Back to Journals » Clinical Ophthalmology » Volume 14
Effectiveness of Subconjunctival Cyclosporine in Treatment of Acute Allergic Conjunctivitis in a Rat-Model
Authors Awara A , Atiba A , Helal D, Elbedewy H
Received 31 December 2019
Accepted for publication 29 January 2020
Published 13 February 2020 Volume 2020:14 Pages 431—435
DOI https://doi.org/10.2147/OPTH.S244287
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Amr Awara,1 Ayman Atiba,2 Duaa Helal,3 Hazem Elbedewy1
1Ophthalmology Department, Tanta University, Tanta, Egypt; 2Department of Surgery, Anesthesiology and Radiology, Faculty of Veterinary Medicine, Kafrelsheikh University, Kafrelsheikh, Egypt; 3Pathology Department, Tanta University, Tanta, Egypt
Correspondence: Amr Awara
Ophthalmology Department, Tanta University, Tanta 31511, Egypt
Tel +2-01274005384
Fax +20403415008
Email [email protected]
Background: Eye allergy is widely spread worldwide. The treatment includes topical anti-histamines, steroids and non-steroidal drugs. Steroids are the first choice by many ophthalmologists, but unfortunately they may cause serious side effects. Cyclosporine A (CsA) is an immunomodulator drug that can improve eye allergy and reduce the need for steroids; however, topical preparation of CsA is difficult because of the lipophilic nature of the drug.
Methods: An experimental study included 16 rats with induced allergy were divided into 2 groups. Group 1: allergic non-treated (6 rats), and Group 2: allergic treated with 0.5 mL subconjunctival CsA 1% (10 rats). Half of each group was sacrificed at 24 hrs and the other half at 1 week. Conjunctival hyperemia and eosinophilic cell count were assessed at each time.
Results: Group 2 (CsA treated) showed significantly lower hyperemia score and eosinophilic count at both 24 hrs and 1 week. No ocular complications were noted.
Conclusion: Subconjunctival CsA was safe and effective in treating ocular allergy through improving conjunctival hyperemia and reducing eosinophilic cell count with no significant ocular side effects.
Keywords: eye allergy, cyclosporine A, eosinophilia, subconjunctival
Introduction
Ocular allergy has a variable clinical presentation; ranging from allergic rhinoconjunctivitis, seasonal allergy to vernal or giant papillary conjunctivitis.1
Because there is no gold standard to treatment; ophthalmologists still have to tailor treatment to each patient. The mainstay of treatment of ocular allergy is the topical anti-inflammatory, anti-histamine and anti-allergic eye drops. These drugs can effectively reduce the symptoms and signs of allergy.2
Topical steroids are highly effective drugs; however, they have serious side effects as they may elevate the intraocular pressure in steroid responders and facilitate ocular infection as they are local immunosuppressants. They can also induce cataracts and cause delayed wound healing.3
The non-steroidal agents are anti-inflammatory drugs that also have a good effect on ocular allergy.4 One of the non-steroidal anti-inflammatory drugs is Cyclosporin A (CsA). It is an immunomodulator which acts through inhibiting the activation of antigen-dependent T cells. It also can directly inhibit eosinophil and mast cell activation and immune-mediators release.5–8
CsA can be administered systemically as orally or topical as an eye drops. However, the systemic administration can lead to renal side effects9 and the topical preparation of CsA is not easy as it is lipophilic and must be dissolved in an alcohol-oil base.10
To our knowledge, the subconjunctival injection of CsA has not been tried before. This work is studying the safety and efficacy of subconjunctival injection of CsA in an animal model which is supposed to have better clinical results especially with poor compliant cases and long-lasting effect.
Materials and Methods
Ethical Approval
Prior to the commencement of the study, the experiments were approved and conducted in compliance with the animal care and use committee of faculty of veterinary medicine, Kafrelsheikh University, Egypt. Kafrelsheikh University follows the institutional animal care and use committee in Egypt.
Reagents
Compound 48/80 (Sigma-Aldrich, USA) is a mast cell degranulator and condensation product mixture of p-methoxy-N-methyl phenethylamine and formaldehyde.
Experimental Animals
Male Wistar rats aged 10 weeks weighting 180–210 g were used. The animals had ad libitum access to food and water. The animals were housed 3 per cage at 23±2 ºC temperature and relative humidity 57 ±3% and 12 h light dark cycle.
Allergic Model
The allergic model was conducted by using C48/80 (Sigma-Aldrich, USA), which was dissolved in phosphate-buffered saline (PBS). One drop (30 μL) containing 1000 μg C48/80 was applied topically onto the surface of the eye to release the histamine from mast cells. Rats were divided into 2 groups, Group 1: included 6 allergic untreated rats as control group and Group II: included 10 allergic rats treated with subconjunctival injection of 0.5 mL of cyclosporine A 1.25mg/mL (Novartis® Pharmaceuticals Corporation, East Hanover, New Jersey, USA). The injection was prepared by adding one mL of CsA solution used as vial for intravenous infusion (50mg/mL) to 19 mL sterile saline 0.9%, then each rat was subconjunctivally injected with 0.5mL of the prepared solution. Cyclosporine was injected only one time in both eyes after 12–24 hr from the first application of C48/80 which was applied topically once daily afterward till the end of the experiments.
Three rats of group 1 and 5 rats of group 2 were sacrificed at 24 hrs, while the rest of the rats (3 of group 1 and 5 of group 2) were sacrificed after 1 week. Rats sacrificed at 12 h were observed for the hyperemia, chemosis and scored just before the sacrifice, while the rest of the rats were observed for hyperemia and scored every 24 hrs and for one week. The severity of conjunctival hyperaemia was scored. Scoring (none = 0, mild = 1, moderate = 2; severe = 3) was performed by two independent and treatment-blinded ophthalmologists.
Tissue Preparation and Histological Analysis
The eyes, including the conjunctivas from the 2 studied groups were harvested and fixed in 10% buffered formalin processed routinely and embedded in paraffin blocks. Tissue sections (5 mm) were stained with hematoxylin and eosin for histologic examination. Infiltrating eosinophils in the lamina propria and mucosae of the tarsal and bulbar conjunctivas throughout each section were counted. Eosinophilic cell count is expressed as infiltrating eosinophil numbers divided by area (cell/mm2). Any other changes in conjunctiva such necrosis, ulceration were recorded.
Statistical Analysis
Results were analyzed by SPSS V. 23 (SPSS Inc. Released 2015. IBM SPSS statistics for windows, version 23.0, Armonk, NY: IBM Corp.). Student’s t-test was used to compare two normally distributed means of two groups while Mann Whitney’s test was used for the not normally distributed ones. Two-sided p value <0.05 was considered statistically significant.
Results
At 24 hrs, group 2 had significantly lower hyperemia score and eosinophilic count (p <0.001 and 0.005, respectively). This significant difference continued till 1 week where group 2 showed significantly lower hyperemia score and eosinophilic count (p <0.001 for both), this is detailed in Tables 1 and 2 and Figure 1.
 |
Table 1 Hyperemia Score and Eosinophilic Count at 24 hrs in Both Groups |
 |
Table 2 Eosinophilic Count at 1 Week in Both Groups |
 |
Figure 1 The daily hyperemia score in the 2 groups. |
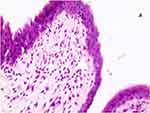
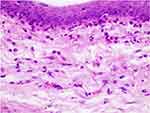
The lamina propria was thickened in both groups due to the inflammatory process as shown in the figures. Representative figures of H&E stained conjunctival sections from groups of rats are presented in [Figure 2]. The section from group 1 (allergic group) showed severe and aggravated infiltration of eosinophils. Group 2 (cyclosporin treated group) showed less amount of inflammatory infiltrate compared to group 1 especially eosinophils without any necrosis, ulceration or other changes [Figures 3 and 4].
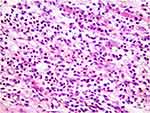 |
Figure 2 H&E stained conjunctival sections from allergic untreated rats with massive infiltration with eosinophils (H&E x 200). |
Discussion
Acute eye allergy ranges from mild allergic reaction to the severe vernal keratoconjunctivitis (VKC) one. VKC can be presented as severe acute episode or acute on top of chronic form. VKC is present all over the world especially in the Mediterranean countries where the climate is mostly warm and windy.11 Patients affected by VKC suffer conjunctival hyperemia, discharge and itching and may end up by vision-threatening corneal complications like shield ulcers.
The pathogenicity of acute eye allergy and VKC is mainly inflammatory with IgE and T-cell mediated reaction with activation of eosinophils, mast, lymphocytes and histamine release.12 Several anti-allergic drug classes are available for treatment of VKC like vasoconstrictors, mast cell stabilizers, steroids, non-steroidal anti-inflammatory and Immunomodulators.13 There is no standard treatment for acute eye allergy or VKC and there are many available options, the selection of which is dependent upon the severity/duration of the disease and the clinical experience of the physician. Many physicians consider Steroids is the standard treatment, however, on the long-term use, they may cause serious side effects.14
CsA is an immunomodulator which acts through inhibiting IL-2 production, blocking Th2 proliferation and histamine release from mast cells.10 In a study done by Ozcan et al, CsA 0.05% decreased the severity of symptoms and clinical signs significantly reduced after 6 months and the need for steroids were reduced, suggesting that CsA at low doses is an effective steroid-sparing agent especially in VKC.15
Variable concentrations of topical cyclosporine have been tried: 2%, 1%, 0.5%, and 0.05%. So far, there is no general consensus regarding the minimum effective concentration of CsA. Burning and irritation are frequent side effects with topical application. Adverse events, such as bacterial or viral infections are rare, while IOP changes have not been reported.13 Systemic absorption of CsA was not detectable by clinical laboratory methods. CsA has large molecular weight with low aqueous solubility which requires specific delivery strategies to improve its bioavailability.16
Effectiveness of topical cyclosporine studied in different trials with variable effect. In a randomized controlled trial, the effects of CsA 0.05% were similar to placebo.8 Conversely, in a clinical prospective and observational study in 594 patients, CsA 0.1% was shown to be effective and safe for the treatment of acute allergy and VKC.17 A recent systematic review and meta-analysis study suggests that topical CsA is effective and safe for the treatment of VKC, since signs and symptoms significantly improve after treatment, regardless of the CsA dosage.18
Limited availability of marketed ophthalmic product containing cyclosporine (commercial eye drops) leads to its preparation in hospital pharmacies by castor or olive oil as solvent with different concentrations and bioavailability for the eye.19
We used CsA 1% (1mg/mL) as one subconjunctival injection instead of topical application. It was effective in reducing the allergic reaction and alleviating the symptoms and signs with no apparent signs of toxicity either on histological or clinical level. The histopathological sections showed a significant decrease in the eosinophilic count than the control group with no effect on the conjunctival tissue.
On the clinical level, there was significant improvement of the conjunctival hyperemia without any signs of conjunctival epithelial toxicity (such as cloudy swelling, cytoplasmic vacuolation, necrosis or ulceration).
In this study subconjunctival injection of cyclosporin, 1mg/mL after induction of acute allergic conjunctivitis was very rapid as it appeared after 1day. The short-term effect at 1 week was recorded.
Conclusion
Subconjunctival CsA injection proved clinically and histologically to be effective with no conjunctival toxic effect in controlling acute allergic conjunctivitis. Value of subconjunctival route in acute allergy and refractory cases is delivering large dose with longer lasting effect than topical application which require frequent instillation because of tearing, blinking and tear film turn over. Also, it avoids hazards of supratarsal steroid injections with serious side effects.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Del Río-Navarro BE, Sienra-Monge JJ, Castellanos A, Williams-Gotti MJ. Allergic conjunctivitis. Bol Med Hosp Infant Mex. 1992;49(4):201–204.
2. Mantelli F, Santos MS, Petitti T, et al. Systematic review and metaanalysis of randomised clinical trials on topical treatments for vernal keratoconjunctivitis. Br J Ophthalmol. 2007;91:1656–1661. doi:10.1136/bjo.2007.122044
3. Keklikci U, Dursun B, Cingu A. Topical cyclosporine a 0.05% eyedrops in the treatment of Vernal Keratoconjunctivitis– randomized placebo-controlled trial. Adv Clin Exp Med. 2014;23(3):455–461. doi:10.17219/acem/37145
4. Bonini S, Coassin M, Aronni S, Lambiase A. Vernal keratoconjunctivitis. Eye. 2004;18:345–351. doi:10.1038/sj.eye.6700675
5. Nussenblatt RB, Palestine AG. Cyclosporine A: immunology, pharmacology and therapeutic uses. SurvOphthalmol. 1986;31:159–169.
6. Borel JF, Baumann G, Chapman I, et al. In vivo pharmacological effects of cyclosporin and some analogues. Adv Pharmacol. 1996;35:115–246.
7. Whitcup SM, Chan CC, Luyo DA, Bo P, Li Q. Topical cyclosporine inhibits mast cell-mediated conjunctivitis. Invest Ophthalmol Vis Sci. 1996;37:2686–2693.
8. Daniell M, Constantinou M, Vu HT, Taylor HR. Randomised controlled trial of topical ciclosporin A in steroid dependent allergic conjunctivitis. Br J Ophthalmol. 2006;90:461–464. doi:10.1136/bjo.2005.082461
9. Fahr A. Cyclosporin clinical pharmacokinetics. Clin Pharmacokinet. 1993;24(6):472–495. doi:10.2165/00003088-199324060-00004
10. Utine CA, Stern M, Akpek EK. Clinical review: topical ophthalmic use of cyclosporin A. Ocul Immunol Inflamm. 2010;18(5):352–361. doi:10.3109/09273948.2010.498657
11. Leonardi A, Sathe S, Bortolotti M, Beaton A, Sack R. Cytokines, matrix metalloproteases, angiogenic and growth factors in tears of normal subjects and vernal keratoconjunctivitis patients. Allergy. 2009;64(5):710–717. doi:10.1111/all.2009.64.issue-5
12. Leonardi A. Vernal keratoconjunctivitis: pathogenesis and treatment. Prog Retin Eye Res. 2002;21(3):319–339. doi:10.1016/S1350-9462(02)00006-X
13. Leonardi A. Management of Vernal Keratoconjunctivitis. Ophthalmol Ther. 2013;2:73–88. doi:10.1007/s40123-013-0019-y
14. Lambiase A, Leonardi A, Sacchetti M, Deligianni V, Sposato S, Bonini S. Topical cyclosporine prevents seasonal recurrences of vernal keratoconjunctivitis in a randomized, double-masked, controlled 2-year study. J Allergy Clin Immunol. 2011;128(4):896–7 e9. doi:10.1016/j.jaci.2011.07.004
15. Ozcan AA, Ersoz TR, Dulger E. Management of severe allergic conjunctivitis with topical cyclosporin a 0.05% eyedrops. Cornea. 2007;26(9):1035–1038. doi:10.1097/ICO.0b013e31812dfab3
16. Czogalla A. Oral cyclosporine A- the current picture of its liposomal and other delivery systems, cell. Mol Biol Lett. 2009;14:139–152.
17. Ebihara N, Ohashi Y, Uchio E, et al. A large prospective observational study of novel cyclosporine 0.1% aqueous ophthalmic solution in the treatment of severe allergic conjunctivitis. J Ocul Pharmacol Ther. 2009;25(4):365–372. doi:10.1089/jop.2008.0103
18. Wan KH, Chen LJ, Rong SS, et al. Topical cyclosporine in the treatment of allergic conjunctivitis: a meta-analysis. Ophthalmology. 2013;120:2197–2203. doi:10.1016/j.ophtha.2013.03.044
19. Lallemand F, Schmitt M, Bourges JL, Gurny R, Benita S. Cyclosporin A delivery to the eye: a comprehensive review of academic and industrial efforts. Eur J Pharma Biopharm. 2017;117:14–28.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.