Back to Journals » Clinical Ophthalmology » Volume 15
Effectiveness of Elevated Intraocular Pressure as a Criterion for Glaucoma Referral After 6 Years of Follow-Up
Authors Nilsson AG , Peters D
Received 30 April 2021
Accepted for publication 6 July 2021
Published 16 July 2021 Volume 2021:15 Pages 3041—3049
DOI https://doi.org/10.2147/OPTH.S318068
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Andreas G Nilsson,1 Dorothea Peters2
1Department of Ophthalmology, Skåne University Hospital, Malmö, Sweden; 2Department of Clinical Sciences Malmö, Ophthalmology, Lund University, Malmö, Sweden
Correspondence: Andreas G Nilsson Email [email protected]
Purpose: To evaluate the long-term predictive value of the need to treat patients referred by optometric practitioners, regarding glaucoma, in Malmö, Sweden, using intraocular pressure (IOP) as the primary referral criterion.
Patients and Methods: This retrospective study included 94 of 108 (87%) individuals referred to the Skåne University Hospital in Malmö, Sweden, for elevated IOP during 2012– 2013. Data were extracted from patient records by the end of 2019. Positive outcome was defined as glaucoma, treated suspected glaucoma or treated ocular hypertension (OH) at referral or during the follow-up period. Positive predictive values (PPV) were calculated using different hypothetical thresholds for age and IOP-levels. Long-term follow-up was used to evaluate whether the first visit diagnoses would change over time, and if this would affect the effectiveness of the referrals.
Results: Elevated IOP was the only referral criterion in 84% (n=79). In 28 patients (35%) among the IOP-only referrals, no ocular disease was found, and 26 patients (33%) had a positive outcome at the first visit. Median follow-up time was 6.4 years. PPV according to diagnosis after follow-up was 42% (95% CI: 32– 54%) for IOP-only referrals. Including thresholds of ≥ 45 years of age in combination with an IOP of ≥ 25 mmHg in the referral criteria would have reduced the number of IOP-only referrals by 27% (21 of 79), and increased the PPV to 57% (95% CI: 45– 71%) at the last visit. No positive outcome would have been missed, among those that were followed-up after the first visit, when applying these thresholds for referral, over a follow-up period of six years.
Conclusion: Using only elevated IOP as referral criterion showed a poor accuracy for predicting those that require IOP lowering treatment. The long-term follow-up allowed us to verify the applicability of higher hypothetical threshold requirements on age and IOP for glaucoma referrals from optometric practices.
Keywords: optometric practices, long-term, guidelines, ophthalmology, Sweden
Introduction
Glaucoma is sometimes called the silent thief of sight, due to its often asymptomatic nature until the late stages of the disease. According to several population-based studies, between 40% and 95% of people with glaucoma are unaware of their condition.1–7 Hence, many glaucoma patients present with advanced stages of glaucomatous visual field defects (VFD). Late presentation is still a major risk factor for lifetime visual impairment and blindness due to glaucoma.8–10 There is currently no cost-effective screening method for glaucoma, and glaucoma suspects are often detected through opportunistic case finding by optometric practitioners (ie optometrists and opticians). Several studies over the past decade have reported that a high number of patients referred for glaucoma by optometric practitioners do not have any eye disease,11–14 leading to unnecessary health care costs and patient stress. Recent studies have shown that it is possible to reduce the number of false-positive referrals by introducing different glaucoma referral filtering services,11,12,15 while others have proposed higher intraocular pressure (IOP) thresholds for IOP-only referrals.13,14 One limitation of such studies is that the positive or negative outcome is defined by the results of the first visit to the ophthalmologist. Therefore, patients deemed to require monitoring after referral are often defined as having a positive outcome, even when they are not given any treatment. This group will probably include a high number of patients with a relatively low risk of developing glaucoma, and the positive health effect of following these individuals is likely to be small. Improved knowledge is therefore needed on the long-term predictive value of referrals from optometric practitioners in order to identify reasonable and safe criteria for referral. As of today, there are no national referral guidelines, regarding glaucoma, for optometric practitioners in Sweden.
The aim of this study was to investigate the predictive value of elevated IOP as a referral criterion by optometric practitioners in identifying glaucoma cases, and to evaluate the effects of applying different hypothetical age and IOP thresholds for referral on the long-term outcome. The results of this study will hopefully contribute to the development of more efficient guidelines for glaucoma referrals.
Patients and Methods
The Ethical Review Board of Lund University, Sweden, approved this study, and it was undertaken in accordance with the tenets of the Declaration of Helsinki. Written consent is not required by the Ethical Review Board, as is common practice in Sweden regarding retrospective studies. Patient data confidentiality was secured by de-identifying it and storing it in a locked vault.
This was a retrospective study using data from the records of patients followed at the Department of Ophthalmology at the Skåne University Hospital in Malmö, Sweden. All incoming referrals from optometric practitioners to the Glaucoma Outpatient Department, for elevated IOP, in individuals aged 18 years or older, during the years 2012–2013 were reviewed for inclusion eligibility. Referrals concerning patients with an established diagnosis of glaucoma, and patients with a primary ocular disease other than glaucoma were not eligible. Referrals in which data were lacking, making them impossible to categorize, were excluded. All eligible patients were informed about the study by letter, and given the opportunity to decline participation. Two patients chose not to participate, and another two did not receive the letter, having moved abroad.
Clinical data were extracted from the patient records from the first visit to the Glaucoma Outpatient Department after referral until 31 Dec. 2019. The data collected included age, gender, and eye status at the first and last visits, including IOP, presence of pseudoexfoliations (PEX), and the results of visual field (VF) examinations, including mean deviation (MD) (in dB), Visual Field Index (in %). Only the results of VF examinations using a Humphrey Field Analyser (Carl Zeiss Meditec, Dublin, CA, USA) 24-2, with the SITA Standard or SITA Fast program were included.
Attention was focused on the first hospital visit and the last hospital visit after follow-up. A resident in ophthalmology (A.G.N.) reviewed the diagnoses made during these visits by consulting the patients’ records. If these did not agree with our definitions of glaucoma, the diagnoses were re-defined after consultation with a glaucoma specialist (D.P.).
Open-angle glaucoma with or without exfoliation syndrome was defined as follows:
- A glaucomatous VFD repeated in the same area of the field on at least two consecutive VF measurements with Glaucoma Hemifield Test (GHT) results “outside normal limits” in at least one of the tests and at least “borderline” in the other test.
or
- One VF examination in which VFD was consistent with glaucoma and GHT results “outside normal limits” combined with corresponding glaucomatous optic nerve head (ONH) damage either found in fundus photography or described in the patient’s records.
Structural glaucomatous damage was defined clinically using all available information on the cup shape and depth, as well as the neuroretinal rim in relation to the disc size and shape (eg a notch in the ONH was considered structural glaucomatous damage, but large cupping alone was not).
Patients in which a suspicious ONH appearance was noted in their records and/or inconsistent VFD findings were defined as glaucoma suspects.
Ocular hypertension (OH) was defined as an IOP > 21 mmHg in either eye measured at the Glaucoma Outpatient Department, with a normal ONH appearance according to their records (including, when available, ONH optical coherence tomography (OCT) findings and fundus images) and no signs of glaucomatous VFD. According to our clinical routines, the IOP of patients with a slightly elevated IOP who also had a thick central corneal thickness (CCT) was corrected according to the Ehlers correction factor.16 If the corrected IOP was found to be below 22 mmHg, the patient was not defined as having OH.
Positive outcome was defined as definite open-angle glaucoma, treated suspected glaucoma, or treated OH.
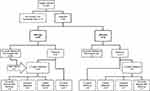
Eligible individuals were assessed and sorted into two sub-groups (Figure 1): one group in which the referral was based solely on an elevated IOP (henceforth referred to as the IOP-only group), and the other in which other examinations had also been conducted (referred to as the IOP-plus group). Information from the IOP-plus group could include VF findings and/or optic nerve-related observations. The IOP of the eye with the highest value measured for each specific patient was used in the analyses, since this was most probably the reason for the referral. In cases where multiple measurements were performed at the optometric practice, the mean value for the eye with the highest IOP value was used.
When a patient was found to have no further need for monitoring or treatment at the first visit to the Glaucoma Outpatient Department or at a later visit, no further action was taken. Those patients were defined as no ocular disease at first hospital visit and no ocular disease after follow-up, respectively.
All others were divided into two groups according to their follow-up time: follow-up < six years or ≥ six years (Figure 1). Cases with a follow-up time < six years were divided into certain diagnosis or uncertain diagnosis. Certain diagnoses denote those patients with a positive outcome prior to being lost to follow-up (mainly due to changing their eye care provider) and patients who died during follow-up. For these patients, the last documented diagnosis was used in the evaluation of the outcome at the last visit.
Statistical Analysis
Positive predictive values (PPV) were calculated for all patients, and for the IOP-only group and the IOP-plus group, separately. PPV was calculated using the results after follow-up. Various hypothetical age and IOP-thresholds were evaluated to investigate the effect on positive outcomes missed and negative outcomes avoided in the IOP-only group.
The Mann–Whitney-U test was used for non-parametric tests, and Fisher’s exact test for nominal values. Statistical analysis was performed with SPSS version 25 (SPSS/IBM, NY, USA). Statistical significance was defined as p≤0.05.
Results
The baseline characteristics of all the patients included in the study, and in the IOP-only and IOP-plus groups, respectively, are given in Table 1.
 |
Table 1 Baseline Characteristics of All Referrals, IOP-Only and IOP-Plus Referrals |
Information in Referrals
Thirty-four different optometric practices referred patients within the study. Only one of those stated that the referral was sent by an optometrist; however, the title of the person referring was not always added to the referral letter. The practices sent between 1 and 12 referrals per practice, 10 of them sent 3 or more referrals during the period of time evaluated. In the IOP-only group, the methods used at the optometric practices were stated as being non-contact tonometry (NCT) in 27 (34%) patients, and Icare® rebound tonometry in nine (11%) patients. The IOP measurement method was not specified in the remaining 43 (54.5%) referrals. Fifty-nine (64%) of all referrals stated only one IOP measurement prior to referral. None of the referrals mentioned PEX or measurements of the CCT. In five (5%) of all referrals, it was specified that the patient had a family history of glaucoma, while no information was given on the family history of the remaining referrals.
Among the IOP-plus referrals, IOP had been measured with NCT in three cases (20%), Icare® rebound tonometry in two cases (13%), Goldmann applanation tonometry in one case (7%), and in nine cases (60%) the method used was not specified. The ONH was described as having been examined using a slit lamp in ten patients (67%), OCT was performed on the ONH in two patients (13%), and a measurement with a Heidelberg Retina Tomograph was included in the referral of one patient (7%). A VF examination had been conducted at the optometric practice in six patients (40%), printouts of which were included in four cases (27%), and in the remaining two (13%) only a description of the results was given. In eight (53%) of the IOP-plus referrals, a glaucoma expert ophthalmologist affiliated with the optometric practice had been consulted prior to the referral to the hospital. This was not the case for any of the patients in the IOP-only group.
Findings at the Glaucoma Outpatient Department
Table 2 presents the findings at the Glaucoma Outpatient Department at the first visit and after follow-up.
 |
Table 2 Outcome and Diagnoses at First Hospital Visit and Last Visit After Follow-Up for All Referrals, IOP-Only and IOP-Plus Referrals |
The elevated IOP leading to referral could not be confirmed with Goldmann applanation tonometry at the first visit to the hospital in 34% of the patients (CCT measurements were not considered). The corresponding results for the IOP-only and IOP-plus groups were 37% and 20%, respectively. Ten (11%) patients were classified as having a negative outcome at the first visit, and changed to a positive outcome during follow-up. Seven of these were in the IOP-only group, and three in the IOP-plus group. In total, 21 patients (22%) had shown PEX at some point during follow-up.
Nine patients (10%) with negative outcome at the first visit were categorized as having an uncertain diagnosis after follow-up due to not reaching ≥ six years’ follow-up; eight in the IOP-only group (10%), and one in the IOP-plus group (7%) (Figure 1). Five of these nine patients (56%) were diagnosed with OH and four (44%) had suspected glaucoma. The median IOP measured at the optometric practice was 28 mmHg (23–37 mmHg) in the nine patients with uncertain diagnosis, compared to 28 mmHg (22–47 mmHg) in the remaining 85 patients (p=0.443, Mann–Whitney U-test, 2-tailed).
After excluding patients without any ocular disease at the first visit, who did not return with a new referral (n=27), the median follow-up time was 69 months (range 1–92 months) for all remaining referrals (n= 67), 69 months (1–92 months) for the IOP-only group (n=53) and 80.5 months (12–86 months) for the IOP-plus group (n=14). After excluding patients where the follow-up revealed no ocular disease (n=9), deceased patients (n=3), patients who were discharged as they had become blind (one patient with herpetic trabeculitis) or due to severe dementia (one patient), and patients with an uncertain diagnosis (n=9), the median follow-up time was 79 months (1–92) for all referrals (n=44), 77 months (1–92) for the IOP-only group (n=34) and 81.5 months (61–86) for the IOP-plus group (n=10).
Effects of Hypothetical Age and IOP Thresholds on IOP-Only Referrals
Prior to applying any hypothetical thresholds, the PPV was 42% (95% CI: 32–54%) for the IOP-only group after follow-up.
None of the patients younger than 45 years of age at referral was defined as having a positive outcome at the first visit or had developed a positive outcome after follow-up (Figure 2). Applying an age threshold of ≥45 years as a requirement for referral would have increased the PPV to 46% (95% CI: 35–58%). An uncertain diagnosis was made of one patient younger than 45 years due to being lost to follow-up. Hence, in the worst-case scenario (where all patients with uncertain diagnosis were classified as having positive outcomes during follow-up) we would have missed one positive outcome (1%).
A combination of age ≥45 years and an IOP threshold of ≥25 mmHg would have led to a PPV of 57% (95% CI: 45–71%). Four of the patients given an uncertain diagnosis would not have been referred using this combination of thresholds, meaning four missed positive outcomes (5%) in the worst-case scenario. However, one of these four patients was followed for 69 months showing only moderate OH, not requiring treatment.
If the IOP threshold had been increased further, to ≥27 mmHg, the PPV would have been 59% (95% CI: 45–75%), but nine (11%) positive outcomes would have been missed. Further five patients with an uncertain diagnosis would also have been missed, leading to a total of 14 missed positive outcomes (18%) in the worst-case scenario (Figure 2).
Discussion
The overall predictive value of referrals from optometric practitioners was relatively low, and more than one patient in three in the IOP-only group was found to have no ocular disease.
As the prevalence of glaucoma increases with age,17 it has been suggested in American guidelines that all patients above 40 years of age should be offered screening for elevated IOP.18 However, no patient younger than 45 years at referral developed a positive outcome in the present study, indicating that the recommended age threshold for screening with IOP alone at optometric practices could be increased.
Additional examinations, other than IOP, prior to referral have been found to increase the PPV of the referrals in previous studies.13,14,19 This was also the case in our study. However, the fact that 53% of the IOP-plus referrals had been evaluated by a glaucoma specialist prior to referral to the hospital should be taken into account.
In roughly one third of the IOP-only patients, the elevated IOP could not be confirmed with Goldman applanation tonometry at the hospital. Regression to the mean is likely one reason for this finding, as many optometric practices did not repeat the IOP measurement prior to the referral. Additionally, five patients with a CCT-corrected IOP < 22 mmHg were not diagnosed as OH and were not followed further. It is possible that none of these patients would have been referred to the hospital if repeated IOP measurements had been made, or the CCT had been measured at the optometric practice prior to referring the patient. The European Glaucoma Society does not recommend the use of correction factors for CCT measurements, but since this was a retrospective study, CCT-corrected IOP was taken into consideration, as this was the routine employed at the hospital at the time. However, none of the patients not diagnosed with OH, due to having thick corneas, were referred again during the follow-up period, supporting the suggestion that filtering out patients with only slightly elevated IOP in combination with a thick cornea could be beneficial in glaucoma screening.
The long-term follow-up of this study is a strength in that it improved the reliability of the diagnosis. We can therefore be more confident that no patients in need of treatment would have been missed through raising the age and IOP thresholds for referrals. However, there is always a risk that a patient may eventually develop a positive outcome, even after six years of follow-up. Re-defining the diagnosis used in the journals for each patient according to specific definition criteria makes the diagnosis more reliable, despite the retrospective approach.
The retrospective nature of this study, where a high amount of data is missing, especially from the referrals, can be seen as an obvious limitation. There are also a small number of patients with uncertain diagnoses due to being lost to follow-up. There is thus a risk of missing patients with positive outcomes during follow-up when using higher thresholds. However, based on calculations according to a worst-case scenario, we consider the suggested thresholds for age and IOP to be reasonable. Also, the number of patients included in the study is relatively small. Another limitation is that optometric practitioners in the county can refer patients to our hospital or to a number of private ophthalmologists. There were a number of private ophthalmologists in the Malmö area in 2012–2013, but the majority of patients were referred to the hospital. Patients may also move from one clinic to another at any time. This means that we do not know whether a patient who is no longer attending our hospital has been referred to a private clinic and developed glaucoma after their last visit to our department. However, this is regarded as unlikely since we found in a recent study20 that the majority of optometric practitioners based their choice of referral unit primarily on geographical location, and often chose to refer patients living in the same area to the closest clinic. We assume that this was also the case in 2012–2013. Two patients returned to the hospital with new referrals after having been discharged previously, supporting this theory.
The results of our study regarding the high proportion of referred individuals found not to have any eye disease at the hospital are similar to other recent studies.12–14 However, the IOP thresholds suggested for referral in some previous studies were higher than those considered here.13,14 Using the IOP threshold of ≥27 mmHg, suggested by Founti et al,14 would have led to more than one patient in ten with a positive outcome being missed in our study. A possible explanation for this is the high prevalence of PEX in Sweden compared to other countries. In our study, more than one fourth of all patients were diagnosed with PEX. Patients with PEX have an increased risk of developing glaucoma21,22 and IOP-lowering treatment is recommended at IOP ≥25 mmHg.23 We were also able to designate patients without the need for treatment for at least six years as negative outcome, despite them being monitored, which was not the case in previous studies. This means that patients who were designated a negative outcome in our study would have been unnecessarily designated a positive outcome in a study without long-term follow-up, possibly forcing them to decrease their hypothetical thresholds to include these patients.
In the region of Malmö, an elevated IOP of > 21 mmHg was accepted by the hospital as the single reason for a glaucoma referral, but additional assessment could be made prior to referral according to local routines of the different optometric practitioners. The optometrist education in Sweden did not start until 2008. Therefore, it is estimated that not more than just over one hundred optometrists were working in the entire country in the years 2012–2013, and probably very few optometric practices were staffed by optometrists. The number of optometrists has increased since then, and today approximately 15% of opticians have been further educated to become optometrists.
We received 108 referrals regarding elevated IOP in patients without any prior glaucoma diagnosis during the years of 2012–2013. In another study evaluating more recent referrals from optometric practices, also carried out at the Department of Ophthalmology in Malmö,20 a similar number of referrals were seen during a single year in 2019. Hence, the number of referrals to the hospital has almost doubled since 2012–13 most certainly because of a rising and aging population.
Conclusion
In conclusion, the predictive value of glaucoma referrals based solely on elevated IOP from optometric practitioners in Sweden was relatively low, and a high proportion of patients referred were found not to have any eye disease. The predictive value was higher for the referral refinement group. The application of a minimum age of 45 years and an IOP threshold of ≥25 mmHg would have led to more efficient use of resources, without missing any glaucoma cases or individuals in need of IOP-lowering treatment for up to six years after the initial referral. National guidelines for glaucoma referrals, including these requirements, are needed in Sweden to ensure that the limited resources in glaucoma health care are used more wisely.
Abbreviations
IOP, intraocular pressure; POAG, primary open-angle glaucoma; PEXG, exfoliation glaucoma.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Sommer A, Tielsch JM, Katz J, et al. Relationship between intraocular-pressure and primary open angle glaucoma among white and black-Americans - the Baltimore eye survey. Arch Ophthalmol. 1991;109(8):1090–1095. doi:10.1001/archopht.1991.01080080050026
2. Klein BE, Klein R, Sponsel WE, et al. Prevalence of glaucoma. The Beaver Dam eye study. Ophthalmology. 1992;99(10):1499–1504. doi:10.1016/S0161-6420(92)31774-9
3. Dielemans I, Vingerling JR, Wolfs RC, Hofman A, Grobbee DE, de Jong PT. The prevalence of primary open-angle glaucoma in a population-based study in The Netherlands. The Rotterdam study. Ophthalmology. 1994;101(11):1851–1855. doi:10.1016/S0161-6420(94)31090-6
4. Weih LM, Nanjan M, McCarty CA, Taylor HR. Prevalence and predictors of open-angle glaucoma: results from the visual impairment project. Ophthalmology. 2001;108(11):1966–1972. doi:10.1016/S0161-6420(01)00799-0
5. Topouzis F, Coleman AL, Harris A, et al. Factors associated with undiagnosed open-angle glaucoma: the Thessaloniki Eye Study. Am J Ophthalmol. 2008;145(2):327–335. doi:10.1016/j.ajo.2007.09.013
6. Karvonen E, Stoor K, Luodonpaa M, et al. Prevalence of glaucoma in the Northern Finland Birth Cohort Eye Study. Acta Ophthalmol. 2019;97(2):200–207. doi:10.1111/aos.13912
7. McCann P, Hogg R, Wright DM, et al. Glaucoma in the Northern Ireland Cohort for the Longitudinal Study of Ageing (NICOLA): cohort profile, prevalence, awareness and associations. Br J Ophthalmol. 2020;104(11):1492–1499. doi:10.1136/bjophthalmol-2019-315330
8. Forsman E, Kivela T, Vesti E. Lifetime visual disability in open-angle glaucoma and ocular hypertension. J Glaucoma. 2007;16(3):313–319. doi:10.1097/IJG.0b013e318033500f
9. Peters D, Bengtsson B, Heijl A. Factors associated with lifetime risk of open-angle glaucoma blindness. Acta Ophthalmol. 2014;92(5):421–425. doi:10.1111/aos.12203
10. Ernest PJ, Busch MJ, Webers CA, et al. Prevalence of end-of-life visual impairment in patients followed for glaucoma. Acta Ophthalmol. 2013;91(8):738–743. doi:10.1111/j.1755-3768.2012.02555.x
11. Ratnarajan G, Newsom W, French K, et al. The impact of glaucoma referral refinement criteria on referral to, and first-visit discharge rates from, the hospital eye service: the Health Innovation & Education Cluster (HIEC) Glaucoma Pathways project. Ophthalmic Physiol Opt. 2013;33(2):183–189. doi:10.1111/opo.12029
12. Huang J, Yapp M, Hennessy MP, et al. Impact of referral refinement on management of glaucoma suspects in Australia. Clin Exp Optom. 2020;103(5):675–683. doi:10.1111/cxo.13030
13. de Vries MM, Stoutenbeek R, Muskens RP, Jansonius NM. Glaucoma screening during regular optician visits: the feasibility and specificity of screening in real life. Acta Ophthalmol. 2012;90(2):115–121. doi:10.1111/j.1755-3768.2011.02355.x
14. Founti P, Topouzis F, Hollo G, et al. Prospective study of glaucoma referrals across Europe: are we using resources wisely? Br J Ophthalmol. 2018;102(3):329–337. doi:10.1136/bjophthalmol-2017-310249
15. Gunn PJG, Marks JR, Konstantakopoulou E, et al. Clinical effectiveness of the Manchester glaucoma enhanced referral scheme. Br J Ophthalmol. 2019;103(8):1066–1071. doi:10.1136/bjophthalmol-2018-312385
16. Ehlers N, Bramsen T, Sperling S. Applanation tonometry and central corneal thickness. Acta Ophthalmol. 1975;53(1):34–43. doi:10.1111/j.1755-3768.1975.tb01135.x
17. Tham YC, Li X, Wong TY, Quigley HA, Aung T, Cheng CY. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology. 2014.
18. Prum BE
19. Keenan J, Shahid H, Bourne RR, White AJ, Martin KR. Cambridge community optometry glaucoma scheme. Clin Exp Ophthalmol. 2015;43(3):221–227. doi:10.1111/ceo.12398
20. Landgren K, Peters D. A prospective study on effectiveness of elevated intraocular pressure as a criterion for glaucoma referrals by optometric practitioners in Sweden. Acta Ophthalmol. 2021. doi:10.1111/aos.14764
21. Grødum K, Heijl A, Bengtsson B. Risk of glaucoma in ocular hypertension with and without pseudoexfoliation. Ophthalmology. 2005;112(3):386–390. doi:10.1016/j.ophtha.2004.09.024
22. Ekström C. Risk factors for incident open-angle glaucoma: a population-based 20-year follow-up study. Acta Ophthalmol. 2012;90(4):316–321. doi:10.1111/j.1755-3768.2010.01943.x
23. Heijl A, Alm A, Bengtsson B, et al.The glaucoma guidelines of the Swedish Ophthalmological Society. Acta Ophthalmol Suppl (Oxf). 2012;(251):1–40. doi:10.1111/j.1755-3768.2012.02415.x
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.