Back to Journals » Journal of Pain Research » Volume 10
Effects of decompression on behavioral, electrophysiologic, and histomorphologic recovery in a chronic sciatic nerve compression model of streptozotocin-induced diabetic rats
Authors Wang PH , Yang CC, Su WR, Wu PT, Cheng SC, Jou IM
Received 25 October 2016
Accepted for publication 11 January 2017
Published 20 March 2017 Volume 2017:10 Pages 643—652
DOI https://doi.org/10.2147/JPR.S125693
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Michael Schatman
Ping-Hui Wang,1 Cheng-Chang Yang,2 Wei-Ren Su,3 Po-Ting Wu,3 Shun-Chien Cheng,1 I-Ming Jou4
1Department of Orthopedics, Chi-Mei Medical Center, 2Institute of Basic Medical Sciences, College of Medicine, National Cheng Kung University, 3Department of Orthopedics, National Cheng Kung University Hospital, Tainan, 4Department of Orthopedics, E-Da Hospital, I-Shou University, Kaohsiung, Taiwan
Purpose: To determine susceptibility to decompression surgery in diabetic and nondiabetic peripheral neuropathy using a chronic compression neuropathy model.
Materials and methods: Twenty-four streptozotocin-induced diabetic rats were randomly divided into three groups: group I, chronic compression of the left sciatic nerve for 4 weeks with decompression; group II, similar without decompression; and group III, sham exposing the sciatic nerve only. The other 24 nondiabetic rats were assigned to groups IV–VI, which received compression–decompression, compression, and the sham operation, respectively. Mixed-nerve-elicited somatosensory evoked potentials (M-SSEPs) and compound muscle action potentials (CMAPs) were measured to verify the compression neuropathy in the posttreatment follow-up. Behavioral observations in thermal hyperalgesia tests were quantified before electrophysiologic examinations. Treated and contralateral nerves were harvested for histomorphologic analysis.
Results: Chronic compression of sciatic nerve induced significant reduction of amplitude and increment of latency of M-SSEP and CMAP in both diabetic and nondiabetic rats. Diabetic group changes were more susceptible. Decompression surgery significantly improved both sensory and motor conduction, thermal hyperalgesia, and the mean myelin diameter of the rat sciatic nerve in both diabetic and nondiabetic groups. Near full recovery of motor and sensory function occurred in the nondiabetic rats, but not in the diabetic rats 8 weeks postdecompression.
Conclusion: Behavioral, electrophysiologic, and histomorphologic findings indicate that decompression surgery is effective in both diabetic and nondiabetic peripheral neuropathy.
Keywords: compression, decompression, streptozotocin, sciatic nerve, diabetes, rat
Introduction
Diabetic neuropathy is one of the musculoskeletal complications of diabetes mellitus. It has been established that the incidence of neuropathy is ~50%–70% in diabetic patients.1–3 Diabetic neuropathy-impaired sensory, motor, and autonomic functions result in substantial morbidity, and mortality, such as recurrent foot infections, ulcers, amputation and Charcot’s joint.4 The treatment is often resource-intensive and long-term and impairs the quality of life and psychosocial function of patients.5,6
Median mononeuropathy is the most common peripheral mononeuropathy in diabetic patients. It is estimated that 20%–30% of diabetic patients develop either symptomatic or asymptomatic carpal tunnel syndrome.1,7 Median mononeuropathy in diabetes seems to be a neuropathic, entrapment disease.8 Surgical decompression of the transverse carpal ligament with or without neurolysis is one of the choices in management of carpal tunnel syndrome. The decompression surgery for carpal tunnel syndrome may be required at a 4–14 times greater frequency in diabetic patients than in the general population.9 The results of carpal tunnel decompression in diabetic patients are controversial. Some studies have shown the results of surgery to be similar in both diabetic and normal patients.10–12 Others have shown a less favorable response in diabetic patients.13,14
In streptozotocin (STZ)-induced diabetic rats, hyperglycemia-induced endoneurial edema increases endoneurial pressure with the cessation of circulation at the epineurial level and makes the peripheral nerve more susceptible to compression at anatomical areas where narrowing normally occurs.15–17 Decreased capillary blood flow, nerve conduction velocity, and pain threshold have been demonstrated in STZ-induced diabetic rats.18 Clinical diabetic neuropathy is based on internal diabetic nerve lesions and external compression of peripheral nerve structures. Many animal studies have demonstrated that early decompression at the onset of diabetes can minimize the development of diabetic neuropathy.19–21 The researches in these animal studies did neurolysis before the onset of diabetic neuropathy, which is different from a clinical situation, where patients undergo surgeries when they are symptomatic.19 However, the effect of decompression surgery in the long-term compression of STZ-induced diabetic rats has rarely been studied.
The purpose of this study is to determine the susceptibility of decompression surgery in diabetic and nondiabetic peripheral neuropathy. The chronic compression neuropathy model was applied to the sciatic nerve of STZ-induced diabetic rats. Behavioral, electrophysiologic, and histomorphologic responses were evaluated.
Materials and methods
Animals, STZ induction, and grouping
The experiment was carried out under the control of the Institutional Animal Care and Use Committee, National Cheng Kung University, Taiwan in accordance with the guidelines on animal experiments at National Cheng Kung University Hospital. We used 8-week-old male Wistar rats with an initial body weight of 250–330 g in this study. Diabetes was induced with a single 60 mg/kg intravenous injection of STZ dissolved in normal saline adjusted in a citric acid buffer to pH 4.0 (Sigma, St. Louis, MO, USA) via the femoral vein. The nondiabetic group rats received an equal volume of the vehicle only. One week after the STZ administration, rats with plasma glucose concentrations of 16 mmol/L were selected as the diabetic group. Both nondiabetic and diabetic rats had free access to rat chow and water. After 8 weeks, all of the rats were randomly divided into experimental groups and treated with silicon tubing compression with or without decompression procedures. Twenty-four STZ-induced diabetic rats were randomly assigned to one of three groups, with eight rats in each group. In the case of groups I and II, chronic compression with silicone wrapping with three ligation sutures was employed. After 4 weeks of compression, group I underwent decompression by releasing the ligation and group II had a similar exposing operation without release. Group III served as the control after the sham operation exposing the sciatic nerve only. The nondiabetic rats without STZ induction were assigned to groups IV–VI, which received compression–decompression, compression, and the sham operation, respectively. The rats were housed two per cage under controlled light and temperature conditions and were fed standard rat chow and water. After surgery, each rat was housed individually and checked daily for signs of infection or dehydration.
Animal preparation and nerve compression and decompression surgical procedures
Compression procedure using silastic tubing with ligation
All surgical procedures were done using 50 mg/kg of intraperitoneal (IP) sodium pentobarbital anesthesia (Nembutal; Abbott, North Chicago, IL, USA). The depth of the anesthesia was determined by assessing the withdrawal reflex upon tail pinch, and subsequent doses of pentobarbital were administered as necessary to maintain adequate anesthetic depth. The rats were intramuscularly premedicated with gentamycin (8 mg/kg; Yung Shin Pharmaceutical Industrial Co., Ltd., Taichung, Taiwan). Core temperature was monitored with rectal probes connected to a multichannel thermometer (Portable Hybrid Recorder, model 3087; Yokogawa Hokushin Electric, Tokyo, Japan) and maintained within 37°C–38°C using a homoeothermic animal blanket (Jerboa Scientific, New Taipei City, Taiwan) until recovery. The rat was then placed in a prone position with extended hips. The surgical procedures were done under aseptic conditions after both gluteal regions were shaved and swabbed with Betadine (Sinphar Pharmaceutical Co., Ltd., I-Lan, Taiwan) solution. An incision was made from the left sciatic notch to the distal thigh. The subcutaneous tissue was bluntly dissected under the skin to expose the biceps femoris muscle. A 20 mm segment of sciatic nerve was freed from its investing fascia with the aid of a 2.5× surgical microscope (Axio Imager 2; Carl Zeiss Microscopy GmbH, Oberkochen, Germany). For the sham, an identical surgery was then performed on the right side. The sciatic nerve was separated over a 20 mm length from the surrounding tissues; a silastic tube was split longitudinally and then slipped around the sciatic nerve, whose extrinsic and intrinsic blood was not disturbed. Three 8-0 nylon sutures were placed to close the silastic tube. The tube diameter approximated the diameter of the nerve. The date of the ligated silastic tubing procedure was regarded as day 0 for both diabetic and nondiabetic groups.
Decompression procedure by releasing ligatures on silastic tubing
The groups I and IV rats underwent decompressive release procedures 4 weeks after the ligated silastic tubing procedure with the induction of chronic compressive sciatic nerve neuropathy, which was confirmed with an electrophysiologic and functional evaluation.
Recording of sensory and motor evoked potentials
The electrophysiologic data were collected, stored, and analyzed on an electrodiagnostic device (Neuropack Z; Nihon Kodan, Tokyo, Japan). Two electrophysiologic surveillance systems were setup and recorded prior to, immediately after, 2 weeks and 1, 2, and 3 months postoperatively after the behavioral examinations (walking track tests). The animals were prepared as mentioned in the electrophysiologic surveillance procedures. After the rat was anesthetized, the rat was then placed in a prone position with its head fixed firmly in a stereotactic frame. A 2 cm longitudinal incision was made in the back, from the thoracolumbar (T-L) junction to the upper lumbar spine. Then, two stimulating and recording systems were setup.
Ascending evoked potentials elicited from bilateral lower extremities
Spinal somatosensory evoked potentials (SSEPs) were recorded using bipolar needle electrodes. The recording cathode was placed in the T-L interspinous ligament; a corresponding reference electrode was placed in subcutaneous tissue just proximal to the recording electrode, and a ground electrode was placed in the pelvic girdle ipsilateral to the side stimulated. Stimulation was delivered with subcutaneous needle electrodes placed from the medial ankle to the tibial nerve just medial to both ankles. Square pulse impulses 0.2 ms in duration with an intensity five times greater than the threshold of visible potential was used. The stimulation rate was 5 impulses per second, with 20 repetitions. The recording was filtered for data from 10 to 5000 Hz; recording time was 20 ms.
Descending compound muscle action potential (CMAP)
CMAP was recorded from monopolar myographic needle electrodes placed in each belly of the bilateral gastrocnemius muscles by stimulation of the spinal cord at T12-L3 using needle electrodes in the interspinous ligament. The acquisition parameters were similar to those for the SSEP, but the presentation rate of stimulation decreased to 1/s, and the low and high linear range of the filter was between 1 and 2000 Hz. A ground electrode was placed subcutaneously between the stimulus and the recording site. At least three sequential single-sweep runs (i.e., without averaging) with similar waveforms were recorded to check and verify the consistency of the responses.
Behavioral examinations
The thermal pain test was run on the same schedule as the electrophysiologic examination (pretreatment and posttreatment at 2 weeks and 1, 2, and 3 months). Before testing with a thermal pain response measurement device (UgoBasile, Comerio, Italy) to which the rats were acclimated for 10 min, individual measurements were repeated four or five times, and the mean value in seconds was then calculated as the thermal pain threshold.
Killing, perfusion fixation, and histopathologic examination
After the final behavioral and electrophysiologic analysis was performed 3 months after the operation, each rat was anesthetized with an overdose of pentobarbital (100 mg/kg; IP). All rats were perfused transcardially with normal saline containing 0.002% NaNO2 and 0.002% heparin, followed by a fixative containing 4% paraformaldehyde in 0.01 M phosphate-buffered saline (pH 7.4). The nerves were bluntly dissected from the dorsal root ganglion to a point distal to the peroneal/tibial nerve divisions.
The sciatic nerve was harvested 5 mm from the epicenter proximally and distally and then immersed in 4% paraformaldehyde overnight. Tissue samples were fixed with 1% osmium tetroxide for 2 h and then dehydrated with graded alcohol and embedded in resin. One micrometer thick sections were collected, and the myelin was observed (magnification 200×) under a microscope (Axio Imager 2; Carl Zeiss Microscopy GmbH). Five random views of a sciatic nerve cross-section were photographed. The mean diameter and number of myelin sheaths were manually calculated by two researchers blinded to each other’s results.
Statistical analysis
Statistical analyses of the body weight, electrophysiologic examinations, and thermal hyperalgesia tests of each decompression group were compared with both the normal controls and the operated but nondecompression controls. These were done using a repeated-measure two-way analysis of variance (ANOVA) with a post hoc Kruskal–Wallis test. The myelin sheath mean diameter and number were done using an one-way ANOVA and then the Kruskal–Wallis test. Additionally, each group was compared with each time period (baseline, immediate, 2, 4 weeks, and 2 and 3 months after operation); and each treatment group was compared with the others using a one-way ANOVA. Significance was set at P<0.05.
Results
General postoperative conditions
The general conditions of all the rats were good. They showed steady body-weight gains throughout the 3-month observation period . There were no significant differences among the three experimental subgroups of diabetic and nondiabetic rats. However, the body-weight gains were less in the diabetic groups (Table 1).
Electrophysiologic findings
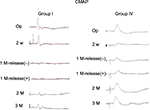
In this study, mixed-nerve-elicited SSEP (M-SSEP) and CMAP monitoring were successful, the M-SSEPs at the T-L junction interspinous ligament were consistent and stable; they showed a major negative wave preceded by a small positive wave. CMAPs were also consistently obtained from gastrocnemius muscles with large amplitude and consistent latency. There were neither significant differences between the right and left lower limbs in amplitude and latency, nor were there between the nondiabetic and diabetic groups in basic preoperative recordings. As expected, except for noncompression right side of rats in sham groups III and VI, the compressive left side of rats in groups I, II, IV, V showed amplitude loss and latency prolongation of the M-SSEP and CMAP on the experimental side after the compressive operation (Figures 1 and 2). All compressive experimental groups were statistically similar in this regard, which indicated that equally consistent damage was induced by this injury model. The amplitude and latency of the M-SSEP and CMAP for all groups after the operation for different time points for 3 months are given in Tables 2–5.
  | Table 2 Comparison of amplitude change of SSEPs between all groups Notes: All values represent relative values (mean ± SD) of peak amplitude with percentage of compression/noncompression side of the same operated rat. *Represents the range of change. Different superscript letters and numbers indicate a statistically significant difference across groups1,2 or time pointsa–d by analysis of variance (Kruskal–Wallis test). Group I: diabetic compression-decompression; II: diabetic compression; III: diabetic sham; IV: nondiabetic compression-decompression; V: nondiabetic compression; VI: nondiabetic sham. Abbreviations: ND, not done; NS, no significant; SD, standard deviation; SSEPs, spinal somatosensory evoked potentials. |
  | Table 3 Comparison of latency change of SSEPs between all groups Notes: All values represent relative values (mean ± SD) of peak amplitude with percentage of compression/noncompression side of the same operated rat. *Represents the range of change. Different superscript letters and numbers indicate a statistically significant difference across groups1–3 or time pointsa–d by analysis of variance (Kruskal–Wallis test). Group I: diabetic compression-decompression; II: diabetic compression; III: diabetic sham; IV: nondiabetic compression-decompression; V: nondiabetic compression; VI: nondiabetic sham. Abbreviations: ND, not done; NS, no significant; SD, standard deviation; SSEPs, spinal somatosensory evoked potentials. |
  | Table 4 Comparison of amplitude change of CMAPs between all groups Notes: All values represent relative values (mean ± SD) of peak amplitude with percentage of compression/noncompression side of the same operated rat. *Represents the range of change. Different superscript letters and numbers indicate a statistically significant difference across groups1–4 or time pointsa–d by analysis of variance (Kruskal–Wallis test). Group I: diabetic compression-decompression; II: diabetic compression; III: diabetic sham; IV: nondiabetic compression-decompression; V: nondiabetic compression; VI: nondiabetic sham. Abbreviations: CMAPs, compound muscle action potentials; ND, not done; NS, no significant; SD, standard deviation. |
  | Table 5 Comparison of latency change of CMAPs between all groups Notes: All values represent relative values (mean ± SD) of peak amplitude with percentage of compression/noncompression side of the same operated rat. *Represents the range of change. Different superscript letters and numbers indicate a statistically significant difference across groups1–3 or time pointsa–e by analysis of variance (Kruskal–Wallis test). Group I: diabetic compression-decompression; II: diabetic compression; III: diabetic sham; IV: nondiabetic compression-decompression; V: nondiabetic compression; VI: nondiabetic sham. Abbreviations: CMAPs, compound muscle action potentials; ND, not done; NS, no significant; SD, standard deviation. |
At 8 and 12 weeks, postdecompression rats in groups I and IV showed that the amplitude loss and latency prolongation improved significantly the M-SSEP and CMAP. In contrast, groups II and V showed no change. However, compared with precompression baseline levels, the significant amplitude and latency improvement persisted more in the nondiabetic rats (group IV) than in the diabetic rats (group I). Moreover, at 8 weeks postdecompression there were no differences between the nondiabetic group (group IV) and the sham group (group VI), but still had significant difference between the diabetic group (group I) and the sham group (group III). These findings suggested incomplete but significant recovery of neural conduction of both sensory and motor tracts in the diabetic group and full recovery in the nondiabetic group.
Behavioral observation and functional assessment using thermal hyperalgesia test
A thermal hyperalgesia test showed significant sciatic functional impairment in the four compressive experimental groups (groups I, II, IV, and V) compared with preoperative levels. The paw withdrawal thresholds for noxious thermal stimuli were significantly lower in the four experimental groups as compared to the sham groups (groups III and VI) at 2 and 4 weeks postcompression, which is consistent with compression-induced thermal hyperalgesia. Rats in the nondecompression groups (groups II and V) showed progressive and significant decreases in thermal hyperalgesia for the entire 12 weeks of follow-up. Rats in decompression groups I and IV showed progressive and significant improvement in thermal hyperalgesia postdecompression at 4 and 8 weeks but did not achieve their precompression level (Table 6).
Histologic observation
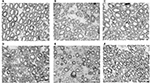
Pathologic tests of the tissue from the diabetic and nondiabetic compression rats showed significant morphologic changes as compared to tests of tissue from the sham rats (Figure 3). There were numerous small diameter myelinated fibers and evidence of demyelination (Figure 4).
The mean diameter of the myelinated fiber was significantly decreased in both the nondiabetic and diabetic rat groups (sham/group VI: 6.68±0.60 μm; nondiabetic compression/group V: 4.23±0.13 μm; diabetic compression/group II: 2.54±0.17 μm). The difference between the compression and decompression groups was significant (nondiabetes: 4.23±0.13 μm vs 7.58±0.27 μm [group V vs IV]; diabetes: 2.54±0.17 μm vs 4.27±0.37 μm [group II vs I]). The mean myelinated fiber diameters without compression in the diabetic group were less than those in the nondiabetic group (nondiabetes/group VI: 6.68±0.60 μm; diabetes/group III: 5.919±0.55 μm; Figure 5).
Discussion
STZ induces non-ketones hyperglycemia, which is similar to diabetes in some animal species. Due to the hyperglycemia effect, the body weights of STZ-induced diabetic rats have been found to be significantly lower than those in nondiabetic rats.22,23 This is compatible with our finding of less body-weight gains in diabetic groups.
The results of the study demonstrated chronic compression of a significant sciatic nerve-induced reduction in amplitude and increment of latency of the M-SSEP and CMAP in both the diabetic and nondiabetic rats. The diabetic groups were more susceptible to change. Decompression surgery significantly improved both sensory and motor conduction and improved thermal hyperalgesia and the mean myelin diameter of the sciatic nerve in both groups of rats. The recovery of motor and sensory function was significant 4 weeks after decompression, and there was complete recovery in nondiabetic rats but not in diabetic rats 8 weeks postdecompression. This finding suggests that nerve function was still under the influence of hyperglycemia and thus lowered the recovery rate of the diabetic rats.
Histophysiologic studies of STZ-induced diabetic rats have revealed reductions in average myelin surface, the myelin/axon ratio, increased endoneurial space, a reduction in the velocity of conduction, and a lower pain threshold.18,24 Similar findings for striking losses of myelinated fiber have also been noted in human diabetic neuropathies.25 The pathophysiologic findings of diabetic neuropathy are similar in humans and STZ-induced diabetic rats. There are many chronic nerve compression animal models. Chronic nerve compression models in rats indicate progressive epineurial and perineurial fibrosis and thinning of the myelin based on the duration of compression. The changes seen in rats are identical to these seen in human beings.26 Locally ligated silastic-tubing induced entrapment is one of the models. The experimental findings in the model used in our prior study showed progressive and consistent neurologic dysfunction with a decline in amplitude and a prolongation of latency after compression.27 We used this model in STZ-induced diabetic rats because it may meet the criteria for mimicking the pathogenesis and clinical entrapment neuropathy of carpal tunnel and cubital tunnel syndrome.28,29
Diabetes impairs glucose metabolism and induces musculoskeletal complications, including connective tissue disorders, neuropathy, and vasculopathy. The pathogenesis of diabetic neuropathy is complex and includes microvascular damage, metabolic insult, and immune-neuronal interactions.30 Diabetes has been shown to impair acetylcholine-induced vasodilatation of arterioles and to cause reduction in endoneurial blood flow.31 A disruption of blood nerve barrier function might cause increased endoneurial fluid pressure and perineurial edema. It has been noted that nerve tissue is then replaced by fibrotic tissue, which causes changes in large myelinated fiber at the peripheral of fascicle and the node of Ranvier.32 These processes slow motor and sensory nerve conduction. Persist hyperglycemia generates excess nicotinamide adenine dinucleotide and leads to an overload in the electron transport chain, causing oxidation stress damage to mitochondria and activation of poly (adenosine diphosphate-ribase) polymerase (PARP). A combination of PARP with hexosamine and protein kinase C activation induces inflammation and neural dysfunction. The conjoining of PARP, advanced glycation end product activation, results in redox imbalance, gene expression disturbance, and further oxidation stress. Finally, there is more inflammation and neuronal dysfunction.4,33
The double crush concept states that nerves subjected to metabolic or mechanical compressions at one site are more prone to experience damage at another site.34 Diabetes induced decreases in axoplasmic blood flow and neuronal dysfunction in nerves would act as the first crush.16 Ligated silastic-tubing-induced chronic nerve entrapment might represent a normal anatomic contraction such as occurs in the transverse carpal ligament for carpal tunnel syndrome at the wrist or in Osborne’s ligament for cubital tunnel syndrome at the elbow. The double crush hypothesis can explain why the diabetic nerve is more susceptible than a nondiabetic nerve, as confirmed with our study, where diabetic groups were shown to be more susceptible to a reduction in amplitude and increments of M-SSEP and CMAP latency. In our study, diabetic group I compared with group II exhibited significant behavioral and neurophysiologic recovery. This indicated the effect that decompression surgery released the second crush in the diabetic group. A more significant decompression effect was also demonstrated in nondiabetic group IV than in diabetic group I. This indicated that the first crush effect still influenced the nerve recovery in the diabetic rats.
Diabetic neuropathy was found to evoke an irreversible change. Diabetes induced microangiopathy at the peripheral nerve, radial stress, attenuated inflammation, retrograde neuron loss/attenuated cell body response, and impaired neurotrophic support, etc. This impaired peripheral nerve regeneration and regeneration of abnormal nerves.35,36 Surgical outcomes for nerve decompression among diabetic patients have been shown to be variable. A Cochrane review study in the decompression of superimposed nerve compression of the lower limbs in patients with symmetrical diabetic peripheral neuropathy failed to identify a definitive result.37 The outcomes of surgical decompression of carpal tunnel syndrome in diabetic patients have also been variable. Some clinical and electrophysiologic results have showed less than favorable results.13,14,38,39 However, others have found some beneficial outcomes.10,11,40 In our study, thermal hyperalgesia recovery after decompression surgery occurred in both the nondiabetic and diabetic groups. However, these results were not compatible with the complete sensory and motor conduction improvement in nondiabetic group. Thermal hyperalgesia is a reflex activity. The changes in reflex activity might be due to alterations in motor and sensory processing.41 The process of motor control involves a complex nerve action.42 This might have influenced the complete functional recovery. However, our results were compatible with most studies focusing on functional and electrophysiologic findings in clinical settings.
Limitations of this study
One limitation of our study is the lack of a long-term end-point that might have given us the opportunity to draw another and stronger conclusion about the long-term therapeutic effects of decompression surgery. However, because evidence from clinical practice indicates that there are no consistent long-term results of decompression surgery, the clinical applicability of this type of research is undoubtedly limited. A second limitation is the rat sciatic nerve silastic-tubing chronic nerve compression model is a well-established nerve injury model, but it may not be applicable to human carpal tunnel syndrome because the mechanism of human entrapment neuropathy is not like direct compression on the rat sciatic nerve. A third limitation is that when we interpreted the histologic results of this study, we used only hematoxylin and eosin staining. We used eosin staining in lieu of more advanced histochemical and immunohistochemical techniques and electron microscopy to detect the changes. A fourth limitation is that we used STZ-induced diabetic rats without hyperglycemia control in this study which is unlike some clinical conditions.
Conclusion
In conclusion, electrophysiologic deterioration of the sciatic nerve was attenuated; behavioral function improved, and the mean myelin diameter of the myelinated fiber augmented significantly in the decompression groups with changes found in the nondiabetic rats. Additional studies are necessary for a better understanding of the mechanisms involved. Our most important finding is that decompression surgery is effective in diabetic peripheral neuropathy.
Acknowledgments
This work was supported by grants from the Chi-Mei Medical Center and National Cheng Kung University (CMNCKU10417), Taiwan.
Disclosure
The authors report no conflicts of interest in this work.
References
Dyck PJ, Kratz KM, Karnes JL, et al. The prevalence by staged severity of various types of diabetic neuropathy, retinopathy, and nephropathy in a population-based cohort: the Rochester Diabetic Neuropathy Study. Neurology. 1993;43(4):817–824. | ||
Young MJ, Boulton AJ, MacLeod AF, Williams DR, Sonksen PH. A multicentre study of the prevalence of diabetic peripheral neuropathy in the United Kingdom hospital clinic population. Diabetologia. 1993;36(2):150–154. | ||
Abbott CA, Malik RA, van Ross ER, Kulkarni J, Boulton AJ. Prevalence and characteristics of painful diabetic neuropathy in a large community-based diabetic population in the U.K. Diabetes Care. 2011;34(10):2220–2224. | ||
Edwards JL, Vincent AM, Cheng HT, Feldman EL. Diabetic neuropathy: mechanisms to management. Pharmacol Ther. 2008;120(1):1–34. | ||
Gordois A, Scuffham P, Shearer A, Oglesby A, Tobian JA. The health care costs of diabetic peripheral neuropathy in the US. Diabetes Care. 2003;26(6):1790–1795. | ||
Vileikyte L, Rubin RR, Leventhal H. Psychological aspects of diabetic neuropathic foot complications: an overview. Diabetes Metab Res Rev. 2004;20 (Suppl 1):S13–S18. | ||
Gamstedt A, Holm-Glad J, Ohlson CG, Sundström M. Hand abnormalities are strongly associated with the duration of diabetes mellitus. J Intern Med. 1993;234(2):189–193. | ||
Fitzgibbons PG, Weiss AP. Hand manifestations of diabetes mellitus. J Hand Surg Am. 2008;33(5):771–775. | ||
Makepeace A, Davis WA, Bruce DG, Davis TM. Incidence and determinants of carpal tunnel decompression surgery in type 2 diabetes: the Fremantle Diabetes Study. Diabetes Care. 2008;31(3):498–500. | ||
Mondelli M, Padua L, Reale F, Signorini AM, Romano C. Outcome of surgical release among diabetics with carpal tunnel syndrome. Arch Phys Med Rehabil. 2004;85(1):7–13. | ||
Thomsen NO, Cederlund R, Rosén I, Björk J, Dahlin LB. Clinical outcomes of surgical release among diabetic patients with carpal tunnel syndrome: prospective follow-up with matched controls. J Hand Surg Am. 2009;34(7):1177–1187. | ||
Thomsen NO, Rosén I, Dahlin LB. Neurophysiologic recovery after carpal tunnel release in diabetic patients. Clin Neurophysiol. 2010;121(9):1569–1573. | ||
Pagnanelli DM, Barrer SJ. Outcome of carpal tunnel release surgery in patients with diabetes. Neurosurg Focus. 1997;3(1):e12. | ||
Ozkul Y, Sabuncu T, Kocabey Y, Nazligul Y. Outcomes of carpal tunnel release in diabetic and non-diabetic patients. Acta Neurol Scand. 2002;106(3):168–172. | ||
Tuck RR, Schmelzer JD, Low PA. Endoneurial blood flow and oxygen tension in the sciatic nerves of rats with experimental diabetic neuropathy. Brain. 1984;107(Pt 3):935–950. | ||
Dellon AL, Mackinnon SE, Seiler WA IV. Susceptibility of the diabetic nerve to chronic compression. Ann Plast Surg. 1988;20(2):117–119. | ||
Jakobsen J. Peripheral nerves in early experimental diabetes: expansion of the endoneurial space as a cause of increased water content. Diabetologia. 1978;14(2):113–119. | ||
Walker D, Carrington A, Cannan SA, et al. Structural abnormalities do not explain the early functional abnormalities in the peripheral nerves of the streptozotocin diabetic rat. J Anat. 1999;195(Pt 3):419–427. | ||
Dellon AL, Dellon ES, Seiler WA IV. Effect of tarsal tunnel decompression in the streptozotocin-induced diabetic rat. Microsurgery. 1994;15(4):265–268. | ||
Kale B, Yüksel F, Celiköz B, Sirvanci S, Ergün O, Arbak S. Effect of various nerve decompression procedures on the functions of distal limbs in streptozotocin-induced diabetic rats: further optimism in diabetic neuropathy. Plast Reconstr Surg. 2003;111(7):2265–2272. | ||
Siemionow M, Sari A, Demir Y. Effect of early nerve release on the progression of neuropathy in diabetic rats. Ann Plast Surg. 2007;59(1):102–108. | ||
Akbarzadeh A, Norouzian D, Mehrabi MR, et al. Induction of diabetes by Streptozotocin in rats. Indian J Clin Biochem. 2007;22(2):60–64. | ||
Deeds MC, Anderson JM, Armstrong AS, et al. Single dose streptozotocin-induced diabetes: considerations for study design in islet transplantation models. Lab Anim. 2011;45(3):131–140. | ||
Zemp C, Bestetti G, Rossi GL. Morphological and morphometric study of peripheral nerves from rats with streptozotocin-induced diabetes mellitus. Acta Neuropathol. 1981;53(2):99–106. | ||
Dyck PJ, Giannini C. Pathologic alterations in the diabetic neuropathies of humans: a review. J Neuropathol Exp Neurol. 1996;55(12):1181–1193. | ||
O’Brien JP, Mackinnon SE, MacLean AR, Hudson AR, Dellon AL, Hunter DA. A model of chronic nerve compression in the rat. Ann Plast Surg. 1987;19(5):430–435. | ||
Wang PH, Tsai CL, Lee JS, Wu KC, Cheng KI, Jou IM. Effects of topical corticosteroids on the sciatic nerve: an experimental study to adduce the safety in treating carpal tunnel syndrome. J Hand Surg Eur. 2011;36(3):236–243. | ||
Gupta R, Steward O. Chronic nerve compression induces concurrent apoptosis and proliferation of Schwann cells. J Comp Neurol. 2003;461(2):174–186. | ||
Gupta R, Nassiri N, Hazel A, Bathen M, Mozaffar T. Chronic nerve compression alters Schwann cell myelin architecture in a murine model. Muscle Nerve. 2012;45(2):231–241. | ||
Zychowska M, Rojewska E, Przewlocka B, Mika J. Mechanisms and pharmacology of diabetic neuropathy – experimental and clinical studies. Pharmacol Rep. 2013;65(6):1601–1610. | ||
Coppey LJ, Davidson EP, Dunlap JA, Lund DD, Yorek MA. Slowing of motor nerve conduction velocity in streptozotocin-induced diabetic rats is preceded by impaired vasodilation in arterioles that overlie the sciatic nerve. Int J Exp Diabetes Res. 2000;1(2):131–143 | ||
Mackinnon SE, Dellon AL, Hudson AR, Hunter DA. Chronic nerve compression – an experimental model in the rat. Ann Plast Surg. 1984;13(2):112–120. | ||
Obrosova IG. Diabetes and the peripheral nerve. Biochim Biophys Acta. 2009;1792(10):931–940. | ||
Upton AR, McComas AJ. The double crush in nerve entrapment syndromes. Lancet. 1973;2(7825):359–362. | ||
Yasuda H, Terada M, Maeda K, et al. Diabetic neuropathy and nerve regeneration. Prog Neurobiol. 2003;69(4):229–285. | ||
Kennedy JM, Zochodne DW. Impaired peripheral nerve regeneration in diabetes mellitus. J Peripher Nerv Syst. 2005;10(2):144–157. | ||
Chaudhry V, Russell J, Belzberg A. Decompressive surgery of lower limbs for symmetrical diabetic peripheral neuropathy. Cochrane Database Syst Rev. 2008;16(3):CD006152. | ||
Haupt WF, Wintzer G, Schop A, Löttgen J, Pawlik G. Long-term results of carpal tunnel decompression. Assessment of 60 cases. J Hand Surg Br. 1993;18(4):471–474. | ||
Al-Qattan MM, Manktelow RT, Bowen CV. Outcome of carpal tunnel release in diabetic patients. J Hand Surg Br. 1994;19(5):626–629. | ||
Jenkins PJ, Duckworth AD, Watts AC, McEachan JE. The outcome of carpal tunnel decompression in patients with diabetes mellitus. J Bone Joint Surg Br. 2012;94(6):811–814. | ||
Baliki M, Calvo O, Chialvo DR, Apkarian AV. Spared nerve injury rats exhibit thermal hyperalgesia on an automated operant dynamic thermal escape task. Mol. 2005;26:1–18. | ||
Latash ML, Levin MF, Scholz JP, Schöner G. Motor control theories and their applications. Medicina (Kaunas). 2010;46(6):382–392. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.