight Training for Breast Cancer Survivors (WTBS) study. Cancer. 2006;106(9):2076–2083. | ||
Sprod LK, Janelsins MC, Palesh OG, et al. Health-related quality of life and biomarkers in breast cancer survivors participating in tai chi chuan. J Cancer Surviv. 2012;6(2):146–154. | ||
Saarto T, Penttinen HM, Sievannen H. Effectiveness of a 12-month exercise program on physical performance and quality of life of breast cancer survivors. Anticancer Res. 2012;32:3875–3884. | ||
Duijts SF, Faber MM, Oldenburg HS, van Beurden M, Aaronson NK. Effectiveness of behavioral techniques and physical exercise on psychosocial functioning and health-related quality of life in breast cancer patients and survivors – a meta-analysis. Psychooncology. 2011;20(2):115–126. | ||
Ferrer RA, Huedo-Medina TB, Johnson BT, Ryan S, Pescatello LS. Exercise interventions for cancer survivors: a meta-analysis of quality of life outcomes. Ann Behav Med. 2011;41(1):32–47. | ||
Fong DY, Ho JW, Hui BP, et al. Physical activity for cancer survivors: meta-analysis of randomised controlled trials. BMJ. 2012;344:e70. | ||
Speck RM, Courneya KS, Masse LC, Duval S, Schmitz KH. An update of controlled physical activity trials in cancer survivors: a systematic review and meta-analysis. J Cancer Surviv. 2010;4(2):87–100. | ||
Schmitz KH, Holtzman J, Courneya KS, Mâsse LC, Duval S, Kane R. Controlled physical activity trials in cancer survivors: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2005;14(7):1588–1595. | ||
Brown JC, Huedo-Medina TB, Pescatello LS, et al. The efficacy of exercise in reducing depressive symptoms among cancer survivors: a meta-analysis. PLoS One. 2012;7(1):e30955. | ||
Brown JC, Huedo-Medina TB, Pescatello LS, Pescatello SM, Ferreer RA, Johnson BT. Efficacy of exercise interventions in modulating cancer-related fatigue among adult cancer survivors: a meta-analysis. Am Association Cancer Res. 2010;10(1158):1055–9965. | ||
Spence RR, Heesch KC, Brown WJ. Exercise and cancer rehabilitation: a systematic review. Can Treat Rev. 2010;36(2):185–194. | ||
Buffart LM, van Uffelen JG, Riphagen II. Physical and psychosocial benefits of yoga in cancer patients and survivors, a systematic review and meta-analysis of randomized controlled trials. BMC Cancer. 2012;12:559. | ||
Nichols HB, Trentham-Dietz A, Egan KM, et al. Body mass index before and after breast cancer diagnosis: associations with all-cause, breast cancer, and cardiovascular disease mortality. Cancer Epidemiol Biomarkers Prev. 2009;18(5):1403–1409. | ||
Goodwin PJ, Ennis M, Pritchard KI, et al. Fasting insulin and outcome in early-stage breast cancer: results of a prospective cohort study. J Clin Oncol. 2002;20(1):42–51. | ||
Kleinberg DL, Wood TL, Furth PA, Lee AV. Growth hormone and insulin-like growth factor-I in the transition from normal mammary development to preneoplastic mammary lesions. Endocr Rev. 2009;30(1):51–74. | ||
Bertram LA, Stefanick ML, Saquib N, et al. Physical activity, additional breast cancer events, and mortality among early-stage breast cancer survivors: findings from the WHEL Study. Cancer Causes Control. 2011;22(3):427–435. | ||
Sternfeld B, Weltzien E, Quesenberry CP, et al. Physical activity and risk of recurrence and mortality in breast cancer survivors: findings from the LACE study. Cancer Epidemiol Biomarkers Prev. 2009;18(1):87–95. | ||
Adamsen L, Quist M, Andersen C, et al. Effect of a multimodal high intensity exercise intervention in cancer patients undergoing chemotherapy: randomised controlled trial. BMJ. 2009;339:b3410. | ||
Ahmed RL, Thomas W, Yee D, Schmitz KH. Randomized controlled trial of weight training and lymphedema in breast cancer survivors. J Clin Oncol. 2006;24(18):2765–2772. | ||
Andrykowski MA, Beacham AO, Jacobsen PB. Prospective, longitudinal study of leisure-time exercise in women with early-stage breast cancer. Cancer Epidemiol Biomarkers Prev. 2007;16(3):430–438. | ||
Kwan ML, Sternfeld B, Ergas IJ, et al. Change in physical activity during active treatment in a prospective study of breast cancer survivors. Breast Cancer Res Treat. 2012;131(2):679–690. | ||
Valenti M, Porzio G, Aielli F, et al. Physical exercise and quality of life in breast cancer survivors. Inter J Med Sci. 2008;5(1):24–28. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.






