Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 11
Effects of Caralluma russeliana stem extract on some physiological parameters in streptozotocin-induced diabetic male rats
Authors Zari TA , Al-Thebaiti MA
Received 24 May 2018
Accepted for publication 3 August 2018
Published 12 October 2018 Volume 2018:11 Pages 619—631
DOI https://doi.org/10.2147/DMSO.S167293
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ming-Hui Zou
Talal A Zari, Mesfer A Al-Thebaiti
Department of Biological Sciences, Faculty of Science, King Abdulaziz University, Jeddah, Saudi Arabia
Purpose: The aim of this study was to investigate the effects of Caralluma russeliana stem extract on some physiological parameters in streptozotocin induced diabetes in male Wistar rats after 8 weeks.
Materials and methods: The experimental rats were randomly assigned into four groups. Rats of group 1 were normal controls. Rats of group 2 were diabetic controls. Rats of group 3 were diabetic rats treated with C. russeliana stem extract. Rats of group 4 were non-diabetic rats, subjected to C. russeliana stem extract.
Results: The lowest body weight gain was noticed in diabetic rats of group 2. Serum glucose, triglycerides, cholesterol, low-density lipoprotein cholesterol, very low-density lipoprotein cholesterol, alanine aminotransferase, aspartate aminotransferase, ALP, total bilirubin, creatinine, blood urea nitrogen (BUN) and uric acid levels were significantly elevated in diabetic rats of group 2; however, total serum protein, albumin and high-density lipoprotein cholesterol were significantly reduced in diabetic rats of group 2.
Conclusion: Treatments with C. russeliana stem extract in diabetic rats revealed notable diminishing and protecting effects of physiological modifications. Therefore, this study revealed the significance of using C. russeliana stem extract as a promising remedial agent to treat diabetes and its complications.
Keywords: Caralluma russeliana, diabetes, streptozotocin, weight loss, glucose, lipids, liver enzymes, kidney function, rats
Introduction
Diabetes mellitus (DM) is a chronic metabolic syndrome characterized by elevated blood glucose levels with disturbance of carbohydrate, lipid and protein metabolism due to the insufficient insulin secretion, inadequate insulin action, or a combination of both.1 DM is a disorder influencing 366 million persons worldwide, a number that might increase to 552 million by 2030.2 The complications of DM are classified as microvascular (retinopathy, neuropathy and nephropathy) or macrovascular (cardiovascular and cerebrovascular diseases).3 Glycemic control in type 2 DM is currently complex despite the availability of many pharmacological agents and growing concerns about their potential adverse effects.4–7 Furthermore, elevated glycemic management is hard to accomplish, and previous studies have revealed numerous causes contributing to inadequate management among diabetic persons.8–10 The International Diabetes Federation has verified that Saudi Arabia is one of the top 10 countries with the maximum DM rates in adults worldwide.11 In addition, the incidence in Saudi Arabia has increased speedily in recent years.12 Diabetes may be induced by particular destruction of β-cells of the pancreas with a single, fast injection of streptozotocin (STZ). STZ has been utilized as a diabetogenic agent in animals.13–17 There is currently no adequate efficient treatment to heal diabetes. DM control by insulin has a number of problems such as insulin resistance.18 Furthermore, in chronic administration, it causes fatty liver, anorexia nervosa and brain atrophy.19
Medicinal herbs and plants have commonly been a significant tool for discovering new therapies to cure human diseases. Diverse herbs have traditionally been used for diabetes management. Many investigators have documented the antidiabetic properties of several plants.20 The plants of genus Caralluma are generally distributed in Africa, Asia, Southeast Europe, Canary Islands, Arabian Peninsula and South Africa.21,22 The species of genus Caralluma (family Apocynaceae) are xerophytic plants. Formerly, genus Caralluma is a member of family Asclepiadaceae.23 Caralluma species are small, erect and fleshy plants. They are mostly succulent perennial herbs, some of which are documented as edible species.24 Different medicinal utilizations of Caralluma species have been reported in the Indian and Arabic traditional medicine such as in the therapy of cancer, diabetes, inflammation, tuberculosis, skin rashes, scabies, fever, snake and scorpion bites.25–28 Most general utilizations of these plants have been documented as a famine food without any negative effect recorded. In general, species of Caralluma contain several components such as pregnane glycosides, flavone and megastigmane glycosides and different esters, which confirm their medicinal value.29–31 Antidiabetic, anticancer, antioxidant, anti-inflammatory, antieczemic, antimicrobial and antifungal characteristics of the different extracts of Caralluma demonstrated their pharmacological significance.31,32
In this study, Caralluma russeliana was collected from the outskirts of Taif city in Saudi Arabia during 2015. There is little research about the antidiabetic activities of this species. Therefore, we hoped 1) to distinguish antidiabetic properties of this species and 2) to demonstrate a relationship between its traditional uses and scientific research, postulating that the tested physiological parameters, for the first time, would improve in diabetic animals after administration of C. russeliana stem extract. Several species of Caralluma contain a number of bioactive components, which confirm their medicinal value and their pharmacological significance. They possess antidiabetic, anticancer, antioxidant, anti-inflammatory and antimicrobial characteristics. In addition, a number of Caralluma species have been reported in the traditional medicine for the treatment of various diseases such as diabetes, cancer, inflammation, tuberculosis, skin rashes, scabies and fever.25–32 Thus, the aims of this study are to examine the effects of C. russeliana stem extract on certain physiological parameters in male Wistar rats with STZ-induced diabetes after 8 weeks and to prove a relationship between the traditional utilizations and scientific research.
Materials and methods
Animals
Eighty adult male Wistar rats (180.1–219.5 g) were used in this study. The rats were obtained from the Animal Unit of King Fahd Medical Research Center, King Abdulaziz University, Jeddah. Animals were acclimated to the laboratory circumstances for 1 week before the start of the experiments. All rats were kept in standard plastic cages and sustained under controlled laboratory situations of humidity (55%±10%), temperature (24°C±1°C) and light (12/12-hour light/dark cycle). They were fed ad libitum on normal commercial pellet diet and had free access to water. All experiments were performed according to ethical guidelines of the animal care and use committee of King Abdulaziz University, Saudi Arabia. The research was approved by the committee of King Abdulaziz University, Jeddah, Saudi Arabia.
Extraction of C. russeliana stems
Fresh C. russeliana stems were collected from Taif city outskirts in Saudi Arabia during July 2015. The plant was scientifically defined by the herbarium of the Department of Biological Sciences, Faculty of Science, King Abdulaziz University, Jeddah. A voucher specimen (No 9721) has been deposited in the herbarium of Department of Biological Sciences, Faculty of Science, King Abdulaziz University. The collected samples were totally washed and then dried at room temperature and stored in dry containers until extraction. The method of Al-Attar and Abu Zeid33 was used to prepare the extract of C. russeliana stem with some alterations. The aqueous extracts were prepared every 2 weeks. The dried samples of C. russeliana (150 g) were powdered and then added to 6 L of hot water. After 5 hours, the mixture was gradually boiled for 1 hour. After that, the mixture was cooled at room temperature, and it was coarsely blended in an electric mixer for 20 minutes. Then, plant solutions were filtered using 250 mm filter papers (Whatman, Maidstone, UK). The filtrates were finally evaporated in an oven at 40°C to make dried residues (active principles). With regard to the powdered samples, the yield mean of C. russeliana stem extract was 20.2%. Afterward, this extract was stored in a refrigerator for the following experiments.
Diabetes induction
DM was induced in rats by intraperitoneal (i.p.) injection of STZ (Sigma-Aldrich Co., St Louis, MO, USA) at a single dose of 60 mg/kg body weight dissolved in saline solution after overnight fasting. Afterward, STZ-injected animals had free access to water and food. Diabetes was permitted to develop and become stable in these STZ-treated animals over 4 days. They were considered diabetic rats if their fasting blood glucose levels were over 300 mg/dL.
Study design
The experimental animals were randomly assigned into four groups, each group consisting of 20 rats. The groups were treated as follows:
- Rats of group 1 were the normal control.
- Diabetic rats of group 2 were the diabetic control.
- Diabetic rats of group 3 were orally supplemented with C. russeliana stem extract at a dose of 300 mg/kg body weight/day.
- Non-diabetic rats of group 4 were orally supplemented with C. russeliana stem extract at a dose of 300 mg/kg body weight/day.
In this study, the extract dose (300 mg/kg body weight/day) refers to the weight of the concentrated plant extract. All experimental treatments continued for 8 weeks.
Weight determinations
The weights of rats were determined at the beginning of the experimental period and after 8 weeks using a digital balance. The experimental rats were also noted for abnormality signs during the study.
Blood serum analyses
After 8 weeks, rats were fasted for 8 hours; water was unrestricted, and then blood samples were collected from the orbital venous plexus of diethyl ether anaesthetized rats into non-heparinized tubes. Then, blood specimens were centrifuged at 2,500 rpm for 15 minutes, and the clear samples of blood serum were separated and stored at –80°C. The serum samples were utilized to measure the levels of glucose, total protein, albumin, triglycerides, cholesterol, high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), very low-density lipoprotein cholesterol (VLDL-C), alanine aminotransferase (ALT), aspartate aminotransferase (AST), ALP, total bilirubin, creatinine, blood urea nitrogen (BUN) and uric acid. All these parameters were determined using an automatic analyzer (Architect c8000 Clinical Chemistry System, Abbott, IL, USA).
Statistical analyses
Data were analyzed using SPSS Package for windows, version 13.0 (IBM Corporation, Armonk, NY, USA). Results were presented as mean±standard error of the mean (SE). Comparisons among groups were carried out using one-way ANOVA, followed by a least-significant difference (LSD) test. P<0.05 was considered to be significant.
Results
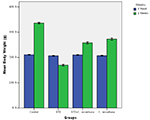
Body weights of all experimental groups after 8 weeks are shown in Table 1 and Figure 1. The maximum weight gain was noted in normal control rats (+59.2%) after 8 weeks. A significant decrease (–17.9%) in weight gain was noticed in diabetic rats fed on normal diet. Weight gain change was +22.8% in diabetic rats supplemented with C. russeliana extract. The percentage change in weight gain in rats treated with C. russeliana extract was +31.6%. Compared to group 1, there were significant decreases in weight of group 2 (P<0.0001), group 3 (P<0.0001) and group 4 (P<0.0001).
The measured levels of serum glucose in control (group 1), STZ (group 2), STZ plus C. russeliana extract (group 3) and C. russeliana extract (group 4)-treated rats are given in Table 2 and Figure 2A. A significant rise in the level of serum glucose was noted in diabetic rats of group 2 (+369.2%, P<0.0001) compared to normal control rats of group 1. Insignificant alterations were observed in serum glucose levels in diabetic (group 3) and non-diabetic (group 4) animals treated with C. russeliana extract compared to normal control rats (group 1).
Compared to control rats (group 1), a significant decline in the level of serum total protein was observed in diabetic animals of group 2 (–11.9%, P=0.007). There were no significant variations in serum total protein levels in rats of STZ plus C. russeliana extract (group 3) and C. russeliana extract-treated rats (group 4) compared to normal control animals of group 1 (Table 2 and Figure 2B).
There was a significant decrease in serum albumin level in the diabetic rats of group 2 (–15.4%, P=0.004). However, there were no significant variations in serum albumin levels in diabetic rats (group 3) and non-diabetic rats (group 4) compared to rats of group 1 (Table 2 and Figure 2C).
There was an increase in serum cholesterol level (P<0.0001) in diabetic rats (group 2) compared to other groups. However, serum cholesterol levels were significantly reduced in diabetic rats of group 3 (P=0.007) and non-diabetic group 4 (P<0.0001) compared to control rats of group 1. Furthermore, serum cholesterol level was significantly declined (P<0.0001) in non-diabetic rats of group 4 compared with other groups (Table 2 and Figure 3A).
Compared with control rats (group 1), the level of serum triglycerides was significantly elevated in diabetic rats (group 2) (+100%, P<0.0001). Insignificant changes in the levels of serum triglycerides were noticed in rats of groups 3 and 4 compared to normal rats of group 1 (Table 2 and Figure 3B).
Serum HDL-C level was significantly declined in rats of group 2 (–25.8%, P=0.01) and group 3 (P=0.041) compared to normal control rats of group 1 (Table 2 and Figure 3C).
The level of serum LDL-C was statistically evoked in diabetic rats of group 2 (+121.5, P<0.0001) compared with normal control rats of group 1. Insignificant change was noticed in the serum LDL-C level in rats of groups 3 and 4 (Table 2 and Figure 3D).
Noticeable increase in serum VLDL-C was observed in diabetic rats (group 2) (+97%, P<0.0001) as compared to normal control rats (group 1). However, this value was insignificantly altered in rats of groups 3 and 4 compared to normal control rats of group 1 (Table 2 and Figure 3E).
Compared to normal control rats (group1), statistical increase in the level of serum ALT was noted in diabetic rats of group 2 (+77.3%, P<0.0001). Furthermore, the ALT level was statistically unchanged in rats of groups 3 and 4 compared to normal control rats of group 1 (Table 2 and Figure 4A).
Serum AST level was significantly increased in diabetic rats of group 2 (+20.2%, P<0.0001). This value was statistically unaltered in rats of groups 3 and 4 compared to normal control rats of group 1 (Table 2 and Figure 4B).
As shown in Table 2 and Figure 4C, serum ALP levels were significantly elevated in diabetic rats of group 2 (+185.5%) P<0.0001) and group 3 (+23.5%, P=0.006) compared to normal control rats (group 1). However, there was no significant change in the serum ALP level in non-diabetic rats (group 4) compared to normal control rats (group 1).
Table 2 and Figure 4D show the serum total bilirubin level in all groups. Serum total bilirubin level was statistically evoked in diabetic rats of group 2 (+58.3%, P<0.0001) compared to normal control rats (group1), while there were no significant changes in serum total bilirubin levels in rats of groups 3 and 4. The measured levels of serum creatinine in all groups are shown in Table 2 and Figure 5A. Compared to control rats, serum creatinine level was increased in diabetic rats of group 2 (+38.3%, P<0.0001). Insignificant alterations were found in serum creatinine levels in rats of groups 3 and 4.
Serum BUN level was significantly increased in diabetic rats of group 2 (+147.2, P<0.0001) compared with control level in normal rats (group 1). Serum BUN levels were remarkably unchanged in rats of groups 3 and 4 (Table 2 and Figure 5B)
Table 2 and Figure 5C represent serum uric acid levels in all groups. Serum uric acid level was statistically enhanced in diabetic rats of group 2 (+46.2%, P=0.002) compared to normal control rats (group 1). Insignificant alterations in serum uric acid levels were noticed in diabetic rats of group 3 and non-diabetic rats of group 4 compared to normal control rats (group 1).
Discussion
DM is a global epidemic syndrome that occurs worldwide. The elevated incidence of DM motivated the investigators to search for antidiabetic agents in the traditional medicine. The use of traditional remedies against DM has yielded good results. Herbal medicines are utilized extensively due to their comparatively low costs and their much lower side effects. It has been demonstrated that many active ingredients derived from plants have an antidiabetic activity.34 Leaves, stems, roots, barks, flowers, seeds and fruits may all be constituents of herbal medicines.35–40 Several Caralluma species have been confirmed very efficient to treat DM such as C. attenuata,41 C. tuberculata,42 C. sinaica and C. edulis that can lead to a considerable decline in glucose concentrations.43
In this study, a significant decline in weight gain in STZ diabetic rats was noticed after 8 weeks. Similarly, these results are supported by several studies in STZ-diabetic animals.44–47 The body weight reduction in diabetic animals may be due to protein loss as a result of lack of carbohydrates as a source of energy.48 Increase in food consumption and reduction in body weight were observed in diabetic rats compared to normal rats, which reveals a polyphagic state and weight loss because of extreme destruction of tissue proteins.49 Moreover, this study showed a considerable decline in weight gain in non-diabetic rats treated with C. russeliana stem extract compared to normal control rats. Caralluma species such as C. indica, C. attenuata, C. fimbriata and C. tuberculata have anti-obesity activity.50 C. fimbriata extract (100 mg/kg/day) has considerably decreased the increase in weight and lipid concentrations as compared to the control group fed on cafeteria diet. Therefore, this plant may be helpful in obesity treatment,51 because it is able to reduce appetite and avoid fat deposition. It obstructs the creation of acetyl co-enzyme A and malonyl co-enzyme A, which are the basic components of fat synthesis.52 The appetite suppressing action of C. fimbriata has been attributed to the active constituent pregnane glycosides. C. fimbriata might downregulate ghrelin creation in the stomach and neuropeptide-Y in the hypothalamus, leading to appetite suppression resulting in decreasing obesity.51 Rising evidence implies that taking this plant for 60 days may reduce hunger, calorie intake and waistline.53 Investigators compared baseline indicators of obesity such as serum lipids, glucose, calorie intake, anthropometric measurements and appetite suppression with those after 60 days of taking of C. fimbriata extract.54 The findings demonstrated decline in food consumption, body weight, body mass index, hip circumference and body fat. Comparable outcomes have been documented by Lawrence and Choudhary.55 In another study, it is proved that C. fimbriata has not only the anti-obesogenic but also anti-atherosclerotic abilities.56 In India, Caralluma species are edible and are used in the medicine. C. fimbriata in India is utilized to suppress appetite and to treat diabetes, pain, inflammation and fever. C. tuberculata is eaten and is generally utilized to treat diabetes, rheumatism, leprosy and as antipyretic.57,58 C. attenuata is eaten raw to treat diabetes and its juice together with black pepper is used to treat migraine.59 C. adscendens and C. umbellata have been documented for their antilipidemic activity. C. fimbriata has also revealed antiobesity activity.50
The present elevated levels of serum glucose, triglycerides, cholesterol, LDL-C and VLDL-C with the reduced levels of total protein, albumin and HDL-C in group 2 diabetic rats reveal disturbances in carbohydrates, lipid and protein metabolism as a result of DM. Similar findings were observed by many experimental DM investigations.13,14,44,46,47,60,61 Hypoproteinemia and hypoalbuminemia are linked with liver and kidney dysfunctions. Albumin is produced by the liver. It is a chief synthetic protein and is a marker of liver’s capability to synthesize proteins.62 Albumin is reliant on protein consumption and subject to feedback control by the plasma albumin concentration. Little albumin is filtered through the glomeruli and a large amount of that is reabsorbed by proximal tubule cells and degraded by their lysosomal enzymes into fragments returning to the circulation.63 In DM, the level of circulating albumin is reduced. The degradation of albumin and relative volume of extravascular distribution in DM also decline by around 35%.64
DM and hyperlipidemia are two chief factors that intervene in the development of cardiovascular disease. Treatments of these situations are important modalities in heart disease prevention.65–67 Faults in the action of insulin and elevations in glucose may cause greater amounts of lipoproteins in the blood. Even little elevations in lipids in such diabetic patients are linked with a considerable rise in cardiovascular diseases.68 The main plasma lipid constituents such as cholesterol and triglycerides do not circulate in the free state but are transported as complexes with lipoprotein. Any trouble in the metabolism of lipoprotein is revealed in the lipid profile and liver. As the liver has a main role in the metabolism of lipoprotein, any defect in its action results in lipid profile modifications. A number of factors might play a role in the accumulation of lipids in the liver. The accumulation of fat in the liver might take place if there is a disturbance in the production of lipoprotein, particularly its apoprotein division.69 Increased levels of plasma HDL-C defend the arterial wall from the development of atherosclerotic plaque enhanced by reverse cholesterol transport.70 Plasma HDL-C levels are changed by several means, including the absorption of complete HDL particle.71,72 Several disturbances in metabolic and regulatory mechanisms, owing to insulin shortage, are accountable for the noted lipid accumulation.73 Insulin secretion impairment leads to promoted lipid metabolism from the adipose tissue to the blood. In addition, it has been observed that diabetic rats treated with insulin demonstrate normalized lipid profile.74 Furthermore, it is recognized that in uncontrolled diabetes, there will be elevation in cholesterol, triglycerides, LDL-C and VLDL-C with reduction in HDL-C, which leads to the coronary artery disease observed in some diabetic individuals.75,76 Diabetes has odd lipid metabolism as insulin shortage in the body due to STZ caused harm to β cells in the pancreas. Insulin may trigger lipoprotein lipase, the enzyme lipoprotein solver. In DM, lipoprotein lipase activity reduced as a result of the elevation of concentrations of blood lipoproteins.77
In this study, serum cholesterol level was significantly reduced in non-diabetic rats treated with C. russeliana stem extract compared with other groups. These results revealed that C. russeliana stem extract may affect the metabolic processes of carbohydrates and lipids owing to their chemical constituents. Cholesterol-decreasing activity of C. adscendens aqueous extract showed hypolipidemic action.78 Treatment with C. fimbriata aqueous extract at three different doses may considerably reduce total cholesterol and LDL cholesterol levels compared to controls. In addition, C. umbellata has revealed antilipidemic action, which may be owing to its active components.62 Plant sterols (phytosterols) and plant stanols (phytostanols) are a vast group of compounds that are presented exclusively in plants. Williams and Gokool78 reported that there are several methods in which plant sterols and stanols stop absorption and, therefore, lower cholesterol. Thus, cholesterol is excreted through feces.
This study demonstrated that STZ induced an increase in serum ALT, AST, ALP and total bilirubin levels in rats, because necrosis or damage to the membrane discharges them into circulation, which is consistent with the formerly reported results.80 Serum concentrations of these compounds are very liable markers in liver disease diagnosis.81 Diabetic persons have elevated liver function test abnormalities than non-diabetic patients.82,83 The high levels of these parameters were pinpointing cellular leakage and functional integrity loss of the cell membranes.84 C. diazielli extract has significantly decreased serum liver enzyme levels (AST, alanine transaminase, ALP) in rats with fructose-induced diabetes.85
This study examined the kidney function by measuring serum creatinine, BUN and uric acid concentrations. The current increase in these biochemical parameters proved renal dysfunction in untreated diabetic rats. Creatinine, BUN and uric acid are protein metabolism waste products that require to be excreted through the kidney, consequently an obvious elevation of these compounds, as noticed in this study, validates a signal of kidney functional damage.86 Hyperglycemia excites oxidative abuse in renal tubular epithelial cells and that damage starts tubulointerstitial fibrosis, a distinctive characteristic of diabetic nephropathy, which then progressively leads to renal failure.87,88 It is reported that nephropathy develops in 30%–40% of the diabetic patients and has globally become an important reason of end-stage renal failure.89,90 Diabetic nephropathy is distinguished by structural and functional abnormalities.91 Defective glycemic control and accumulation of advanced glycation end products (AGEs) play a major role in diabetic nephropathy development.90 Furthermore, Mestry et al91 showed that untreated STZ-diabetic rats exhibit a marked damage in renal function, which is proved by the increase in serum creatinine, BUN and uric acid levels.
A number of Caralluma species demonstrated antihyperglycemic action of their extracts or fractions.93 Abdel-Sattar et al92 evaluated the antihyperglycemic effects of C. quadrangula indigenous to Saudi Arabia on STZ-induced diabetic rats. The outcomes revealed a considerable decline in the levels of blood glucose in diabetic-treated rats after the treatment of most extracts and fractions of C. quadrangula and glibenclamide. They concluded that their study confirmed the traditional use of this species to treat DM. Its extract has been utilized in Saudi traditional medicine for the treatment of diabetes, melasma, freckles, vitiligo and in cases of thirst and hunger.
In this study, treatments with C. russeliana stem extract in diabetic rats revealed significant declining and defending effects of physiological modifications. The medicinal values of Caralluma depend on their phytochemical constituents that generate beneficial physiological activities. In Caralluma, the main and distinctive phytochemical components are pregnane glycosides, flavone glycosides, megastigmane glycosides, triterpenes, bitter principles and saponins.29,30
The aerial parts of C. russeliana from Saudi Arabia contain several phytochemical compounds such as acylated pregnane glycosides, russeliosides A–D (1–4), flavone glycoside, luteolin 4′-O-β-D-neohesperidoside, russeliosides, 14β-benzoyloxy-15β-isovaleroyloxy-16β-hydroxypregn-20-on-3-O-(β-d-3-O-methyl-6-deoxyoleandrosopyranosyl-(1→4)-β-d-cymaropyranosyl-(1→4)-β-d-cymaropyranoside) (1) and 14β-isovaleroyloxy-15β-benzoyloxy-16β-hydroxypregn-20-on-3-O-(β-d-3-O-methyl-6-deoxyoleandrosopyranosyl-(1→4)-β-d-cymaropyranosyl-(1→4)-β-d-cymaropyranoside) (2).25,31,94,95 A number of Caralluma species have been utilized to treat different diseases, such as rheumatism, diabetes, cancer, tuberculosis, leprosy, scabies, fever, inflammation, snake and scorpion bites, as antiseptic and disinfectant.96
C. sinica administration in varied doses to healthy animals can cause a considerable decline in the level of glucose.97 C. attenuata and C. edulis extracts had hypoglycemic activities and provide synergistic influence in combination with phlorizin extract that usefully alters the levels of blood insulin, blood and urine glucose as well as glucose transport and aids in weight loss.98 Abdel-Sattar et al99 studied the potential and mechanisms of the antidiabetic activity of different C. tuberculata extracts in STZ-induced diabetic rats. Both methanolic extract and the remaining water fractions demonstrated the highest strength, where methanolic extract had greater activity. The main mechanism for the noticed antihyperglycemic activity of methanolic extract might be credited to improved skeletal muscle use of glucose, hepatic gluconeogenesis inhibition and insulin secretion stimulation.99 Latha et al100 demonstrated that oral administration of C. fimbriata methanol extract to STZ-induced diabetic rats at a dose of 100 and 200 mg/kg body weight caused a considerable decrease in blood glucose at varied treatment periods. The methanol extract-treated diabetic rats were notably improved from diabetic and renal toxicity as well as hepatotoxicity, by analyzing certain factors such as glycosylated hemoglobin, plasma insulin, body weight, total protein, ALP, serum glutamic oxaloacetic transaminase and serum glutamic pyruvic transaminase. Moreover, the histopathological outcome of methanol extract-treated rats proved the noteworthy recovery of liver and kidney damage. They concluded that the study revealed the therapeutic influence of methanol extract for DM and its associated complications. Poodineh and Nakhaee101 evaluated the antidiabetic influences of two doses of C. tuberculata suspension and its safety on STZ-induced diabetic rats. C. tuberculata-treated groups displayed a considerable improvement in aberrations of oral glucose tolerance test, hematological and biochemical parameters compared to the diabetic control group. In addition, C. tuberculata at both doses (100 and 200 mg/kg) revisited considerably DM-induced modifications in lipid profile excluding the level of HDL-C that was notably elevated at a dose of 200 mg/kg. No significant variation was found in hematological, kidney and liver parameters between normal control and normal rats getting C. tuberculata. Therefore, the authors concluded that this plant might be useful for improving hyperlipidemia, hyperglycemia and hematological alterations excited by DM. Furthermore, it could guard the kidney and liver against complications as a result of DM without any poisonous effects.
Conclusion
The data of this study demonstrated a significant effect of C. russeliana stem extract on certain physiological parameters in STZ-induced diabetes in male rats after 8 weeks. The lowest body weight gain was noticed in diabetic rats of group 2. Serum glucose, triglycerides, cholesterol, LDL-C, VLDL-C, ALT, AST, ALP, total bilirubin, creatinine, BUN and uric acid levels were significantly elevated in diabetic rats of group 2; however, serum total protein, albumin and HDL-C levels were significantly reduced. Treatments with C. russeliana stem extract in diabetic rats revealed notable decreasing and protecting effects of physiological modifications. Therefore, this study revealed the significance of using C. russeliana stem extract as a potential therapeutic agent to treat DM and its complications. Finally, further research is needed to establish the efficacy of various extracts of this plant and their constituents to treat DM and to clarify their action mechanisms on diabetic models.
Acknowledgments
We would like to thank the committee of King Abdulaziz University, Jeddah, Saudi Arabia, for the approval of the experiments in this study. Moreover, we owe many thanks to King Abdulaziz City for Science and Technology for the financial support of this study under Grant no 1-38-067.
Author contributions
TZ and MA-T contributed toward data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
World Health Organization [webpage on the Internet]. Diabetes. World Health Organization; 2013. Available from: http://www.who.int/mediacentre/factsheets/fs312/en/index.html. Accessed November 21, 2017. | ||
Whiting DR, Guariguata L, Weil C, Shaw J. IDF Diabetes Atlas: Global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res Clin Pract. 2011;94(3):311–321. | ||
Stumvoll M, Goldstein BJ, van Haeften TW. Type 2 diabetes: principles of pathogenesis and therapy. The Lancet. 2005;365(9467):1333–1346. | ||
Matthews DR, Tsapas A, Tsapas A. Four decades of uncertainty: landmark trials in glycaemic control and cardiovascular outcome in type 2 diabetes. Diabetes and Vascular Disease Research. 2008;5(3):216–218. | ||
Blonde L. Current antihyperglycemic treatment guidelines and algorithms for patients with type 2 diabetes mellitus. Am J Med. 2010;123(3):S12–S18. | ||
Nyenwe EA, Jerkins TW, Umpierrez GE, Kitabchi AE. Management of type 2 diabetes: evolving strategies for the treatment of patients with type 2 diabetes. Metabolism. 2011;60(1):1–23. | ||
Yudkin JS, Richter B, Gale EAM. Intensified glucose control in type 2 diabetes—whose agenda? The Lancet. 2011;377(9773):1220–1222. | ||
Cheneke W, Suleman S, Yemane T, Abebe G. Assessment of glycemic control using glycated hemoglobin among diabetic patients in Jimma University specialized hospital, Ethiopia. BMC Res Notes. 2016;9(1):96. | ||
Angamo MT, Melese BH, Ayen WY. Determinants of glycemic control among insulin treated diabetic patients in Southwest Ethiopia: hospital based cross sectional study. PLoS One. 2013;8(4):e61759. | ||
Ghazanfari Z, Niknami S, Ghofranipour F, Larijani B, Agha-Alinejad H, Montazeri A. Determinants of glycemic control in female diabetic patients: a study from Iran. Lipids Health Dis. 2010;9(1):83. | ||
Boutayeb A, Boutayeb W, Lamlili MEN, Boutayeb S. Indirect cost of diabetes in the Arab region. Int J Diabetol Vasc Dis Res. 2013;1(4):24–28. | ||
Alharbi NS, Almutari R, Jones S, Al-Daghri N, Khunti K, de Lusignan S. Trends in the prevalence of type 2 diabetes mellitus and obesity in the Arabian Gulf States: Systematic review and meta-analysis. Diabetes Res Clin Pract. 2014;106(2):e30–e33. | ||
Zari TA, Al-Attar AM. Effects of ginger and clove oils on some physiological parameters in streptozotocin-diabetic and non-diabetic rats. J. Med. Sci. 2007;7(2):267–275. | ||
Al-Attar AM, Zari TA. Influences of crude extract of tea leaves, Camellia sinensis, on streptozotocin diabetic male albino mice. Saudi J Biol Sci. 2010;17(4):295–301. | ||
Shirali S, Zahra Bathaie S, Nakhjavani M. Effect of crocin on the insulin resistance and lipid profile of streptozotocin-induced diabetic rats. Phytother Res. 2013;27(7):1042–1047. | ||
Hsu TC, Chiu CC, Lin HL, et al. Attenuated effects of deep-sea water on hepatic apoptosis in STZ-induced diabetic rats. Chin J Physiol. 2015;58:197–205. | ||
Emordi JE, Agbaje EO, Oreagba IA, Iribhogbe OI. Antidiabetic and hypolipidemic activities of hydroethanolic root extract of Uvaria chamae in streptozotocin induced diabetic albino rats. BMC Complement Altern Med. 2016;16(1):468. | ||
Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27(5):1047–1053. | ||
Yaryura-Tobias JA, Pinto A, Neziroglu F. Anorexia nervosa, diabetes mellitus, brain atrophy, and fatty liver. Int J Eat Disord. 2001;30(3):350–353. | ||
Ghorbani A. Best herbs for managing diabetes: a review of clinical studies. Brazi J Pharm Sci. 2013;49(3):413–422. | ||
Gilbert MG. A review of Caralluma R. Br. and its segregates. Bradleya. 1990;8(8):1–32. | ||
Meve U, Liede S. Subtribal Division of Ceropegieae (Apocynaceae-Asclepiadoideae). Taxon. 2004;53(1):61–72. | ||
Bensuzan K. Taxonomy and conservation status of Moroccan stapeliads (Apocynaceae-Asclepiadoideae-Ceropegieae-Stapeliinae. Vol. 31. Rabat: Bull Inst Sci Univ; 2009:67–77. | ||
Naik MR, Krishnamurthy YL. Xerophyte Caralluma stalagmifera var. longipetala (Asclepiadaceae): a new record to the flora of Karnataka, India. Journal of Threatened Taxa. 2012;4(6):2656–2659. | ||
Abdel-Sattar E, Harraz FM, Al-Ansari SMA, et al. Antiplasmodial and antitrypanosomal activity of plants from the Kingdom of Saudi Arabia. J Nat Med. 2009;63(2):232–239. | ||
de Leo M, de Tommasi N, Sanogo R, et al. New pregnane glycosides from Caralluma dalzielii. Steroids. 2005;70(9):573–585. | ||
Oyama M, Iliya I, Tanaka T, Iinuma M. Five New Steroidal Glycosides fromCaralluma dalzielii. Helv Chim Acta. 2007;90(1):63–71. | ||
Aruna V, Kiranmai C, Karuppusamy S, Pullaiah T. Micropropagation of three varieties of Caralluma adscendens via nodal explants. J Plant Biochem Biotechnol. 2009;18(1):121–123. | ||
Bader A, Braca A, de Tommasi N, Morelli I. Further constituents from Caralluma negevensis. Phytochemistry. 2003;62(8):1277–1281. | ||
Braca A, Bader A, Morelli I, et al. New pregnane glycosides from Caralluma negevensis. Tetrahedron. 2002;58(29):5837–5848. | ||
Abdel-Sattar E, Ahmed AA, Hegazy MEF, Farag MA, Al-Yahya MAA. Acylated pregnane glycosides from Caralluma russeliana. Phytochemistry. 2007;68(10):1459–1463. | ||
Waheed A, Barker J, Barton SJ, et al. Novel acylated steroidal glycosides from Caralluma tuberculata induce caspase-dependent apoptosis in cancer cells. J Ethnopharmacol. 2011;137(3):1189–1196. | ||
Al-Attar AM, Abu Zeid IM. Effect of tea (Camellia sinensis) and olive (Olea europaea L.) leaves extracts on male mice exposed to diazinon. Biomed Res Int. 2013;2013(2):1–6. | ||
Prabhakar P, Doble M. A target based therapeutic approach towards diabetes mellitus using medicinal plants. Curr Diabetes Rev. 2008;4(4):291–308. | ||
Abdel-Rahim EA, El-Beltagi HS. Alleviation of hyperlipidemia in hypercholesterolemic rats by lentil seeds and apple as well as parsley in semi-modified diets. Adv food sci. 2011;33(1):2–7. | ||
Mohamed AA, Khalil AA, El-Beltagi HES. Antioxidant and antimicrobial properties of kaff maryam (Anastatica hierochuntica) and doum palm (Hyphaene thebaica. Grasas y Aceites. 2010;61(1):67–75. | ||
Ujowundu CO, Kalu FN, Emejulu AA, Nkwonta CG, Nwosunjoku EC. Evaluation of the chemical composition of Mucuna utilis leaves used in herbal medicine in Southeastern Nigeria. Afr. J. Pharm. Pharmacol. 2010;4(11):811–816. | ||
Tiwari P, Kumar B, Kaur M, Kaur G, Kaur H. Phytochemical screening and extraction: a review. Int Pharm Sci. 2011;1:98–106. | ||
Zari TA. Bioactivity of plant essential oils. In: Rai MK, Cordell GA, Martinez JL, Marinoff M, Rastrelli L, editors. Medicinal Plants: Biodiversity and Drugs. CRC Press, Boca Raton, FL, USA; 2012:599–562. | ||
Kumar AS, Kavimani S, Jayaveera KN. A review on medicinal plants with potential antidiabetic activity. Int J Phytopharmacol. 2011;2(2):53–60. | ||
Ali H, Sannai J, Sher H, Rashid A. Ethnobotanical profile of some plant resources in Malam Jabba valley of Swat, Pakistan. J Med Plants Res. 2011;5(18):4676–4687. | ||
Wadood AB, Wadood NO, Shah SA. Effects of Acacia arabica and Caralluma edulis on blood glucose levels of normal and alloxan diabetic rabbits. J Pak Med Assoc. 1989;39(8):208–212. | ||
Zari TA, Al-Logmani AS. Long-term effects of Cinnamomum zeylanicum Blume oil on some physiological parameters in streptozotocin-diabetic and non-diabetic rats. BLACPMA. 2009;8(4):266–274. | ||
Salahuddin M, Jalalpure SS, Gadge NB. Antidiabetic activity of aqueous bark extract of Cassia glauca in streptozotocin-induced diabetic rats. Can J Physiol Pharmacol. 2010;88(2):153–160. | ||
Al-Logmani A, Zari T. Long-term effects of Nigella sativa L. oil on some physiological parameters in normal and streptozotocin-induced diabetic rats. J Diabetes Mellitus. 2011;01(03):46–53. | ||
Zhang Y, Feng F, Chen T, Li Z, Shen QW. Antidiabetic and antihyperlipidemic activities of Forsythia suspensa (Thunb.) Vahl (fruit) in streptozotocin-induced diabetes mice. J Ethnopharmacol. 2016;192:256–263. | ||
Chen V, Ianuzzo CD. Dosage effect of streptozotocin on rat tissue enzyme activities and glycogen concentration. Can J Physiol Pharmacol. 1982;60(10):1251–1256. | ||
Chatterjea MN, Shinde R. Textbook of Medical Biochemistry. 5th ed. New Delhi: Jaypee Brothers; 2002. | ||
Adnan M, Jan S, Mussarat S, et al. A review on ethnobotany, phytochemistry and pharmacology of plant genus C. aralluma R. Br. J Pharm Pharmacol. 2014;66(10):1351–1368. | ||
Ambadasu B, Dange SV, Walli RS, Worlikar PS. Effect of Caralluma fimbriata extract on appetite and lipid profile in rats fed with hypercalorie/cafeteria diet. Int J Pharma Biosci. 2013;4:788–793. | ||
Naingade SS, Jadhav AS, Surve SB. Caralluma fimbriata: an overview. Int J Pharm Bio Sci. 2013;3(1):281–286. | ||
Astell KJ, Mathai ML, Mcainch AJ, Stathis CG, Su XQ, Xq S. A pilot study investigating the effect of Caralluma fimbriata extract on the risk factors of metabolic syndrome in overweight and obese subjects: a randomised controlled clinical trial. Complement Ther Med. 2013;21(3):180–189. | ||
Kuriyan R, Raj T, Srinivas SK, Vaz M, Rajendran R, Kurpad AV. Effect of Caralluma Fimbriata extract on appetite, food intake and anthropometry in adult Indian men and women. Appetite. 2007;48(3):338–344. | ||
Lawrence RM, Choudhary S. Caralluma fimbriata in the treatment of obesity. In: 12th Annual World Congress of Anti-Aging Medicine; 2004; Las Vegas, USA. | ||
Tanko Y, Daniel PA, Mohammed KA, Jimoh A, Yerima M, Mohammed A. Effect of ethanolic extract of Caralluma diazielli on serum lipid profiles on fructose induced diabetes in wistar rats. Scholar Res. 2013;4:162–166. | ||
Chopra RN, Nayar SL, Chopra IC. Glossary of Indian Medicinal Plants. New Delhi: C SIR; 1956. | ||
Ahmad MM, Shaikh MM. Improvement in glucose tolerance by Caralluma tuberculata, Acacia nilotica and Papaver somniferum. Pak J Zool. 1989;21:325–332. | ||
Ramesh M, Nageshwar Rao Y, Appa Rao AVN, Rao YN, Rao AA, Ramesh M, Rao YN, Rao AA, Prabhakar MC, Rao CS, Muralidhar N, Reddy BM. Antinociceptive and anti-inflammatory activity of a flavonoid isolated from Caralluma attenuata. J Ethnopharmacol. 1998;62(1):63–66. | ||
Hebi M, Farid O, Ajebli M, Eddouks M. Potent antihyperglycemic and hypoglycemic effect of Tamarix articulata Vahl. in normal and streptozotocin-induced diabetic rats. Biomed Pharmacother. 2017;87:230–239. | ||
Sadri H, Goodarzi MT, Salemi Z, Seifi M. Antioxidant effects of biochanin A in streptozotocin induced diabetic rats. Braz Arch Biol Technol. 2017;60. | ||
Johnston A. Spices as influencers of body metabolism: an over view of three decades of research. Food Res Int. 1999;38:77–86. | ||
Al-Hashem F. Camel’s milk protects against aluminum chloride-induced toxicity in the liver and kidney of white albino rats. Am J Biochem Biotechnol. 2009;5(3):98–109. | ||
Murtiashaw MH, Baynes JW, Thorpe SR. Albumin catabolism in diabetic rats. Arch Biochem Biophys. 1983;225(1):256–262. | ||
Steiner G. The diabetes atherosclerosis intervention study (DAIS): a study conducted in cooperation with the World Health Organization. Diabetologia. 1996;39(12):1655–1661. | ||
Goldberg IJ. Diabetic Dyslipidemia: causes and consequences. J Clin Endocrinol Metabol. 2001;86(3):965–971. | ||
Spratt KA. Managing diabetic dyslipidemia: aggressive approach. J Am Osteopath Assoc. 2009;109(5_suppl_1):S2–7. | ||
Snipelisky D, Ziajka P. Diabetes and hyperlipidemia: a direct quantitative analysis. World J Cardiovasc Dis. 2012;2(1):20–25. | ||
Martin DW, Mayes PA, Rodwell VM. Harpers Review of Biochemistry. Maruzen Asian Ed. Singapore: Large Medical Publication; 1983. | ||
Fielding CJ, Fielding PE. Molecular physiology of reverse cholesterol transport. J Lipid Res. 1995;36(2):211–228. | ||
Krieger M. Charting the fate of the “good cholesterol”: identification and characterization of the high-density lipoprotein receptor SR-BI. Annu Rev Biochem. 1999;68(1):523–558. | ||
Williams DL, Connelly MA, Temel RE, Swarnakar S, Phillips MC, Rothblat GH. Scavenger receptor BI and cholesterol trafficking. Curr Opin Lipidol. 1999;10(4):329–339. | ||
Rajalingam R, Srinivasan N, Govindarajulu P. Effects of alloxan induced diabetes on lipid profiles in renal cortex and medulla of mature albino rats. Indian J Exp Biol. 1993;31:577–579. | ||
Pathak RM, Ansari S, Mahmood A. Changes in chemical composition of intestinal brush border membrane in alloxan induced chronic diabetes. Indian J Exp Biol. 1981;19(5):503–509. | ||
Palumbo PJ. Metformin: effects on cardiovascular risk factors in patients with non–insulin-dependent diabetes mellitus. J Diabetes Complications. 1998;12(2):110–119. | ||
Arvind K, Pradeepa R, Deepa R, Mohan V. Diabetes & coronary artery disease. Indian J Med Res. 2002;116:163–176. | ||
Johnston CS, Gaas CA. Vinegar: medicinal uses and antiglycemic effect. Med Gen Med. 2006;8(2):61. | ||
Sakore S, Patil SD, Surana S. Hypolipidemic activity of Caralluma adscendens on triton and methimazole induced hyperlipidemic rats. Pharmtechmedica. 2012;1(1):49–52. | ||
Williams E, Gokool N. Primary care: an update on the role of plant sterols and stanols in the management of hypercholesterolaemia. CNMag. 2005;5:36–38. | ||
Kew MC. Serum aminotransferase concentration as evidence of hepatocellular damage. Lancet. 2000;355(9204):591–592. | ||
de David C, Rodrigues G, Bona S, et al. Role of quercetin in preventing thioacetamide-induced liver injury in rats. Toxicol Pathol. 2011;39(6):949–957. | ||
Neuschwander-Tetri B, Caldwell SH. Nonalcoholic steatohepatitis: Summary of an AASLD Single Topic Conference. Hepatology. 2003;37(5):1202–1219. | ||
Harris EH. Elevated liver function tests in type 2 diabetes. Clin Diabetes. 2005;23(3):115–119. | ||
Rajesh MG, Latha MS. Preliminary evaluation of the antihepatotoxic activity of Kamilari, a polyherbal formulation. J Ethnopharmacol. 2004;91(1):99–104. | ||
Tanko Y, Daniel PA, Mohammed KA, Jimoh A, Yerima M, Mohammed A. Effect of ethanolic extract of Caralluma diazielli on serum lipid profiles on fructose induced diabetes in wistar rats. Scholar Res. 2013;4:162–166. | ||
Panda NC. Kidney. In: Textbook of biochemistry and human biology. 2nd ed. India: Prentice-Hall; 1999:290–296. | ||
Morcos M, Sayed AAR, Bierhaus A, et al. Activation of tubular epithelial cells in diabetic nephropathy. Diabetes. 2002;51(12):3532–3544. | ||
Lin Y, Berg AH, Iyengar P, et al. The hyperglycemia-induced inflammatory response in adipocytes: the role of reactive oxygen species. J Biol Chem. 2005;280:4617–4626. | ||
Schena FP, Gesualdo L. Pathogenetic mechanisms of diabetic nephropathy. J Am Soci Nephrol. 2005;16(3 suppl 1):S30–S33. | ||
Tanios BY, Ziyadeh FN. Emerging therapies for diabetic nephropathy patients: beyond blockade of the Renin-Angiotensin system. Nephron Extra. 2012;2(1):278–282. | ||
Forbes JM, Coughlan MT, Cooper ME. Oxidative stress as a major culprit in kidney disease in diabetes. Diabetes. 2008;57(6):1446–1454. | ||
Mestry SN, Dhodi JB, Kumbhar SB, Juvekar AR. Attenuation of diabetic nephropathy in streptozotocin-induced diabetic rats by Punica granatum Linn. leaves extract. J Tradit Complement Med. 2017;7(3):273–280. | ||
Abdel-Sattar E, El-Maraghy SA, El-Dine RS, Rizk SM. Antihyperglycemic activity of Caralluma quadrangula in streptozotocin-induced diabetic rats. Bullet Faculty Pharmacy Cairo Univ. 2017;55(2):269–272. | ||
Abdul-Aziz Al-Yahya M, Abdel-Sattar E, Guittet E. Pregnane glycosides from Caralluma russeliana. J Nat Prod. 2000;63(10):1451–1453. | ||
Abdel-Mogib M, Raghib HM. Two new pregnane glycoside diesters from Caralluma russeliana. Nat Prod Res. 2013;27(14):1287–1292. | ||
Al-Massarani SM, Bertrand S, Nievergelt A, et al. Acylated pregnane glycosides from Caralluma sinaica. Phytochemistry. 2012;79:129–140. | ||
Halaweish FT, Huntimer E, Khalil AT. Polyoxy pregnane glycosides from Caralluma retrospiciens. Phytochem Anal. 2004;15(3):189–194. | ||
Habibuddin M, Daghriri HA, Humaira T, Qahtani MSA, Hefzi AAH. Antidiabetic effect of alcoholic extract of Caralluma sinaica L. on streptozotocin-induced diabetic rabbits. J Ethnopharmacol. 2008;117(2):215–220. | ||
Mahmood T, Muhammad SA, Shinwari ZK. Molecular and morphological characterization of Caralluma species. Pak J Bot. 2010;42(2):1163–1171. | ||
Abdel-Sattar EA, Abdallah HM, Khedr A, Abdel-Naim AB, Shehata IA. Antihyperglycemic activity of Caralluma tuberculata in streptozotocin-induced diabetic rats. Food Chem Toxicol. 2013;59:111–117. | ||
Latha S, Rajaram K, Suresh Kumar P. Hepatoprotective and antidiabetic effect of methanol extract of Caralluma fimbriata in streptatozocin induced diabetic albino rats. Int J Pharm Pharm Sci. 2014;6:665–668. | ||
Poodineh J, Nakhaee A. Hypoglycemic and hypolipidemic effects of Caralluma tuberculata and its safety on liver and kidneys of diabetic rats. Turkish J Biochem. 2016;41(3):136–143. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.