Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 13
Effects of Breastfeeding and Formula Feeding on the Expression Level of FTO, CPT1A and PPAR-α Genes in Healthy Infants
Authors Cheshmeh S , Nachvak SM, Rezvani N, Saber A
Received 2 March 2020
Accepted for publication 12 June 2020
Published 26 June 2020 Volume 2020:13 Pages 2227—2237
DOI https://doi.org/10.2147/DMSO.S252122
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ming-Hui Zou
Sahar Cheshmeh, 1, 2,* Seyed Mostafa Nachvak, 1 Nayebali Rezvani, 3 Amir Saber 1,*
1Department of Nutritional Sciences, School of Nutritional Sciences and Food Technology, Kermanshah University of Medical Sciences, Kermanshah, Iran; 2Student Research Committee, School of Nutritional Sciences and Food Technology, Kermanshah University of Medical Sciences, Kermanshah, Iran; 3Department of Clinical Biochemistry, Kermanshah University of Medical Sciences, Kermanshah, Iran
*These authors contributed equally to this work
Correspondence: Seyed Mostafa Nachvak
Department of Nutritional Sciences, Faculty of Nutritional Sciences and Food Technologies, Kermanshah University of Medical Sciences, Isar Sq., Across from Farabi Hospital, P.O. Box 6719851351, Kermanshah, Iran
Tel +98-8338395885
Fax +98-83 37102002
Email [email protected]
Nayebali Rezvani
Department of Clinical Biochemistry, Kermanshah University of Medical Sciences, P.O. Box 67148-69914, Kermanshah, Iran
Tel +98-9183391265
Email [email protected]
Purpose: The study aimed to investigate the effect of breastfeeding, formula feeding and mix feeding (breastfed plus formula-fed) on the expression level of obesity-predisposing genes including fat mass and obesity-associated (FTO), carnitine palmitoyltransferase 1A (CPT1A), and peroxisome proliferator-activated receptor-α (PPAR-α) in 5- to 6-month-old infants.
Patients and Methods: A total of 150 infants participated in this case–control study. All subjects were healthy infants aged 5– 6 months that divided into 3 groups: breastfed, formula-fed, and mix-fed. The expression level of FTO, CPT1A, and PPAR-α genes in peripheral blood mononuclear cells (PBMC) was evaluated in each group using reverse transcription-polymerase chain reaction (RT-PCR) method.
Results: Our findings showed that the current weight, height, and head circumference of infants in the formula feeding and mix feeding groups were significantly higher than those in the exclusive breastfeeding group. The expression level of FTO and CPT1A genes in formula-fed and mix-fed infants was significantly higher (p< 0.001) than that in breastfed infants, while the expression level of PPAR-α gene was significantly lower (p< 0.05).
Conclusion: Breastfeeding showed modulatory effects on the expression level of obesity-predisposing genes and can protect against obesity and subsequent non-communicable diseases. However, more investigations are required to explain the epigenetic effects of breast milk.
Keywords: breastfeeding, formula feeding, obesity, FTO, CPT1A, PPAR-α, infants
Introduction
Childhood obesity has been recognized as one of the major public health problems in the 21st century and it seems that the global number of overweight/obese infants and young children increased from 32 million in 1990 to 41 million in 2016.1–3 According to the world health organization (WHO) estimation, if the present trend continues, the number of overweight/obese infants and children will increase to 70 million by 2025. This important health problem is affecting most of the low- and middle-income countries in Asia and Africa.1,4 Several studies have demonstrated that overweight and obese children remain obese and overweight during adulthood and are more likely to develop non-communicable diseases like; diabetes, metabolic syndrome (MetS), and cancers at younger age.5 In this way, several preventable and inevitable factors such as; environmentally and genetically factors have been identified in the pathophysiology of early obesity.6,7 Since weight and height gain, as the most important indicators of health status in infants and children, affected by diet and behavioral habits, precise modulation and control of them is a necessity to prevent subsequent chronic disorders.8 Previous studies have shown that diet causes epigenetic changes early in life and may affect health status in adulthood.9,10 Therefore, modulation of the dietary factors may influence the expression level of different genes in the body through epigenetic alterations.10
Different studies showed that some factors like extreme gestational weight gain, gestational diabetes mellitus (GDM), hypertension, maternal dyslipidemia and breastfeeding less than 6 months are associated with maternal and childhood obesity and can be perinatal risk factors for higher BMI in childhood.11 There is a robust relationship between obesity and chronic diseases like diabetes and cancers which is the result of modifications in the expression level of crucial genes for a long time. Numerous studies showed that the gene of transcription factor TCF7L2, PPAR-γ2 receptors, and SLC16A11 gene variants have an important role in the incidence of obesity and type 2 diabetes in different populations. On the other hand, weight loss can modify the expression level of some important genes that involved in the production of different interleukins, cytokines, complement components, acute phase proteins, and molecules.11,12
Breast milk has the appropriate composition and unique ingredients that can provide all of the requirements and ensure the health status of infants. Breastfeeding has been associated with lower risk of chronic and infectious diseases in infants and young children.13,14 There are different mechanisms regarding protective effects of human milk against obesity and its related chronic diseases. Breastfed infants consume low-calorie and protein that leads to a lower insulin response, fat deposition and adipocytes. Moreover, in exclusively breastfed infants the self-regulation and satiation feeling will improve and an infant will stop feeding when is satiated and consume lower amounts of calorie than formula feeding infant.14–17 In addition, breast milk possesses more amounts of bioactive factors, and lower protein and insulin concentrations in comparison to the formula that leads to the reduction of fat deposition. The bioactive factors that exist in human milk are able to promote epidermal growth factor and tumor necrosis factor (TNF) synthesis that prevents adipocyte differentiation in infants.18–21
Numerous studies have been demonstrated that breastfeeding plays a significant role in the health status of infants by affecting specific genes.22,23 Many of the functions and signaling pathways of these obesity-predisposing genes have been identified.24 Some of the important genes involved in the obesity incidence through energy metabolism and body weight regulation are; PPARA, CPT1A, fatty acid synthase (FASN), leptin receptor (LEP-R), and FTO genes.25–28
FTO is the first gene that recognized by genome-wide association studies (GWAS) and is contributed to polygenic human obesity in different ethnicities. The important role of FTO on body composition, and risk of obesity has been proven in children and adults.29–32 According to different studies, FTO has an important role in the association between amino acid levels and mTORC1 function, so that inappropriate FTO gene leads to weakness of mTORC1 signaling pathway.33,34 Also, it seems that the FTO gene has an important role in the proliferation and differentiation of cells through PI3K/Akt signaling pathway and can perform a critical role in the relationship between 5′ AMP-activated protein kinase (AMPK) with the PI3K/AKT/mTOR pathway.35,36
CPT1A is one of the three isoforms of the CPT-1 enzyme, and the mutation of its coding gene predisposes individuals to some metabolic disorders.37 Since the assessment of CPT1A gene expression may be an early biomarker for metabolic alterations like; insulin resistance, fatty liver and obesity, it can be a suitable target to the development of therapeutic agents against metabolic disorders.38 In this manner, Sanchez et al showed that the expression level of LEP-R, insulin receptor (INSR), and CPT1A genes are higher in overweight children compared to normal weight.39 On the other hand, Priego et al demonstrated that the higher expression level of FASN, PPAR-α and INSR genes in breastfed infants is associated with a lower risk of overweight compared to formula-fed infants.40 Moreover, McCrory et al revealed that breastfeeding more than 13 weeks is associated with the significant reduction in risk of being obese at 9 years of age.41 However, the evidence regarding the protective effects of breastfeeding against childhood obesity is inconsistent yet. Previous studies showed that the early life diet significantly affects the health status of infants through making alterations in the expression level of effective genes involved in the regulation of energy homeostasis.13,42 Therefore, the aim of this study was to investigate the effect of different types of feeding, including breastfeeding, formula feeding and mix feeding on the expression level of important genes in the development of obesity and overweight (FTO, CPT1A, and PPAR-α) in infants.
Patients and Methods
Study Design and Subjects
In this case-control study, after applying exclusion criteria, out of 649 infants that were referred to the health centers in Kermanshah city, 150 healthy infants aged 5 to 6 months (boys and girls) were recruited (Figure 1). Since the variance of the FTO and PPAR-α genes was less than the variance of the CPT1A gene, then the variance of this gene was applied to determine the sample size of the study. By assuming a 5% variance range with a 90% power and 5% significance, 38 infants were calculated for each group. Finally, by considering previous studies and possible missing samples, 50 infants were determined for each case and control group.
 |
Figure 1 Flow chart of the study population selection including infant’s recruitment and exclusion criteria. |
Before starting the study, the formal written consent form was completed by parents of all infants. Infants diagnosed with specific diseases and nutritional deficiencies were excluded from the study. All of the eligible infants were divided into 3 groups (n=50) as control (breastfeeding infants) and case groups (formula-fed and mix-fed infants). To reduce potential bias, all of the controls were matched to cases by age, sex, birth weight, birth height and pregnancy term (Figure 1). Also, this study was approved by the ethics committee of Kermanshah University of Medical Sciences, and the study was conducted in accordance with the Declaration of Helsinki.
Anthropometric Measures and Type of Feeding
All of the infants underwent a complete physical checkup and their body mass and height were measured with Seca-233 digital baby scale (Seca), while wearing minimum clothes in sleep position and compared with standard WHO charts.43 The type of infants feeding (breastfeeding, formula feeding or mix feeding) was recorded via parents recall and all of the characteristics of infants and his/her parents including; sex, age, mothers and fathers weight and height, mother’s weight before and after pregnancy, pregnancy term, at birth and current weight (in fasting status), at birth and current height, at birth and current head circumference, history of medications, nutritional interventions and infants’ disease were measured and recorded precisely. Also to reduce recall bias all of the information of infants were re-checked by their previous recorded data.
Quantitative Real-Time PCR for Gene Expression Analysis
For analysis of the gene expression level, the infant’s blood samples (1.5 mL) that provided by health-care practitioners were collected in Ethylenediaminetetraacetic acid (EDTA) coated vials. The blood sample was transferred into polypropylene tubes and stored frozen at −80 °C until needed. Blood samples of cases and controls were analyzed in the same batch and researchers were completely blind about the samples. The expression level of FTO, CPT1A, and PPAR-α genes was determined using the infant’s PBMC by RT-PCR method. Briefly, the total RNA from PBMC was extracted by Trisol Reagent kit (YTzol pure RNA, Iran) according to the manufacturer’s instructions. The extracted RNA quantity and quality were checked in the elution using ND-1000 Nanodrop spectrophotometer (Thermo Fisher Scientific Inc, USA) at 260–280 nm. Also, 1 µg of extracted RNA was employed for the synthesis of complementary DNA (cDNA) using Prime Script RT-Reagent kit (Takara Bio Inc., Tokyo, Japan) according to the manufacturer’s instructions. To amplify intended genes, specific primers were designed and purchased from Metabion Biotechnology Company (Metabion, steinkirchen, Germany) (Table 1). All amplification reactions were performed triplicate for each sample and every experiment mixture (20 µL), containing 10 µL SYBR Green gene expression master mix (Takara Bio Japan, Inc.), 1 µL cDNA (1 µg/µL), 1 µL primer (forward and reverse) and 0.8 µL 6-carboxy-X-rhodamine (ROX as reference dye) was subjected to ABI-step I plus (Applied Biosystems, Forster City, CA, USA) instrument. Thermal cycling condition was as follows: 1 cycle at 95°C for 2 mins followed by 45 cycles at 95°C for 5 s, 60°C for 30 s, and at 72°C for 10 s. To verify the purity of the products, a melting curve was produced after each run according to the manufacturer’s instructions. Interpretation of the results was performed using the Pfaffle method and the threshold cycle (Ct) values that are provided by the instrument’s software (StepOne Software v2.0) were normalized to the expression rate of internal housekeeping control gene, 18s rRNA, and expressed as fold change. Since the quantity of starting RNA may vary greatly between different samples and in order to compensate the variations in PCR efficiency, normalization of target genes was performed.30,44 Also, for the detection of contamination, no template control (NTC) reaction was performed, which included all of the ingredients of the reaction except the target template RNA. All of the changes in the expression level of intended genes were assessed by the REST (Relative Expression Software Tool) software.
 |
Table 1 Primers Sequences for RT-PCR Amplification |
Statistical Analysis
Statistical analysis was performed using the statistical package for the social sciences (SPSS Inc. Chicago, IL, USA version 23.0). All data were obtained from at least three independent experiments and expressed as means ± standard deviations (SD) for quantitative variables and frequency (percent) for qualitative variables. To the assessment of normality, the Kolmogorov–Smirnov test was used. Moreover, chi-square test was used to compare proportions for categorical variables and one-way ANOVA and Kruskal–Wallis tests were performed for analyzing differences between normal and non-normal variables, respectively. Statistical significance was considered as a value of P ≤ 0.05.
Results
In this study, 150 infants including boys 79 (52.7%) and girls 71 (47.3%) were recruited and at the beginning of the study they divided into three equal groups regarding the type of feeding, including breastfed (n=50), formula-fed (n=50), and mix-fed (n=50). The basic characteristics of parents and their infants by types of feeding are given in Tables 2 and 3.
 |
Table 2 Basic Characteristics of Parents by Types of Feeding (Mean ± SD) |
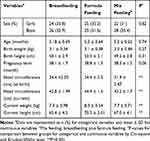 |
Table 3 Basic Characteristics of Infants by Types of Feeding |
According to the results of the study, there were no significant differences between mother’s (p=0.16) and father’s ages (p=0.11), and also father’s weight (p=0.53) and height (p=0.26) between three groups. On the other hand, the mother’s weight before and after pregnancy was significantly different between three groups (p<0.001). Besides, the mother’s weight (p<0.001) and height (p=0.02) were significantly different (Table 2).
Moreover, based on Table 3, a significant difference between current weight (p<0.001) and height (p<0.001), and also head circumference (at birth and current) of infants were observed between three groups (p<0.001).
As shown in Table 4 and Figure 2, the expression level of FTO and CPT1A genes in formula-fed and mix-fed groups were significantly higher (p<0.001) than that in the breastfeeding group, while the expression level of PPAR-α was significantly lower in comparison to breastfeeding group (p<0.05). The comparison between the three groups revealed that the mean values of FTO and CPT1A gene expression in breastfed infants were 3.39 ± 1.1 and 13.51 ± 6.04, respectively, that was significantly lower than two other groups. On the contrary, the expression level of PPAR-α in breastfed infants (85.41 ± 17.84) was significantly higher than that of formula-fed and mix-fed groups (p<0.001).
 |
Table 4 The Expression Level of FTO, PPAR-α and CPT1A Genes in Peripheral Blood Cells in Breastfed Infants, Formula-Fed and Mix-Fed Infants |
According to Table 5, the mean values of FTO (p<0.001), CPT1A (p<0.001) and PPAR-α (p=0.03) gene expression levels in formula-fed group were significantly different compared to the breastfed group. As well, the comparison of gene expression level between breastfed and mix-fed groups was significantly different only for CPT1A (p=0.02) and PPAR-α (p=0.04) genes. The comparison of formula-fed and mix-fed groups about FTO (P=0.16), CPT1A (P=0.09) and PPAR-α (P=0.13) gene expression was not significant (Table 5).
 |
Table 5 The Difference Between Mean Values of FTO, CPT1A and PPAR-α Gene Expression Levels by Types of Feeding |
Discussion
To our knowledge, this is the first investigation that evaluates the effects of breastfeeding and formula feeding on the expression level of FTO, CPT1A and PPAR-α genes in infants. Our results showed that the current head circumference, weight, and height of formula-fed and mix-fed infants were significantly higher than those of exclusively breastfed infants after 5–6 months, while the birth weight of them was not significantly different (Table 3). These findings confirmed the previous findings regarding the protective effects of breastfeeding against excessive weight gain in infants.45–48
Also, the evaluation of three key genes in the obesity pathway revealed that the expression level of FTO and CPT1A genes in the breastfed group was lower than that in the formula-fed and mix-fed groups, while the expression level of PPAR-α in the breastfed group was higher than that in two other groups (Figure 2). The FTO gene is one of the most important genes in relation to obesity and its overexpression leads to increased food intake and body weight. Various studies have shown an association between FTO gene expression with energy expenditure, ghrelin, and leptin hormone levels and the risk of obesity and type 2 diabetes.30,32,49,50 As well, excessive intake of protein and carbohydrates up-regulated the FTO gene expression and exacerbates the process of obesity in susceptible individuals.30
Since the knockdown and overexpression of FTO gene can inhibit and increase the differentiation and adipogenesis of adipocytes, it seems that the appropriate adipocyte differentiation and proliferation are associated with FTO gene expression.51
Different studies showed that a group of single nucleotide polymorphisms (SNPs) in the first intron of the FTO gene predispose individuals to obesity and also carriers of the risk allele of FTO rs9930506 polymorphism are at higher risk for higher BMI and food intake.52 The effect of these important SNPs appears to be associated with postprandial leptin and ghrelin levels which can raise hunger, decrease post-prandial satiety and fullness. In this way, a significant relationship exists between haplotypes in intron 1 of FTO gene and BMI while other variants are associated with abnormal eating behaviors.53,54 Doaei et al suggested that a significant association between the up-regulation of FTO in PBMCs and the increase of skeletal muscle (%SM) in 84 boys aged 12 to 16 years.31 Merkestein et al showed that FTO is able to regulate early-stage adipogenesis in FTO overexpression mice (FTO-4). These adipogenic effects performed through induction of pro-adipogenic short isoform of RUNX1T1 which enhance adipocyte proliferation.55 In the RAINE cohort study, long-term exclusive breastfeeding was associated with lower BMI in carriers of the risk allele of the FTO SNP rs9939609 via regulation of FTO gene and modulation of energy balance during infancy.56 In another cohort study, da Silva et al suggested a significant relationship between FTO gene rs9939609 variants (T/T, T/A, and A/A genotypes) with anthropometric and dietary intake. All children with the A/A genotype had higher BMI than those with the T/A genotype.57 Likewise, other studies suggested that breastfeeding was associated with lower obesity indices like: waist-hip ratio and skinfolds triceps in children.45,58 Our results showed that the type of feeding affects the expression level of obesity-related genes and breastfeeding can be a protective factor against obesity.
Some active biological compounds in the milk have epigenetic effects which can trigger some processes like; DNA methylation, histone modification, and chromatin remodeling.59,60 The overexpression of FTO gene prompts through demethylation of specific cytosine phosphate guanine (CpG) sites. Cow milk can decrease DNA methylation at intron 1 of FTO gene via exosomal miRNA-29s and suppression of DNA methyltransferases (DNMT) which leads to overexpression of FTO gene and resulted in obesity. Also, infant’s formula based on cow milk has high levels of branched-chain amino acids (BCAAs) and glutamine that activates the mammalian target of rapamycin complex 1 (mTORC1) and increases FTO gene expression level.61
In addition, the duration of exclusive breastfeeding interact with SNP of FTO gene and prevent from the high BMI and facilitates the return to normal weight in later life years.56,62 Jurado et al showed that exclusive breastfeeding less than 3 months happens approximately 4 times in children with obesity.63 Similarly, our study confirmed that the consumption of formula feeding for 5–6 months leads to higher weight gain in infants by down-regulation of FTO and CPT1A gene expression. As well, other studies revealed that the expression level of CPT1A gene in obese and overweight children is higher than that in normal weights and also there is a significant association between the expression level of CPT1A and PPAR-α genes with different health parameters, including BMI, triglyceride and cholesterol levels.39,40 In this way, Das et al showed an inverse relationship between decreased methylation at two intronic loci of CPT1A and risk of MetS and its components that proposed the CPT1A as a suitable target for the treatment of MetS.64
In addition, Díaz-Rúa et al after 1 month administration of high fat (HF) and high protein (HP) diets to adult Wistar rats, reported an increase in CPT1A mRNA level in PBMC before major alterations in fat mass, body weight and HOMA-IR index.65 These findings revealed that PBMC is a convenient and proper tissue for assessment of nutritional intervention in positive or negative direction, and is able to swiftly reflect the changes in the health indexes like; HOMA-IR, triglyceride and effective genes such as; CPT1A, cardiotrophin-1 (CT-1), sirtuins1/2 (SIRT1/2), LEP-R and signal-regulatory protein β1 (SIRPβ 1) genes.66 Likewise, we used infant’s PBMC for evaluating the effects of nutritional factors on the expression level of key genes in the metabolic pathways which properly reflected the effect of different types of feeding in infants after 5–6 months. In the present study, we found that the expression level of PPAR-α in the breastfeeding group was significantly higher than that in the two other groups. Indeed, these modulatory effects of breastfeeding confirmed its protective effects against overweight and obesity that perform through up- and down-regulation of protective and predisposing genes.
Also, different studies reported that the expression level of effective genes like: SLC27A2, FASN, PPAR-α, and INSR, in breastfed infants are higher than formula-fed.39,40 PPAR-α which mostly expressed in tissues with a high level of fatty acid catabolism plays a major role in metabolic processes and regulation of obesity. In the prolonged fasting situation with energy deprivation, PPAR-α activates the process of ketogenesis via up-regulation of some genes involved in the fatty acid transportation and β-oxidation. The central role of PPAR-α during fasting is related to up-regulation of different target genes like; PDK4, ACOX1, Acadvl, Hadha, CPT2 and CPT1A that increase plasma ketone body. Also, numerous in-vivo studies showed that PPAR-α deficient mice are more obese with abnormal triglyceride and cholesterol levels. On the other hand, obesity leads to suppression of PPAR-α and its corresponding target gene expression.67 Several longitudinal cohort studies with a large number of participants proved the protective effects of breastfeeding against all risk factors of overweight and obesity.68,69
The unique composition of human milk that contains a balanced proportion of macro- and micro-nutrients plus other biologically active compounds like leptin and ghrelin, seems to be responsible for the numerous beneficial effects of it.58 Leptin and ghrelin that secreted in breast milk can influence the proliferation and differentiation of infant adipocytes and prevent excessive weight gain. Accordingly, human milk possesses lower energy and protein content and has more long-chain polyunsaturated fatty acids (LCPUFAs), cholesterol and non-digestible carbohydrate as prebiotics. Interestingly, together with changes in the diet composition of mothers the composition of breast milk will change and provide the metabolic needs of children in different growing steps.48,70 Generally, these properties modulate some pathways and hormones that resulted in the progress of hunger and satiety self-regulation and slower child growth in breastfed infants.13,71 Detection of possible mechanisms that involved in the protective effects of human milk against developing obesity is difficult, but it seems that some hormones and inflammatory factors such as insulin and insulin-like growth factor I (IGF-I), leptin, adiponectin, ghrelin, resistin, IL-6, and TNF-α, exist in breast milk and affect fat deposition in infants through modifying the appetite, satiety-responsiveness and reducing over-eating risk.18 Also, intake of large amounts of protein and amino acids in the first 2 years of life can decrease lipolysis and increase the secretion of insulin and insulin-like growth factor I (IGF-I), which leads to adipogenic activity and differentiation via autocrine-paracrine pathways.72–74
In addition, formula-fed infants are more likely to empty the bottle in late infancy in comparison to breastfed infants. This may happen when parents encourage infants to finish the contents of the bottle.75 Recent evidence has demonstrated that nutrients and genes have a reciprocal relationship, so that some nutrients and bioactive compounds in human milk directly or indirectly can regulate the expression level of different genes.
The present study had some limitations including low sample size. It seems that the evaluation of more children is required to obtain more accurate results and the monitoring should be conducted in several periods of life. Also, we had not enough information about the consumed milk volume, calories and the components of formula feeding. Therefore, we could not recognize the relationship between calorie intake, formula components and the expression level of target genes. The strength of this study was that the participated infants were divided into three different food groups at the beginning of the study, and sampling was performed before the initiation of the solid foods to evaluate the exact effects of the primary feeding in infants.
In summary, the present study indicates that breastfeeding can decrease the expression level of FTO and CPT1A genes and can increase the expression level of PPAR-α gene. Breastfeeding may exert modulatory effects on the expression level of obesity-predisposing genes and protects against several communicable and non-communicable diseases in infancy and in adult life. Hence, breastfeeding as the best type of feeding for infants with a lot of beneficial effects on health and growth should be recommended to all women and their infants. However, further investigations with larger populations are needed to evaluate other obesity-related genes and elucidate the mechanisms of epigenetic effects of human milk.
Abbreviations
BCAAs, branched-chain amino acids; cDNA, complementary DNA; CPT1A, carnitine palmitoyltransferase IA; Ct, threshold cycle; CT-1, cardiotrophin-1; DNMT, DNA methyltransferases; FASN, fatty acid synthase; FTO, fat mass and obesity-associated; INSR, insulin receptor; LCPUFAs, long-chain polyunsaturated fatty acids; LEP-R, leptin receptor; MetS, metabolic syndrome; mTORC1, mammalian target of rapamycin complex 1; PBMC, peripheral blood mononuclear cell; PPAR-α, peroxisome proliferator-activated receptor-α; RT-PCR, reverse transcription-polymerase chain reaction; SIRPβ 1, signal-regulatory protein β1; SIRT1/2, sirtuins1/2; SNPs, single nucleotide polymorphisms.
Ethics Approval
Ethical approval for the study was obtained from the Kermanshah University of Medical Sciences Ethics Committee with code No. IR.KUMS.REC.1397.069
Acknowledgment
We are grateful to all the parents and infants who took part in this study. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. The financial support of the Kermanshah University of Medical Sciences is gratefully acknowledged.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. World Health Organization. Obesity and overweight fact sheet. June 2016; http://www.who.int/mediacentre/factsheets/fs311/en/.
2. Kumar S, Kelly AS. Review of childhood obesity: from epidemiology, etiology, and comorbidities to clinical assessment and treatment. Mayo Clin Proc. 2017;92(2):251–265. doi:10.1016/j.mayocp.2016.09.017
3. Chooi YC, Ding C, Magkos F. The epidemiology of obesity. Metabolism. 2019;92:6–10. doi:10.1016/j.metabol.2018.09.005
4. Wen LM, Rissel C, He G. The effect of early life factors and early interventions on childhood overweight and obesity 2016. J Obes. 2017;2017:3642818. doi:10.1155/2017/3642818
5. Blüher M. Obesity: global epidemiology and pathogenesis. Nat Rev Endocrinol. 2019;15(5):288–298. doi:10.1038/s41574-019-0176-8
6. Douketis JD, Macie C, Thabane L, et al. Systematic review of long-term weight loss studies in obese adults: clinical significance and applicability to clinical practice. Int J Obes (Lond). 2005;29(10):1153–1167. doi:10.1038/sj.ijo.0802982
7. Gadde KM, Martin CK, Berthoud H-R, et al. Obesity: pathophysiology and Management. J Am Coll Cardiol. 2018;71(1):69–84. doi:10.1016/j.jacc.2017.11.011
8. Campbell KJ, Hesketh KD, McNaughton SA, et al. The extended infant feeding, activity and nutrition trial (InFANT Extend) Program: a cluster-randomized controlled trial of an early intervention to prevent childhood obesity. BMC Public Health. 2016;16(1):166. doi:10.1186/s12889-016-2836-0
9. Tiffon C. The impact of nutrition and environmental epigenetics on human health and disease. Int J Mol Sci. 2018;19(11):3425. doi:10.3390/ijms19113425
10. Block T, El-Osta A. Epigenetic programming, early life nutrition and the risk of metabolic disease. Atherosclerosis. 2017;266:31–40. doi:10.1016/j.atherosclerosis.2017.09.003
11. Skrypnik K, Suliburska J, Skrypnik D, et al. The genetic basis of obesity complications. Acta Sci Pol Technol Aliment. 2017;16(1):83–91. doi:10.17306/j.afs.2017.0442
12. Clément K, Viguerie N, Diehn M, et al. In vivo regulation of human skeletal muscle gene expression by thyroid hormone. Genome Res. 2002;12(2):281–291. doi:10.1101/gr.207702
13. Verduci E, Banderali G, Barberi S, et al. Epigenetic effects of human breast milk. Nutrients. 2014;6(4):1711–1724. doi:10.3390/nu6041711
14. Spatz DL. Preventing obesity starts with breastfeeding. J Perinat Neonatal Nurs. 2014;28(1):41–50. doi:10.1097/jpn.0000000000000009
15. Whitehead RG. For how long is exclusive breast-feeding adequate to satisfy the dietary energy needs of the average young baby? Pediatr Res. 1995;37(2):239–243. doi:10.1203/00006450-199502000-00019
16. Rolland-Cachera MF, Deheeger M, Akrout M, et al. Influence of macronutrients on adiposity development: a follow up study of nutrition and growth from 10 months to 8 years of age. Int J Obes Relat Metab Disord. 1995;19(8):573–578.
17. Lucas A, Sarson DL, Blackburn AM, et al. Breast vs bottle: endocrine responses are different with formula feeding. Lancet. 1980;1(8181):1267–1269. doi:10.1016/s0140-6736(80)91731-6
18. Marseglia L, Manti S, D’Angelo G, et al. Obesity and breastfeeding: the strength of association. Women Birth. 2015;28(2):81–86. doi:10.1016/j.wombi.2014.12.007
19. Plagemann A. ‘Fetal programming’ and ‘functional teratogenesis’: on epigenetic mechanisms and prevention of perinatally acquired lasting health risks. J Perinat Med. 2004;32(4):297–305. doi:10.1515/JPM.2004.055
20. Singhal A. Does Breastfeeding Protect from Growth Acceleration and Later Obesity? In: Issues in Complementary Feeding. Vol. 60. Karger Publishers; 2007:15–29.
21. Hauner H, Rohrig K, Petruschke T. Effects of epidermal growth factor (EGF), platelet-derived growth factor (PDGF) and fibroblast growth factor (FGF) on human adipocyte development and function. Eur J Clin Invest. 1995;25(2):90–96. doi:10.1111/j.1365-2362.1995.tb01532.x
22. Krishnan M, Thompson JMD, Mitchell EA, et al. Analysis of association of gene variants with obesity traits in New Zealand European children at 6 years of age. Mol Biosyst. 2017;13(8):1524–1533. doi:10.1039/c7mb00104e
23. Demerath EW, Choh AC, Czerwinski SA, et al. Genetic and environmental influences on infant weight and weight change: the fels longitudinal study. Am J Hum Biol. 2007;19(5):692–702. doi:10.1002/ajhb.20660
24. Li L, Wang G, Li N, et al. Identification of key genes and pathways associated with obesity in children. Exp Ther Med. 2017;14(2):1065–1073. doi:10.3892/etm.2017.4597
25. Han L, Shen WJ, Bittner S, et al. PPARs: regulators of metabolism and as therapeutic targets in cardiovascular disease. Part I: PPAR-alpha. Future Cardiol. 2017;13(3):259–278. doi:10.2217/fca-2016-0059
26. Houten SM, Wanders RJ. A general introduction to the biochemistry of mitochondrial fatty acid beta-oxidation. J Inherit Metab Dis. 2010;33(5):469–477. doi:10.1007/s10545-010-9061-2
27. Berndt J, Kovacs P, Ruschke K, et al. Fatty acid synthase gene expression in human adipose tissue: association with obesity and type 2 diabetes. Diabetologia. 2007;50(7):1472–1480. doi:10.1007/s00125-007-0689-x
28. Mitchell SE, Nogueiras R, Morris A, et al. Leptin receptor gene expression and number in the brain are regulated by leptin level and nutritional status. J Physiol. 2009;587(Pt 14):3573–3585. doi:10.1113/jphysiol.2009.173328
29. Loos RJ, Bouchard C. FTO: the first gene contributing to common forms of human obesity. Obes Rev. 2008;9(3):246–250. doi:10.1111/j.1467-789X.2008.00481.x
30. Doaei S, Kalantari N, Izadi P, et al. Interactions between macro-nutrients’ intake, FTO and IRX3 gene expression, and FTO genotype in obese and overweight male adolescents. Adipocyte. 2019;8(1):386–391. doi:10.1080/21623945.2019.1693745
31. Doaei S, Kalantari N, Mohammadi NK, et al. Up-regulation of FTO gene expression was associated with increase in skeletal muscle mass in overweight male adolescents. Arch Med Sci. 2019;15(5):1133–1137. doi:10.5114/aoms.2019.87239
32. Doaei S, Jarrahi SM, Moghadam AS, et al. The effect of rs9930506 FTO gene polymorphism on obesity risk: a meta-analysis. Biomol Concepts. 2019;10(1):237. doi:10.1515/bmc-2019-0025
33. Gulati P, Cheung MK, Antrobus R, et al. Role for the obesity-related FTO gene in the cellular sensing of amino acids. Proc Natl Acad Sci U S A. 2013;110(7):2557–2562. doi:10.1073/pnas.1222796110
34. Cheung MK, Gulati P, O’Rahilly S, et al. FTO expression is regulated by availability of essential amino acids. Int J Obes (Lond). 2013;37(5):744–747. doi:10.1038/ijo.2012.77
35. Akbari ME, Gholamalizadeh M, Doaei S, et al. FTO gene affects obesity and breast cancer through similar mechanisms: a new insight into the molecular therapeutic targets. Nutr Cancer. 2018;70(1):30–36. doi:10.1080/01635581.2018.1397709
36. Wu W, Feng J, Jiang D, et al. AMPK regulates lipid accumulation in skeletal muscle cells through FTO-dependent demethylation of N(6)-methyladenosine. Sci Rep. 2017;7(1):41606. doi:10.1038/srep41606
37. Bonnefont J-P, Djouadi F, Prip-Buus C, et al. Carnitine palmitoyltransferases 1 and 2: biochemical, molecular and medical aspects. Mol Aspects Med. 2004;25(5):495–520. doi:10.1016/j.mam.2004.06.004
38. Keung W, Ussher JR, Jaswal JS, et al. Inhibition of carnitine palmitoyltransferase-1 activity alleviates insulin resistance in diet-induced obese mice. Diabetes. 2013;62(3):711–720. doi:10.2337/db12-0259
39. Sanchez J, Priego T, Pico C, et al. Blood cells as a source of transcriptional biomarkers of childhood obesity and its related metabolic alterations: results of the IDEFICS study. J Clin Endocrinol Metab. 2012;97(4):E648–E652. doi:10.1210/jc.2011-2209
40. Priego T, Sanchez J, Pico C, et al. Influence of breastfeeding on blood-cell transcript-based biomarkers of health in children. Pediatr Obes. 2014;9(6):463–470. doi:10.1111/j.2047-6310.2013.00204.x
41. McCrory C, Layte R. Breastfeeding and risk of overweight and obesity at nine-years of age. Soc Sci Med. 2012;75(2):323–330. doi:10.1016/j.socscimed.2012.02.048
42. Mutch DM, Wahli W, Williamson G. Nutrigenomics and nutrigenetics: the emerging faces of nutrition. FASEB J. 2005;19(12):1602–1616. doi:10.1096/fj.05-3911rev
43. Bammann K, Sioen I, Huybrechts I, et al. The IDEFICS validation study on field methods for assessing physical activity and body composition in children: design and data collection. Int J Obes (Lond). 2011;35(Suppl 1):S79–S87. doi:10.1038/ijo.2011.38
44. Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29(9):e45. doi:10.1093/nar/29.9.e45
45. Cope MB, Allison DB. Critical review of the World Health Organization’s (WHO) 2007 report on ‘evidence of the long-term effects of breastfeeding: systematic reviews and meta-analysis’ with respect to obesity. Obes Rev. 2008;9(6):594–605. doi:10.1111/j.1467-789X.2008.00504.x
46. Bider-Canfield Z, Martinez MP, Wang X, et al. Maternal obesity, gestational diabetes, breastfeeding and childhood overweight at age 2 years. Pediatr Obes. 2017;12(2):171–178. doi:10.1111/ijpo.12125
47. Matias SL, Dewey KG, Quesenberry CP
48. Yan J, Liu L, Zhu Y, et al. The association between breastfeeding and childhood obesity: a meta-analysis. BMC Public Health. 2014;14(1):1267. doi:10.1186/1471-2458-14-1267
49. Church C, Moir L, McMurray F, et al. Overexpression of Fto leads to increased food intake and results in obesity. Nat Genet. 2010;42(12):1086–1092. doi:10.1038/ng.713
50. Zhao X, Yang Y, Sun B-F, et al. FTO and obesity: mechanisms of association. Curr Diab Rep. 2014;14(5):486. doi:10.1007/s11892-014-0486-0
51. Jiao Y, Zhang J, Lu L, et al. The FTO gene regulates the proliferation and differentiation of pre-adipocytes in vitro. Nutrients. 2016;8(2):102. doi:10.3390/nu8020102
52. Doaei S, Mosavi Jarrahi SA, Sanjari Moghadam A, et al. The effect of rs9930506 FTO gene polymorphism on obesity risk: a meta-analysis. Biomol Concepts. 2019;10(1):237–242. doi:10.1515/bmc-2019-0025
53. Liu Y, Liu Z, Song Y, et al. Meta-analysis added power to identify variants in FTO associated with type 2 diabetes and obesity in the Asian population. Obesity (Silver Spring). 2010;18(8):1619–1624. doi:10.1038/oby.2009.469
54. Cha SW, Choi SM, Kim KS, et al. Replication of genetic effects of FTO polymorphisms on BMI in a Korean population. Obesity (Silver Spring). 2008;16(9):2187–2189. doi:10.1038/oby.2008.314
55. Merkestein M, Laber S, McMurray F, et al. FTO influences adipogenesis by regulating mitotic clonal expansion. Nat Commun. 2015;6(1):6792. doi:10.1038/ncomms7792
56. Abarin T, Wu Y Y, Warrington N, et al. The impact of breastfeeding on FTO-related BMI growth trajectories: an application to the Raine pregnancy cohort study. Int J Epidemiol. 2012;41(6):1650–1660. doi:10.1093/ije/dys171
57. da Silva CF, Zandona MR, Vitolo MR, et al. Association between a frequent variant of the FTO gene and anthropometric phenotypes in Brazilian children. BMC Med Genet. 2013;14(1):34. doi:10.1186/1471-2350-14-34
58. Breastfeeding S. Breastfeeding and the use of human milk. Pediatrics. 2012;129(3):e827–e841. doi:10.1542/peds.2011-3552
59. Tammen SA, Friso S, Choi SW. Epigenetics: the link between nature and nurture. Mol Aspects Med. 2013;34(4):753–764. doi:10.1016/j.mam.2012.07.018
60. Liotto N, Miozzo M, Giannì ML, et al. Early nutrition: the role of genetics and epigenetics. Pediatr Med Chir. 2009;31(2):65–71.
61. Melnik BC. Milk: an epigenetic amplifier of FTO-mediated transcription? Implications for Western diseases. J Transl Med. 2015;13:385. doi:10.1186/s12967-015-0746-z
62. Oddy WH, Li J, Landsborough L, et al. The association of maternal overweight and obesity with breastfeeding duration. J Pediatr. 2006;149(2):185–191. doi:10.1016/j.jpeds.2006.04.005
63. Sandoval Jurado L, Jiménez Báez MV, Olivares Juárez S, et al. Lactancia materna, alimentación complementaria y el riesgo de obesidad infantil. Aten Primaria. 2016;48(9):572–578. doi:10.1016/j.aprim.2015.10.004
64. Das M, Sha J, Hidalgo B, et al. Association of DNA methylation at CPT1A locus with metabolic syndrome in the genetics of lipid lowering drugs and diet network (GOLDN) study. PLoS One. 2016;11(1):e0145789. doi:10.1371/journal.pone.0145789
65. Diaz-Rua R, Palou A, Oliver P. Cpt1a gene expression in peripheral blood mononuclear cells as an early biomarker of diet-related metabolic alterations. Food Nutr Res. 2016;60(1):33554. doi:10.3402/fnr.v60.33554
66. Marti A, Morell-Azanza L, Rendo-Urteaga T, et al. Serum and gene expression levels of CT-1, IL-6, and TNF-alpha after a lifestyle intervention in obese children. Pediatr Diabetes. 2018;19(2):217–222. doi:10.1111/pedi.12561
67. Kersten S. Integrated physiology and systems biology of PPARalpha. Mol Metab. 2014;3(4):354–371. doi:10.1016/j.molmet.2014.02.002
68. Quigley MA. Re: “Duration of breastfeeding and risk of overweight: a meta-analysis. Am J Epidemiol. 2006;163(9):870–872. doi:10.1093/aje/kwj134
69. Hunsberger M, Lanfer A, Reeske A, et al. Infant feeding practices and prevalence of obesity in eight European countries - the IDEFICS study. Public Health Nutr. 2013;16(2):219–227. doi:10.1017/s1368980012003850
70. Skrypnik D, Bogdański P, Zawiejska A, et al. Role of gestational weight gain, gestational diabetes, breastfeeding, and hypertension in mother-to-child obesity transmission. Pol Arch Intern Med. 2019;129(4):267–275. doi:10.20452/pamw.4426
71. Agostoni C, Baselli L, Mazzoni MB. Early nutrition patterns and diseases of adulthood: a plausible link? Eur J Intern Med. 2013;24(1):5–10. doi:10.1016/j.ejim.2012.08.011
72. Socha P, Grote V, Gruszfeld D. Milk protein intake, the metabolic-endocrine response, and growth in infancy: data from a randomized clinical trial. Am J Clin Nutr. 2011;94(suppl_6):1776S–1784S. doi:10.3945/ajcn.110.000596
73. Jones AP, Simson EL, Friedman MI. Gestational undernutrition and the development of obesity in rats. J Nutr. 1984;114(8):1484–1492. doi:10.1093/jn/114.8.1484
74. Lucas A, Boyes S, Bloom S, et al. Metabolic and endocrine responses to a milk feed in six‐day‐old term infants: differences between breast and cow’s milk formula feeding. Acta Pædiatrica. 1981;70(2):195–200. doi:10.1111/j.1651-2227.1981.tb05541.x
75. Li R, Fein SB, Grummer-Strawn LM. Do infants fed from bottles lack self-regulation of milk intake compared with directly breastfed infants? Pediatrics. 2010;125(6):e1386–e1393. doi:10.1542/peds.2009-2549
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

