Back to Journals » Clinical Pharmacology: Advances and Applications » Volume 16
Effectiveness of Drug Repurposing and Natural Products Against SARS-CoV-2: A Comprehensive Review
Authors Velásquez PA, Hernandez JC , Galeano E, Hincapié-García J, Rugeles MT, Zapata-Builes W
Received 26 August 2023
Accepted for publication 14 November 2023
Published 4 January 2024 Volume 2024:16 Pages 1—25
DOI https://doi.org/10.2147/CPAA.S429064
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Arthur E. Frankel
Paula Andrea Velásquez,1,2 Juan C Hernandez,1,2 Elkin Galeano,3 Jaime Hincapié-García,4 María Teresa Rugeles,2 Wildeman Zapata-Builes1,2
1Grupo Infettare, Facultad de Medicina, Universidad Cooperativa de Colombia, Medellín, Colombia; 2Grupo Inmunovirología, Facultad de Medicina, Universidad de Antioquia UdeA, Medellín, Colombia; 3Grupo Productos Naturales Marinos, Departamento de Farmacia, Facultad de Ciencias Farmacéuticas y Alimentarias, Universidad de Antioquia UdeA, Medellín, Colombia; 4Grupo de investigación, Promoción y prevención farmacéutica, Facultad de Ciencias Farmacéuticas y Alimentarias, Universidad de Antioquia UdeA, Medellín, Colombia
Correspondence: Wildeman Zapata-Builes, Grupo Infettare, Facultad de Medicina Universidad Cooperativa de Colombia, Calle 50 # 40-74, Medellín, Colombia, Tel +57-4 4446065, Ext 4252, Email [email protected]
Abstract: The Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) is a betacoronavirus responsible for the COVID-19 pandemic, causing respiratory disorders, and even death in some individuals, if not appropriately treated in time. To face the pandemic, preventive measures have been taken against contagions and the application of vaccines to prevent severe disease and death cases. For the COVID-19 treatment, antiviral, antiparasitic, anticoagulant and other drugs have been reused due to limited specific medicaments for the disease. Drug repurposing is an emerging strategy with therapies that have already tested safe in humans. One promising alternative for systematic experimental screening of a vast pool of compounds is computational drug repurposing (in silico assay). Using these tools, new uses for approved drugs such as chloroquine, hydroxychloroquine, ivermectin, zidovudine, ribavirin, lamivudine, remdesivir, lopinavir and tenofovir/emtricitabine have been conducted, showing effectiveness in vitro and in silico against SARS-CoV-2 and some of these, also in clinical trials. Additionally, therapeutic options have been sought in natural products (terpenoids, alkaloids, saponins and phenolics) with promising in vitro and in silico results for use in COVID-19 disease. Among these, the most studied are resveratrol, quercetin, hesperidin, curcumin, myricetin and betulinic acid, which were proposed as SARS-CoV-2 inhibitors. Among the drugs reused to control the SARS-CoV2, better results have been observed for remdesivir in hospitalized patients and outpatients. Regarding natural products, resveratrol, curcumin, and quercetin have demonstrated in vitro antiviral activity against SARS-CoV-2 and in vivo, a nebulized formulation has demonstrated to alleviate the respiratory symptoms of COVID-19. This review shows the evidence of drug repurposing efficacy and the potential use of natural products as a treatment for COVID-19. For this, a search was carried out in PubMed, SciELO and ScienceDirect databases for articles about drugs approved or under study and natural compounds recognized for their antiviral activity against SARS-CoV-2.
Keywords: COVID-19, pandemic, coronavirus, medicinal plants
Introduction
The Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) is a betacoronavirus containing positive-sense, single-stranded RNA, which shares genetic similarities with the severe acute respiratory syndrome coronavirus (SARS-CoV) and the Middle East respiratory syndrome coronavirus (MERS-CoV). SARS-CoV-2 is a new strain responsible for the global Coronavirus disease 2019 (COVID-19) pandemic that first emerged in China (Wuhan) in 2019, causing severe respiratory disorders and even death if not treated in time and adequately.1
COVID-19 is a systemic disorder that includes fever, dry cough, fatigue, headache, sore throat, rhinorrhea, dyspnea and anosmia that become significant symptoms for clinical diagnosis. Immunological characteristics include lymphopenia, infiltration of immune cells such as T cells, monocytes, macrophages, NK cells and dendritic cells into the lungs, along with a cytokine storm, leading to acute respiratory distress syndrome (ARDS), and eventually to death.2 Figure 1 summarizes the pathophysiology and multisystem manifestations of COVID-19.3–6
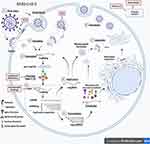
During SARS-CoV-2 entry into the host cell (cells from the lungs, heart, kidneys, nasal and oral mucosa, stomach, small intestine, and colon), the spike glycoprotein (S protein) binds to angiotensin-converting enzyme 2 (ACE2) its cellular receptor. Then, the S protein is cleaved by the cellular transmembrane serine protease (TMPRSS2), allowing the fusion of the viral envelope and the host cell membrane.
In cells expressing low TMPRSS2 or not producing this enzyme, the viral particles can enter by endocytosis. Inside the endosomes, the cathepsins cut the protein S to allow the fusion of the viral envelope and the endosome membrane.7 After this, the viral RNA is released and translated in the cytoplasm, generating polyproteins that are cleaved into individual non-structural proteins (nsps) by viral proteases, the papain-like protease (PL pro) and the chymotrypsin-like protease (3CL pro) better known as major protease (Mpro). The generated viral proteins form the replication-transcription complex (RTC), which includes RNA-dependent RNA polymerase (RdRp).8 Subsequently, the structural proteins are synthesized; these move to the membranes of the endoplasmic reticulum (ER) and to the intermediate compartment between the Golgi and the ER (ERGIC), where they interact with the viral RNA to assemble new viral particles, then they are released by exocytosis (vesicles).9,10
COVID-19 has left more than 6.6 million deaths and approximately 646 million infected worldwide.11 To face the pandemic, preventive measures have been taken against transmission, such as social distancing, hand washing, use of masks, social isolation and the most important strategy, vaccine development,12 to prevent severe disease and death cases. For the COVID-19 treatment, antiviral, antiparasitic, anticoagulant13,14 and other drugs have been reused due to the absence of specific medicaments for this disease; however, two new drugs (baricitinib and sotrovimab) were recently approved to treat symptoms and fight infection, but they are not available for the general population yet. The World Health Organization (WHO) recommends the use of baricitinib in severe cases by increasing the probability of surviving the complications and reducing the need for mechanical ventilation. In contrast, sotrovimab is recommended in moderate cases to prevent it from worsening.15
New drug development is a long process; for this, drug repurposing (use of drugs that have shown to be safe in humans for the treatment of other diseases) emerged as a strategy to treat COVID-19 during the pandemic. However, a systematic experimental screening of a vast pool of drugs for repurposing is impossible because this process becomes very long and expensive.16 To get over these restrictions, one favorable alternative is computational drug repurposing or in silico assays, which use algorithms and database information to designate the best drugs for repositioning.
An enormous advantage of in silico approaches is the large number of molecules that can be evaluated quickly and the possibility of identifying other benefits of the drugs developed for different diseases. Meanwhile, experimental drug repurposing is usually carried out in closely related diseases.17
In the search for an effective treatment, clinical trials for new uses of approved drugs such as chloroquine, hydroxychloroquine, ivermectin, zidovudine, ribavirin, lamivudine, remdesivir, lopinavir/ritonavir and tenofovir/emtricitabine18,19 have been conducted, which were used based on their effectiveness in other viral pathologies such as SARS-CoV, Middle East Respiratory Syndrome (MERS), ARDS, and evidence of in vitro and in silico activity17 against SARS-CoV-2.20 The most recently approved second-use drugs by WHO, baricitinib and sotrovimab, have been previously used in other pathologies. Baricitinib is a Janus kinase inhibitor drug; it works mainly to reduce inflammation and is used to treat rheumatoid arthritis. Sotrovimab is a monoclonal antibody usually administered to transplant recipients, cancer patients and other high-risk groups.21,22
Additionally, new therapeutic options have been sought in natural products (compounds or substances produced by living organisms that humans have not modified)23 that, historically, have been very important for discovery of new treatments for cancer and infectious diseases.24 To treat SARS-CoV-2 infection, it has been found that essential oils25 and plant extracts26–29 have promising results in vitro and in silico trials.
Many natural products that have shown antiviral activity belong to the families of terpenoids, alkaloids, saponins and phenolics (including flavonoids and phenolic acids).8 For instance, hispidulin, quercetin, rutin, saikosaponin D, glycyrrhizin and hesperidin showed in vitro and in vivo antiviral activities against different respiratory viruses, including SARS-CoV-2.30 Terpenoids such as ursolic acid, oleanolic acid and carvacrol are inhibitors of the 3CLpro protease of SARS-CoV-2 using in silico assays and molecular modeling.31,32 Also, traditional medicine can be considered as one of the treatments. For example, in vitro investigations with Lianhuaqingwen (LH), a traditional Chinese medicine, showed a significant reduction in SARS-CoV-2 replication in Vero E6 cells and decreased expression of the pro-inflammatory cytokines.33 Without effective treatments for SARS-CoV-2 infections, natural products might be potential alternative therapies.
This review shows the evidence of drug repurposing efficacy and the potential use of natural products as a treatment for COVID-19. For this, a search was carried out in PubMed, SciELO and ScienceDirect databases for recent review and scientific articles published from 2020 of some drugs under study and natural compounds recognized for their antiviral activity that could be effective in treating SARS-CoV-2 infection. Some search terms were the following: “Antivirals AND SARS-CoV-2”, “Drug repurposing AND SARS-CoV-2”, “Bioactive compounds AND COVID-19” or “Natural products AND SARS-CoV-2”.
Drug Repurposing Strategies for COVID-19 Treatment
Since the beginning of the application of vaccines to prevent COVID-19, the number of infections and deaths due to the disease has gradually decreased; however, many variants have been reported and approved anti-coronavirus drugs are limited, which has become a global challenge. Therefore, searching for new effective therapeutic agents to treat and manage COVID-19 is urgent. Drug repurposing is an effective strategy to identify new drugs with few clinical trials.
The conventional process to discover new drugs is expensive, long (10–15 years), risky and has a low success rate. Drug repurposing or drug repositioning is an effective way to overcome these difficulties and explore new uses of drugs approved or under investigation for their application in new medical prescriptions. Drug repurposing requires shorter investigations; therefore, the development process is less expensive, besides these second-use medicines present a lower risk of binding to different therapeutic targets or going through other pathways.16,34
For these reasons, drug repurposing is a quick alternative to find a treatment for the COVID-19 pandemic. Some drugs act on the RNA genome as nucleotide analogs, such as remdesivir, favipiravir, ribavirin and tenofovir, which are joined with the replicating genome of the virus, stopping the RdRp activity. The protease inhibitors block the proteolytic processing of polypeptides into non-structural proteins. Examples are saquinavir, indinavir, ritonavir and lopinavir.35 Entry blockers block the proteins that participate in the entry of the viral particle into the cell, such as chloroquine, hydroxychloroquine and arbidol, and other drugs have immunomodulatory properties, for which they were proposed as potential agents for the COVID-19 treatment, among these are nitazoxanide, ivermectin, statins, vitamin D and anticoagulants.36
Antiparasitic
Chloroquine (CQ) was synthesized in 1934, and it is an aminoquinoline, a weak base that increases the pH of acid vesicles. It is concentrated in organelles with a low pH, such as endosomes, lysosomes, and Golgi vesicles. CQ interferes with sialic acid synthesis, which participates in the interaction between the virus and the cell, avoiding the recognition and attachment of the virus to the cell. Furthermore, after endocytosis of the viral particle, CQ prevents endosome maturation and, thus, the release of the viral genome into the cytoplasm, preventing viral replication.37
The CQ can affect viral infections in different ways, but its antiviral activity depends mainly on the use of endosomes for viral entry; it has been demonstrated to have antiviral activity against SARS-CoV-2 in primate cells. This drug has been effective and commonly used in treating malaria, amoebiasis, human immunodeficiency virus (HIV) infection and autoimmune diseases with few and mild side effects.38
Hydroxychloroquine (HCQ) is a CQ derivative (Figure 2) and an antiprotozoal drug that penetrates cell membranes, accumulating in the acidic lysosomes and increasing the pH, inhibiting lysosome functions. According to a study by Alsuwaidan et al, this prevents coronavirus entry into target cells and reduces body temperature.39
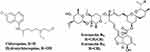 |
Figure 2 Molecular structure of chloroquine, hydroxychloroquine and ivermectin. |
The HCQ is a drug with potential antiviral activity; however, clinical trials done during the pandemic have suggested no benefit for patients with COVID-19, including those treated in an outpatient setting.40,41 In addition, patients treated with HCQ for a non-COVID-19 indication were not associated with preventing infection with SARS-CoV-2.42 Moreover, in another study, it was found the use of HCQ was not associated with decreased COVID-19 mortality among people who received this drug for treatment of rheumatoid diseases prior to experiencing COVID-19.43 Additionally, combined with azithromycin, it was not associated with decreased mortality in patients hospitalized for mild and moderate COVID-19;44,45 furthermore, it did not change the number of days of hospitalization to discharge or death.46
In contrast, observational studies have suggested a clinical benefit in patients treated with azithromycin combined with HCQ or ivermectin in viral shedding, clinical duration, hospitalization, mechanical ventilation, death and post-COVID symptoms; however, these studies are subject to bias and confounding factors.47 Regarding the safety of HCQ, cardiomyopathy causing heart failure has been reported, with fatal cases in long-term therapy with high doses; monitoring of patients and stopping treatment in case of symptoms is recommended. The QT interval prolongation has also been reported; thus, constant monitoring is suggested, especially in patients with risk factors such as a history of prolonged QT interval, ventricular arrhythmias, bradycardia, and other cardiac abnormalities.48,49
CQ and HCQ inhibit the replication of coronaviruses such as SARS-CoV and SARS-CoV-2 in vitro;50–53 however, the clinical study results have not been consistent with the in vitro findings, which suggests that the antiviral mechanisms of CQ and HCQ are not fully understood. Tichauer et al demonstrated in a cell-free in vitro model that CHQ inhibits the function of the ACE2 enzyme in a dose-dependent manner. Still, the interaction between ACE2 and the S protein of SARS-CoV-2 generates structural changes that disrupt the binding of HCQ to ACE2. These findings suggest that there may be variations in the human body that affect the effectiveness of HCQ in clinical trials.37
The Food and Drug Administration (FDA) authorized the emergency use of HCQ for adults hospitalized with COVID-19 on March 28, 2020; however, this permit was revoked on June 15, 2020.54 The results of a clinical study on hospitalized patients with COVID-19 showed that HCQ is not effective in treating the disease, did not reduce death rates or shorten recovery time for patients. These findings are consistent with the results of another study showing that the suggested dose of HCQ is unlikely to inhibit or eliminate SARS-CoV-2.55
Ivermectin (IVM) is a semisynthetic and anthelmintic agent (Figure 2), which attracted the interest of the world scientific community due to some in vitro studies and animal models in which antiviral activity against SARS-CoV-2 was evidenced,56 increasing the demand by the general population. This drug binds to chloride channels in nerve and muscle cells of invertebrate animals and opens these channels, increasing the flow of chloride ions and hyper-polarizing the cell membranes, causing paralysis and death of the invertebrate. It also acts on different intracellular proteins to reduce the replication of some viruses.57 The IVM is a specific inhibitor of nuclear importation, blocking replication of HIV-1 and dengue viruses;58 it also blocks the attachment of the SARS-CoV-2 spike protein to the ACE2 molecule (binding energy −18 kcal/mol) in the in silico assays.59 However, the clinical trials and medical observations report conflicting results on the effectiveness of ivermectin to treat SARS-CoV-2 infection.
A clinical study carried out in adult patients with mild COVID-19 showed that IVM is effective for treating this disease; viral shedding was earlier in the group treated with IVM than in the control group.60 Another study administered IVM with doxycycline to patients with mild-to-moderate disease, and they recovered sooner and fewer of these patients progressed to severe COVID-19.61
Okumuş et al evidenced that IVM can increase clinical recovery, improve laboratory results and reduce mortality in patients with severe symptoms of COVID-19;62,63 also, it was reported a reduction in viral shedding, disease duration, respiratory complications, length of hospitalization or mechanical ventilation, number of deaths and post-COVID manifestations.47
Regarding the dose, an investigation recommended the use of low doses (0.05 and 0.1 mg/kg) of inhaled IVM as a possible treatment in COVID-19 cases because of its safety.64 Still, Krolewiecki et al identified a significant reduction in the number of SARS-CoV-2 viral particles in respiratory secretions of patients with COVID-19 treated with high dose IVM, compared to untreated controls. In addition, no toxicity related to the high doses of ivermectin (0.6 mg/kg/day for 5 days) was observed.65
In contrast, some studies have shown adverse effects or non-clinical improvement in patients treated with IVM. López-Medina et al showed that treatment for five days with IVM in adults with mild COVID-19, compared to the control group, did not significantly improve the symptom resolution time; therefore, the results do not support the treatment of mild COVID-19 with IVM.66 A recent study on 3515 patients found no significant effects of IVM use, and the treatment with IVM does not reduce the incidence of hospitalizations or the length of stay in the ICU of patients diagnosed with COVID-19.67
The IVM is generally safe when prescribed but can generate toxicity in overdose cases or by inappropriate use. The American College of Medical Toxicology collects reports of unfavorable reactions to COVID-19 treatments, and they have received, since August 2021, many reports of patients becoming ill due to the use of IVM to prevent SARS-CoV-2 infection or treat disease; some of these reports included severe toxicity.68 This trend is similar to national data collected by Poison Control Centers and the US Centers for Disease Control and Prevention (CDC), which reported an increase in IVM poisoning cases.69 Clinical manifestations differ in severe cases and may include gastrointestinal symptoms like nausea, vomiting, abdominal pain and diarrhea, furthermore, headache, dizziness, fatigue, visual changes, fast heart rate, low blood pressure, and skin rashes.68,70
Additionally, one of the most important studies supporting IVM as COVID-19 treatment was withdrawn over ethical concerns after independent researchers found discrepancies in the data. The study about the efficacy and safety of IVM in the treatment of COVID-19, managed by Dr Ahmed Elgazzar from Benha University in Egypt, was published on the Research Square website in November and affirmed that patients with COVID-19 treated with IVM early reported substantial recovery, improvement and a reduction of 90% in mortality rate; however, the utility of IVM to treat SARS-CoV-2 infection is questioned after significant irregularities were identified on the paper, leading to the retraction.71,72
Due to these latest and important findings, the FDA has not authorized the use of IVM to prevent or treat COVID-19 in humans; it is approved only for the treatment of some infections caused by intestinal and scalp parasites, but animal IVM products are very different (often highly concentrated) from those approved for humans and taking large doses of this drug is dangerous; an overdose can cause even death.73
Table 1 summarizes the possible therapeutic targets and mechanism of action or antiviral activity of the previous antiparasitics.
 |
Table 1 Drugs: Potential Therapeutic Targets and Their Antiviral Effects |
Antivirals
Among the drugs with antiviral activity used to control the SARS-CoV2 pandemic are zidovudine, lamivudine, abacavir, efavirenz, lopinavir, remdesivir and tenofovir administered with emtricitabine, below are some of the most relevant findings.
Zidovudine (AZT) is a synthetic analog of the nucleoside thymidine (Figure 3) and inhibits reverse transcriptase after being incorporated into newly synthesized viral DNA strand in place of thymidine, acting as a viral DNA chain terminator.90 AZT has been used as a reverse transcriptase (RT) inhibitor of HIV, and it has also been demonstrated its capability to incorporate and terminate nucleotide extension of SARS‐CoV RdRp, which is nearly identical to the SARS‐CoV‐2 RdRp (amino acid similarity of 98%), suggesting that it might also inhibit the SARS-CoV-2 polymerase.91
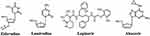 |
Figure 3 Molecular structure of zidovudine, lamivudine, lopinavir and Abacavir. |
In silico trials of screening, molecular modeling and docking of ligands against N protein of SARS-CoV-2 have shown that AZT has a more robust and stable interaction with this protein structure (docking scores of −9.75 and binding free energies of −59.43) than Valganciclovir and Ribavirin, others FDA-approved drug.78
Therefore, AZT can be further evaluated in clinical trials as a potential treatment for COVID-19.
Like AZT, lamivudine is a synthetic nucleoside analog (Figure 3) used to treat HIV infection; it is integrated into the viral DNA chain by the reverse transcriptase of the virus, causing premature termination of viral chain synthesis.80
In vitro, lamivudine exhibited antiviral activity against the D614G strain of SARS-CoV-2 at 100 µM (inhibition percentage of 66.7%) and yielded favorable binding energies with SARS-CoV-2 RdRp (−4.9 Kcal/mol) and 3CLpro (−5.8 Kcal/mol) using bioinformatics methods.92
Other in silico studies showed the ability of lamivudine to bind to SARS-CoV-2 RdRp catalytic site (interacts with serine 682, threonine 687, asparagine 760 and 761) where are incorporated the corresponding natural nucleotide substrates with comparable inhibition constant (Ki) value and similar interactions than remdesivir, tenofovir and emtricitabine.79,93 In addition, lamivudine and lopinavir (Figure 3) bind with high affinity and lower free energy to Mpro of the SARS-CoV-2, which would lead to inhibition of replication and maturation in host cells.82
In a retrospective study, patients with Hepatitis B virus (HBV) infection taking lamivudine treatment showed a lower risk of SARS-CoV-2 infection. Approximately 2% of patients treated with lamivudine were diagnosed with COVID-19 compared to 48.4% of patients not treated with this antiviral. These results suggested that individuals with HBV treated with lamivudine are less susceptible to SARS-CoV-2 infection, probably due to structural similarities of some proteins in both viruses.94 Therefore, this drug is an excellent candidate for effective therapy against COVID-19.
Another synthetic nucleoside analog and an antiviral agent is abacavir (ABC) (Figure 3); its active metabolite, carbovir triphosphate, inhibits the RT activity of HIV-1 by competing with dGTP, the natural substrate, and stopping DNA chain elongation.83 The results presented by Chien et al provided a molecular basis that demonstrated this viral polymerase inhibitor is incorporated by the SARS-CoV-2 polymerase; therefore, it is considered a potential candidate for clinical trials to evaluate its effectiveness as a prophylaxis and treatment of COVID-19.84,95 Tomic et al, using the VINI in silico model for virtual drug screening, demonstrated that the combination of cobicistat-ABC-rilpivirine had the highest predicted efficacy in inhibiting SARS-CoV-2 spike glycoprotein,96 and Del Amo et al reported the incidence of COVID-19 and the severity of this disease in patients with HIV treated with antiretrovirals. They found that patients treated with ABC/lamivudine had a 32% lower risk of being diagnosed with COVID-19 than those who received tenofovir alafenamide/emtricitabine.97 Therefore, more clinical trials on COVID-19 patients with moderately severe and severe conditions are warranted.
Lopinavir is a peptidomimetic (Figure 4) that inhibits the HIV protease, preventing the cleavage of the Gag-Pol polyprotein, thus reducing the number of mature and infectious viral particles.81 It is generally administered with ritonavir (Figure 4), which increases plasma levels of lopinavir and inhibits its metabolism. Lopinavir/ritonavir have been included in drug protocols to prevent and treat COVID-19.98 WHO selected these drugs for evaluation in clinical trials, alone or combined with Ribavirin and interferon beta. It was considered an appropriate second option due to the clinical experience in the context of MERS and the preclinical data that show some clinical benefit, for which they recommend investigating it, especially in severe cases.99
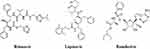 |
Figure 4 Molecular structure of ritonavir, lopinavir and remdesivir. |
Fan-Ngai et al reported that in a Phase 2 trial in adults with COVID-19 early treatment with triple antiviral therapy (lopinavir, ritonavir and ribavirin every 12 h and three doses of interferon beta-1b) was safe and more effective than lopinavir and ritonavir in reducing symptoms, reducing viral-shedding time and hospital stay in patients with mild-to-moderate disease.100 However, some investigations with lopinavir-ritonavir in combination with other drugs have not shown clinical effects. In a randomized controlled trial conducted in hospitalized adults with severe COVID-19, no benefit was observed with lopinavir combined with ritonavir treatment compared to control interventions (arbidol, navaferon or lopinavir-ritonavir + novaferon). In addition, it did not significantly accelerate the clinical recovery of the patient; it did not reduce mortality, or decrease viral RNA detection in throat swabs and did not shorten the duration of SARS CoV-2 shedding.101,102
Another study conducted by Ader et al showed that patients with COVID-19 treated with lopinavir/ritonavir, lopinavir/ritonavir plus IFN-β-1a and hydroxychloroquine had no clinical improvement after 15 and 29 days of clinical follow-up, no decrease in viral shedding time was observed and there were significantly more adverse effects in the group treated only with lopinavir and ritonavir.103 Likewise, other research found that critically ill patients with COVID-19, treated with lopinavir-ritonavir and hydroxychloroquine or combination therapy, presented worse outcomes compared to patients who did not receive antiviral therapy.104 Other studies simply do not recommend the use of these drugs as treatment for hospitalized patients with COVID-19.103,105
Most research does not support using lopinavir-ritonavir for the treatment of COVID-19. Therefore, several clinical studies may be done to evaluate the effectiveness of lopinavir-ritonavir in combination with ribavirin or other drugs.
Remdesivir is a monophosphoramidate prodrug (Figure 4) and an adenosine analog developed initially to treat Ebola virus infections. In several countries, it was the first antiviral approved or authorized for emergency use to treat COVID-19.106
Remdesivir is a delayed translocation inhibitor of the SARS-CoV-2 RdRp; it blocks the translocation after the incorporation of the fourth remdesivir monophosphate (RMP), which leads to a stop in the RNA synthesis process due to a steric clash between the 1′-cyano group of RMP and viral polymerase.85
Beigel et al conducted a double-blind, randomized, placebo-controlled trial of intravenous remdesivir in adults hospitalized with moderate-to-severe COVID-19. They found that remdesivir was better than placebo in reducing recovery time and improving clinical outcomes.107 Furthermore, Olender et al reported more significant recovery in patients with severe COVID-19 and 62% fewer odds of death with remdesivir than standard treatment.108
A meta-analysis of 10 clinical trials found that remdesivir treatment significantly improves patient recovery and lowers the need for mechanical ventilation.109
In a recent study published in 2022, with no hospitalized patients at high risk for COVID-19 progression, the treatment with remdesivir for 3 days was safe and reduced hospitalization or mortality rates by 87% compared to placebo.110
In contrast to previous findings, in a mortality trial recommended by WHO (December 2020), four repurposed antiviral drugs (remdesivir, hydroxychloroquine, lopinavir and interferon beta-1a) were evaluated in patients hospitalized with COVID-19. In this study participated, 405 hospitals in 30 countries and 11,330 randomly selected adults; 2750 received remdesivir, 954 hydroxychloroquine, 1411 lopinavir (without interferon), 2063 interferon (including 651 to interferon plus lopinavir), and 4088 no received trial drug. It was concluded that these drugs did not reduce the mortality rates, time to start ventilation, or duration of hospital stay in patients with COVID-19.20
However, to date, the use of remdesivir is approved by the FDA to treat adults 18 and over and children 28 days of age or older with COVID-19.
Tenofovir and emtricitabine are other nucleoside analogs and viral reverse transcriptase inhibitors (Figure 5). This combination significantly reduced SARS-CoV-2 viral titers in non-hospitalized patients with COVID-19. These findings support the need of larger trials of therapy with tenofovir for the prevention and treatment of COVID-19.111
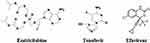 |
Figure 5 Molecular structure of emtricitabine, tenofovir and efavirenz. |
Molecular docking assays showed that tenofovir can tightly bind the RdRp of SARS-CoV-2 (−6.9 Kcal/mol)86 and a study in ferrets found that the group treated with tenofovir and emtricitabine decreased viral titers in nasal washes after 8 days of infection compared to the control group treated with PBS;112 therefore, this combination of drugs could be considered an important alternative for clinical trials.
Efavirenz is a non-competitive inhibitor of the HIV-1 TR (Figure 5) and a candidate for COVID-19 treatment, based on the study using the MT-DTIa drug–target interaction model which identifies commercially available drugs that could act on the SARS-CoV-2 proteins. The results showed that efavirenz has inhibitory potency with a dissociation constant (Kd) of 199.17 nM against the SARS-CoV-2 protease; its activity is better than ritonavir that binds with inhibitory potency with Kd 204,05 nM.87
Although efavirenz is a potent drug available to treat SARS-CoV-2 infection, this drug may cause severe toxicity;113 therefore, limiting its use or searching for treatment alternatives is advisable.
Table 1 summarizes the possible therapeutic targets and mechanisms of action of the previous antivirals.
Other Drugs
People with COVID-19, primarily those with severe disease, may have a series of coagulation disorders consistent with hypercoagulability (caused by virus-induced endothelial dysfunction), elevation of cytokine levels and activation of complement proteins.114 Therefore, these patients have been treated with anticoagulants in many cases. Observational studies evidenced the benefits of anticoagulation with a reduction in mortality, mainly in patients who require mechanical ventilation.115 Likewise, a randomized trial found that anticoagulation with prophylactic doses is equally effective than higher doses in reducing the risk of venous thromboembolism (VTE), even in patients at the intensive care unit (ICU), with trends towards lower rates of bleeding;116 these findings are confirmed by more recent research.117 Another study carried out by Jonmarker et al showed that among critically ill COVID‑19 patients with respiratory failure, prophylactic use of high-dose anticoagulants was associated with a lower incidence of thromboembolic events and lower risk of death compared with lower doses.118
In a recently updated clinical guidance (2022) for anticoagulant therapies in patients with COVID-19, anticoagulant prophylaxis was not recommended in non-hospitalized adult patients with mild symptoms and no other indications for this use.
However, giving at least one prophylactic dose of anticoagulant to all hospitalized patients with COVID-19 is recommended. For critically ill adult patients, prophylaxis with standard doses of anticoagulants is recommended (not intermediate or therapeutic doses). For non-critical ill patients but who are at risk of progressing to severe disease or at risk of developing thromboembolism and without a high risk of bleeding, prophylaxis with therapeutic doses of anticoagulant is recommended.119
In contrast, other studies have found that intermediate and therapeutic doses have a higher risk of bleeding and do not reduce the probability of suffering VTE events or mortality of critically ill patients with COVID-19.117,120 Other clinical investigations showed a high incidence of thrombotic complications in patients with COVID-19 even those with thromboprophylaxis with standard anticoagulants.121,122
The REMAP-CAP investigators showed that in critically ill patients with COVID-19, initial treatment with therapeutic doses of the anticoagulant heparin does not increase the probability of survival after hospital discharge, and it does not significantly decrease the time with respiratory or cardiovascular support compared with routinely administered prophylactic anticoagulants.123
Despite the risk of anticoagulation therapy in some COVID-19 patients, the Anticoagulation Forum (the leading North American organization of anticoagulation providers) recommends the use of anticoagulants in critically or non-critically ill patients at high risk of progressing to severe disease.
Atorvastatin (ATV), a hypolipidemic drug (Figure 6), showed antiviral activity in vitro against the SARS-CoV-2 D614G strain and the emergent delta and mu variants in the Vero E6 cell line. By bioinformatics methods, it was observed that ATV binds to the RdRp and Mpro with binding energies of −6.7 kcal/mol and −7.5 kcal/mol, respectively.50
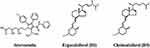 |
Figure 6 Molecular structure of atorvastatin, ergocalciferol (D2) and cholecalciferol (D3). |
The pharmacological use of statins, such as atorvastatin, seems to prevent death in patients infected with SARS-CoV-2 due to its effects on inflammation and oxidative stress, which positively impact cardiovascular disease. Statins modulate the immune response, like immune cell adhesion and migration, antigen presentation, and cytokine production. Moreover, it reduces reactive oxygen species, increases antioxidants, and restores the function and integrity of the endothelium.88
In a study in which the effect of atorvastatin on the replication of SARS-CoV-2 in vitro and in silico was evaluated, it was found through bioinformatics methods that atorvastatin can bind to viral proteins, and its antiviral activity affects the replicative cycle at phases posterior to viral entry.124
A retrospective cohort study in adults with laboratory-confirmed COVID-19 reported lower mortality associated with atorvastatin in patients admitted to the ICU.125 An observational study supports these findings; this study found that statin treatment reduces mortality rates, decreases the need for ICU care, and decreases the need for mechanical ventilation in hospitalized ICU patients.126
Another observational study reported that statin treatment was associated with a decrease in mortality, decreased need for ICU care and a lower need for mechanical ventilation in ICU hospitalized COVID-19 patients,127 but controlled trials are needed considering the observational nature of these studies to confirm this benefit.
In contrast, the most recent observational and retrospective studies published with this drug in more than 1000 patients hospitalized with COVID-19 have shown that previous treatment with statins does not decrease the likelihood of receiving mechanical ventilation and ICU care but reduce the mortality rates of these patients.128,129
Additionally, some studies in patients with COVID-19 treated with statins showed similar mortality to those without statins, but this finding was influenced by a higher prevalence of patients with risk factors for severe COVID-19 presentation.130 These results were confirmed in an observational retrospective study, where statins did not reduce mortality in patients with COVID-19; it was also necessary to closely monitor for adverse effects during hospital admission because patients developed liver cytolysis, rhabdomyolysis and thrombotic and hemorrhagic events more frequently.124 These data do not support using statins as a therapy for hospitalized patients with COVID-19. However, for many researchers, the clinical experience of atorvastatin, its affordable price, availability, safety and known tolerability could be useful in the treatment of COVID-19 and reduce morbidity and mortality of this disease, but randomized controlled trials are needed for can to include atorvastatin as part of treatment, for COVID-19.131
Another drug with positive effects on the immune system is vitamin D (VitD) or ergocalciferol (Figure 6); this promotes intestinal absorption of calcium and phosphate and increases their reabsorption by renal tubules, thus increasing the serum levels to allow bone mineralization.132,133 Besides, adequate levels of VitD in blood also play an effective role in the function of the immune system, which can help to achieve a satisfactory cellular response and to protect against the severity of infections.89,134 Nimavat et al found an association between a low level of VitD and COVID-19 severity.135,136
In geriatric patients hospitalized for COVID-19 and supplemented with vitamin D for 3 months, better survival of these was observed; this result is consistent with observational data collected during the pandemic, where patients with COVID-19 and vitamin D deficiency were found to be more likely to experience severe COVID-19.137 Likewise, lower serum VitD levels also appear to be associated with hospital stay.138,139 On the contrary, higher levels are associated with a lower risk of ICU admission due to COVID-19;140 or administration of high-dose calcifediol, a metabolite of VitD, significantly reduces the need for ICU admission of patients with severe COVID-19.141
These benefits have not been shown in other investigations where VitD supplementation (oral dose) did not reduce mortality, ICU admission rates, or the need for ventilation.142 In addition, the administration of high doses of vitamin D3 in critically ill patients with COVID-19 and deficiency of this vitamin during ICU care did not reduce the need for intubation, length of hospital stay, and hospital mortality.143
In conclusion, calcifediol seems to reduce disease severity, but clinical trials with more significant numbers of patients and appropriately matched groups must show a definitive response.144
Table 1 summarizes the main antiviral mechanisms of previous medications.
Natural Compounds with Potential Use in the COVID-19 Treatment
Currently, few drugs are available to treat COVID-19; therefore, searching for new effective and safe antivirals against this infection is urgent. In this search, molecules with biological activity extracted from plants are being considered to treat coronavirus infection.145 The therapies with herbal medicines or their extracts (secondary metabolites or phytochemicals) are frequently used to treat respiratory diseases and have been very effective in treating flu symptoms;146 therefore, among the treatments proposed for COVID-19, compounds or molecules extracted from plants are promising source for developing drugs.
The primary classification system for these phytochemicals includes four major groups: alkaloids, phenols (including flavonoids and phenolic acids), terpenoids and saponins, families that, due to their antiviral properties, can be used in combination with antiviral drugs to treat infections caused by viruses.30
Alkaloids are low molecular weight nitrogenous compounds mainly produced by plants and animals; they have several biological effects and reported antimicrobial activity; the function is primarily defense against insect and herbivorous animals.147
Uncaria tomentosa, also known as Cat’s claw, produces more than 50 phytochemicals (compounds with biological activity) and is widely used as a treatment by the anti-inflammatory and immunoregulatory properties. Oxindole and its alkaloid derivatives are characteristic of this species, and several therapeutic effects have been discovered. Interestingly, Yepes-Perez et al found that the extract of U. tomentosa inhibited 92.7% of SARS-CoV-2 and decreased 98.6% the cytopathic effect caused by the virus on Vero E6 cells at 25 μg/mL with EC50 calculated by plaque reduction assay of 6.6 μg/mL.53
Another alkaloid, Isogranulatimide, a marine pyrrolocarbazole (Figure 7), is cell-permeable and was extracted from the marine sponge Didemnum conchyliatum, as a cell cycle inhibitor. It was identified by Humayun et al through virtual drug screening with a −6.9 kcal/mol docking score as a candidate to develop novel drugs against the SARS-CoV-2 due to its binding to neuropilin-1, a transmembrane glycoprotein that acts as a co-receptor for several extracellular ligands;148 it has also been determined to act as a co-receptor for SARS-CoV-2 to infect host cells.149
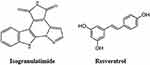 |
Figure 7 Molecular structure of Isogranulatimide and resveratrol. |
Alhadrami et al isolated three indole diketopiperazine alkaloids from the Red Sea-derived Aspergillus fumigatus, neoechinulin A, echinulin, and eurocristatine. Despite the structural similarity among these compounds, only neoechinulin A (Figure 8) showed significant SARS-CoV-2 Mpro inhibitory effects with an IC50 value of 0.47 μM compared to the positive control GC376 with an IC50 value of 0.36 µM. Moreover, in silico analysis showed that the amino acid in the active side of Mpro that contributed to the binding stability of neoechinulin A were LEU-141, ASN-142, GLY-143 and GLU-166 with hydrophobic interaction with HIS-41 (catalytic residue of the enzyme, participating in the hydrolysis of the substrate) which could predict the action mechanism of Neoechinulin A to inhibit SARS-CoV-2 Mpro.150
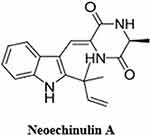 |
Figure 8 Molecular structure of neoechinulin A. |
Polyphenols (made up of phenol units with hydroxyl groups) are plants’ most prevalent bioactive compounds. They can be categorized into flavonoids, phenolic acids, polyphenolic amides and other polyphenolic compounds.151
Mahmoud et al carried out in silico studies of some polyphenols found in Cuphea ignea plant extract, and they found that rutin (glycoside combining the flavonol quercetin and the disaccharide rutinose), myricetin-3-O-rhamnoside (flavonoid) and rosmarinic acid (phenolic ester compound) showed the most promising antiviral activity. Rutin binds to the active site of Mpro by hydrogen bonds with Glu166 and Thr26; myricetin forms H-bonds with the amino acids Glu166 and Met165, and rosmarinic acid with His41 and Met145 residues at the catalytic site of the protease. These results show that the compounds block enzyme function by binding to the Mpro active site with a similar affinity to the N3 control inhibitor.151
The natural compound resveratrol (trans-3,5,4′-trihydroxystilbene), a polyphenolic compound (Figure 7), was described to exhibit antiviral activity against HIV-1, influenza virus, MERS-CoV and SARS-CoV-2. Against SARS-CoV-2 showed an IC50 of 4.48 µM in Vero cells (MOI 0.01)152 and IC50 of 10.6 µM in Vero E6 cells (MOI 0.01).151 Bram et al demonstrated that resveratrol exhibited a dose-dependent antiviral effect and a 50% reduction in the production of viral particles at 66 µM against SARS-CoV-2 in Vero E6 cells (MOI 1) and in differentiated human primary bronchial epithelial cells (PBECs) isolated from healthy volunteers a 99.3% reduction of the viral titer at 150 uM up to 48 h post-infection.153
Resveratrol presents various biological properties, but this compound shows very low solubility and bioavailability; for this reason, large doses are required, generating nausea, vomiting, gastrointestinal symptoms and weight loss, mainly after the use of doses higher than 1g (single dose); moreover, alterations in the levels of liver enzymes have also been reported at doses lower than 1 g.154 For this reason, a micellar 10% resveratrol solubilization formulation (JOTROLTM) has been created to increase the bioavailability of resveratrol via lymphatic system absorption.155 However, the effect of this improved version of resveratrol in patients with COVID-19 must be evaluated.
Suru et al evaluated the pomegranate peel extract (PoPEx) employing in silico and in vitro methods and found that the polyphenols punicalin and punicalagin (Figure 9) block and decrease the interaction of S-glycoprotein and cellular receptor ACE2 with the highest docking scores (−9.25 and −7.79 Kcal/mol, respectively) compared to other compounds extracted from PoPEx. The in vitro results confirmed the in silico predictions; punicalin and punicalagin showed significant percentages of inhibition of viral replication and punicalin the best IC50 (0.14 mg/mL), providing evidence of the antiviral potential of pomegranate polyphenols against SARS-CoV-2.156
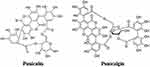 |
Figure 9 Molecular structure of punicalin and punicalagin. |
The curcumin polyphenol and the flavonoids quercetin and hesperidin (Figure 10) were evaluated by Kandeil et al; they observed that curcumin and quercetin had potent inhibitory effects on SARS-CoV-2 replication, at different multiplicities of infection in Vero E6 cells (IC50 = 0.44 and 18.2 µM, respectively) and reduced more than 90% in plaque counts and the copy number of viral RNA. In contrast, hesperidin showed the lowest antiviral activity compared to curcumin and quercetin.157
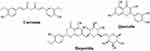 |
Figure 10 Molecular structure of curcumin, hesperidin and quercetin. |
Curcumin affects the SARS-CoV-2 replicative cycle, exhibiting an antiviral effect on different variants and strains, and immunomodulatory properties. A study made by Marin et al showed that curcumin (10 µg/mL) exhibited an in vitro antiviral effect against the DG614 strain and delta variant (99% and 99.8%, respectively). Moreover, it decreased the inflammatory cytokine levels (mRNA and protein) of IL-1β, IL-6, IL-8, MCP-1 and TNF-α in peripheral blood mononuclear cells (PBMC),51 cytokines that lead to the development of ARDS, multiorgan failure and coagulopathy.158
Curcumin has therapeutic potential against different diseases, including cancer and Alzheimer’s, but it has poor oral bioavailability, which limits its effects. Its safety profile was established several years ago. The daily intake value recommended by EFSA (European Food Safety Authority)159 is 3 mg/kg body weight/day. However, in a dose–response study in which study subjects were given between 500 and 12,000 mg (in high single oral doses), they showed side effects such as headache, skin rashes, diarrhea, and yellow stools.160 In another study, people receiving between 450 and 3600 mg for 1 to 3 months experienced nausea, diarrhea, and increased alkaline phosphatase and lactate dehydrogenase.161
To improve its bioavailability, a curcumin-galactomannoside complex (CurQfen) was recently developed, and it was tested in healthy volunteers in whom no adverse effects were observed at a concentration of 1000 mg/day for 3 months.162
It is expected that this new product will be evaluated in patients with COVID-19 to see if this better bioavailability can enhance its anti-inflammatory and antiviral effects.
Flavonoids are secondary metabolites formed by phenolic rings with different modifications and can be found in multiple fruits, vegetables, flowers, grains and their derivatives. These molecules are given many health benefits with anti-oxidative, anti-inflammatory, anti-mutagenic and anti-carcinogenic properties, and modulating important cellular enzymatic functions.114
Quercetin is a flavonoid produced by many fruits and vegetables (Figure 10). It is a promising candidate to treat SARS-CoV-2 infection due to its recognized antiviral activity against many viruses. In silico analyses suggest that this molecule interacts with the SARS-CoV-2 virus and could inactivate it.114 Abian et al recently reported that quercetin inhibited SARS-CoV-2 Mpro enzyme activity by binding to the active site and destabilizing its structure in molecular simulations.163 In addition, other molecular docking studies showed that quercetin could bind to the ACE2 receptor (Asp38 residue), preventing viral particle attachment to the cell.
These results indicate that quercetin is likely to prevent viral entry into lung cells by disrupting the ACE2 cell receptor, thereby inhibiting viral infection.164
Additionally, quercetin can suppress SARS-CoV-2 replication by activating nuclear factor erythroid 2-related factor 2 (NRF2); this transcription factor stimulates the expression of genes responsible for regulating the cellular response against toxic and oxidative substances.114,165 The activation of NRF2 by quercetin suppresses NLRP3 inflammasome, which generates active forms of cytokines IL-1β and IL-18 (inflammatory cytokines), produced in response to cytosolic PAMP and DAMPs.165 Several studies have reported quercetin as a potent NRF2 agonist.166 Olagnier et al demonstrated that the NRF2 antioxidant gene is not expressed in the lungs of patients with COVID-19, and this gene is responsible for inducing an effective antiviral response (IFN-independent) that limits viral replication and reduces the inflammatory response against human pathogenic viruses such as SARS-CoV-2.167
Schetting et al reported that in patients with COVID-19 treated with hydroxychloroquine and antibiotics, the use of nebulized quercetin (20 mg/mL) and N-acetylcysteine (100 mg/mL) improved respiratory symptoms caused by SARS-CoV-2 infection.168 Another study that evaluated the efficacy of quercetin treatment combined with remdesivir and favipiravir (antiviral drugs) in patients with severe COVID-19 showed that although it did not reduce mortality, the length of stay in the ICU or the number of patients admitted there, the treatment with quercetin (1000 mg daily) reduced hospitalization time and serum levels of some analytes such as C-reactive protein (CRP), lactate dehydrogenase and alkaline phosphatase, markers of severity of COVID-19.169 These findings show the importance of conducting more clinical studies to evaluate flavonoids alone or combined therapies to treat this disease.
Quercetin is one of the most studied flavonoids due to its important biological activity in humans and, like curcumin, it is a compound with low bioavailability and solubility in water. It has been observed safe in doses between 100 and 2000 mg administered for 3 months.154
Sumac, an oriental spice of the plant Rhus coriaria, possesses anti-inflammatory activities and recent studies of sumac phytochemicals Hinokiflavone (biflavonoid) and Myricetin (flavonoid) (Figure 11) indicated that their structures have significant energy of interaction and stability with the active site of SARS-CoV-2 Mpro, showing important pharmacokinetic properties and bioavailability.170
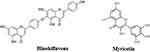 |
Figure 11 Molecular structure of hinokiflavone and myricetin. |
Additionally, hinokiflavone can also bind and block the enzymatic activity of PLpro, the other viral protease, as demonstrated by Li et al through an enzyme assay with an IC50 = 9.5 μM171 and Xia et al confirmed that myricetin strongly inhibits the Mpro of SARS-CoV-2 by in silico (molecular docking) and in vitro (enzymatic assay) assays.172 In the in silico study using docking and molecular dynamics simulation, it was found that myricetin binds to the pocket of SARS-CoV-2 Mpro through four binding sites, His 41, Phe140, Glu166 and Asp187 with −32.98 kcal/mol free energy calculated using the MM/GBSA method. In the in vitro study, they showed that myricetin inhibited the Mpro enzymatic activity in 97.79% (IC50 = 3.684 ± 0.076 μM).172 Furthermore, in the in vivo study carried out by these same researchers in which the anti-inflammatory capacity of myricetin was studied on lung lesions (bleomycin-induced), it was found that myricetin at 100 mg/kg significantly reduces the infiltration and the number of inflammatory cells in damaged lung tissue from mice treated with this flavonoid in a dose-dependent manner.172 Additionally, myricetin inhibited the expression of proinflammatory cytokines such as IL-6, TNF-α, IFN-γ and IL-1α. The anti-inflammatory effect of myricetin at high doses (100 mg/kg) is comparable to that found with the positive control, pirfenidone.172
These results suggest that these flavonoids could be evaluated in vivo for the clinical management of COVID-19.
Terpenes and terpenoids are hydrocarbons that can be seen as a combination of numerous isoprene units; they are aromatic compounds that naturally occur in plants and create signature scents. Terpenes comprise 10–15 carbon atoms and terpenoids are terpenes with some modifications such as removing or relocating methyl groups after adding oxygen atoms.149 A member of this group is Betulinic acid, a plant-derived triterpene (composed of three terpene units) (Figure 12), this exhibited antiviral activity in an enzyme inhibition assay against SARS-CoV by binding to its main protease (IC50 = 10 µM)173 Hence, Alhadrami et al evaluated betulinic acid against SARS-CoV-2, and they observed that it inhibits Mpro at an IC50 = 14.55 µM (± 1.3 µM).174 Previous findings of Wen et al identified in this enzyme a hydroxyl group at C-3 (H-bond donor) essential for inhibiting SARS-CoV-2 Mpro in vitro.173 Moreover, Carino et al found that betulinic acid has robust binding selectivity toward the spike receptor-binding domain’s pocket 1 (RBD) with binding affinity −8.1 kcal/mol and incubating RBD with betulinic acid at a concentration of 0.1 μM, reduces binding of RBD by the immobilized ACE2 receptor.175
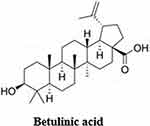 |
Figure 12 Molecular structure of betulinic acid. |
Table 2 summarizes the possible therapeutic targets, mechanisms of action and the IC50 of the previous natural compounds.
 |
Table 2 Natural Compound: Potential Therapeutic Targets and Their Antiviral Effects |
Medicinal Plants for COVID-19 Treatment
Medicinal plants are plants that produce substances or molecules that can be used to heal injuries or treat diseases, and it has been reported that approximately 80% of people in the world use them for these purposes.179
Medicinal plants of traditional Chinese medicine have been used in this country to treat approximately 91.5% of COVID-19 cases and have been shown to improve symptoms and reduce incidence and mortality.180 In Colombia, medicinal plants have been essential for mestizos, indigenous and Afro-Colombians to treat multiple diseases, and during the COVID-19 pandemic they have been used to treat this disease.181
Flórez et al evaluated some plant extracts used in Colombia by indigenous and Afro-American people against SARS-CoV in vitro on the Vero E6 cell line. They reported that Gliricidia sepium inhibited SARS-CoV-2 in vitro by 75.6% at 10 mg/mL, and Piper tuberculatum reduced viral titer by 33.3% at 6 mg/mL after 48h.
These results demonstrate that these plants can be a therapeutic alternative to treat SARS-COV infection.182
Jaimes-Gualdron et al evaluated the in vitro antiviral activity of Corozo (Bactris guineensis) fruit extract and demonstrated that it inhibits the SARS-CoV-2 virus by 88.2% at 15.6 g/L. The extract of this fruit is rich in polyphenols such as anthocyanins (the main flavonoids in fruits and vegetables); these compounds could be responsible for the antiviral activity of corozo because they have shown effectiveness against other respiratory viruses in the past.183
In a Phase II multicenter randomized, double-blind clinical trial on COVID-19 patients, Urueña et al evaluated the effectiveness of an extract of seeds from Caesalpinia spinosa (P2Et) rich in polyphenols. Patients treated with P2Et were discharged on average two days earlier than the control group (7.4 and 9.6 days, respectively). A reduction in the proinflammatory cytokines G-CSF, IL-6 and IL-18 was observed, which is related to the lower recovery of patients at long term. In mice injected with bleomycin, P2Et also decreased lung inflammation, fibrosis, and replication of human coronaviruses in vitro, showing its antiviral activity and anti-inflammatory capacity, two essential mechanisms to control the disease.184
Finally, it is important to note that our research has a couple of limitations. The included studies’ publication bias (overvaluation of the impact of the treatments) is beyond our control. Moreover, the number of studies published in the databases is less than the number of available studies. Some studies included in this review present contradictory results, and personal biases can affect the selection and interpretation of studies.
Conclusions
Limited approved therapies for treating or preventing COVID-19 have led to drug repurposing, allowing the discovery of new and better treatment strategies, thus providing fast and valuable assistance for treating this disease. In addition, in silico methods help to study molecular structures and analyze the interaction of potential inhibitors and their viral targets, facilitating the search for novel preventive and therapeutic agents.
One of the antiparasitic drugs that have been repurposed is the hydroxychloroquine. Although this antiparasitic drug showed antiviral effects during in vitro analysis, in many clinical trials, it was observed that it is inadequate for COVID-19 treatment; whereby the FDA withdrew Emergency Usage Authorization on June 15, 2020.
The anthelmintic agent ivermectin improved the condition of some patients with COVID-19; however, toxicity was reported by the American College of Medical Toxicology, the Poison Control Centers and the US CDC in some patients who became ill after using IVM as prophylaxis or treatment of COVID-19, and for this reason, the FDA did not approve the use of ivermectin to prevent or treat COVID-19.
Among the drugs with antiviral activity reused to control the SARS-CoV2, the better results have been observed for remdesivir; in hospitalized adults, it improved clinical outcomes, showed greater recovery, and reduced odds of death; in no hospitalized patients, remdesivir showed a lower risk of hospitalization or death.
Other drugs like anticoagulants and statins have shown benefits in patients with COVID-19. The anticoagulation forum has recommended anticoagulants for all patients hospitalized with COVID-19, critically ill hospitalized adults and non-critically ill patients at risk of disease progression or thromboembolism. Statins like atorvastatin are associated with significantly reduced mortality in hospitalized patients with COVID-19.
Figure 13 shows the mechanisms of action of the second-use drugs in the replication cycle during SARS-CoV-2 infection.
In silico studies of the viral proteins, RdRp, protein S, and Mpro showed that many plant metabolites have the same affinity as compounds currently used to treat SARS-COV2 infection. Compounds such as resveratrol, curcumin, and quercetin, widely known to have antiviral activity, have demonstrated in vitro antiviral activity against SARS-CoV-2. Among these, a nebulized formulation of quercetin alleviated the respiratory symptoms caused by SARS-CoV-2. This suggests the need for further clinical studies to assess the effectiveness of quercetin alone or in combination with other drugs to treat COVID-19.
Abbreviations
ABC, Abacavir; ACE2, Angiotensin-converting enzyme 2; ALP, Alkaline phosphatase; ARDS, Acute respiratory distress syndrome; ATV, Atorvastatin; AZT, Zidovudine; CDC, Centers for Disease Control and Prevention; COVID19, Coronavirus disease 2019; CQ, Chloroquine; CRP, C-reactive protein; ER, Endoplasmic reticulum; ERGIC, Reticulum-Golgi intermediate compartment; FDA, Food and Drug Administration; HBV, Hepatitis B virus; HCQ, Hydroxychloroquine; HIV, Human immunodeficiency virus; ICU, Intensive care unit; IVM, Ivermectin; Kd, Dissociation constant; Ki, Inhibition constant; LDH, Lactate dehydrogenase; LH, Lianhuaqingwen; MERS-CoV, Middle East respiratory syndrome coronavirus; Mpro, Main protease; NRF2, Nuclear factor erythroid; Nsps, Non-structural proteins; PBECs, Primary bronchial epithelial cells; PBMC, Peripheral blood mononuclear cells; PL pro, Papain-like protease; PoPEx, Pomegranate peel extract; P2Et, Extract of Caesalpinia spinosa; RBD, Receptor-binding domain; RdRP, RNA-dependent RNA polymerase; RMP, Remdesivir monophosphate; RT, Reverse transcriptase; RTC, Replication and transcription complex; SARS-CoV, Severe acute respiratory syndrome coronavirus; SARS-CoV-2, Severe acute respiratory syndrome Coronavirus 2; S protein, Spike protein; TMPRSS2, Transmembrane serine protease 2; VitD, Vitamin D; VTE, Venous thromboembolism; WHO, World Health Organization; 3CL pro, Chymotrypsin-like protease.
Funding
This work was supported by Universidad Cooperativa de Colombia (code INV3159) and Universidad de Antioquia, UdeA.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Pande M, Debanjan Kundu RS. Drugs repurposing against SARS-CoV2 and the new variant B.1.1.7 (Alpha Strain) targeting the spike protein: molecular docking and Simulation studies. Estuar Coast Shelf Sci. 2021;7:107397. doi:10.1016/j.heliyon.2021.e07803
2. Das A, Roy S, Swarnakar S, Chatterjee N. Understanding the immunological aspects of SARS-CoV-2 causing COVID-19 pandemic: a therapeutic approach. Clin Immunol. 2021;231:108804. doi:10.1016/j.clim.2021.108804
3. Manta B, Sarkisian AG. Fisiopatología de la enfermedad COVID-19. Odontoestomatologia. 2022;24:1–19. doi:10.22592/ode2022n39e312
4. Jessie H, Hume AJ, Abo KM, et al. SARS-CoV-2 infection of pluripotent stem cell- derived human lung alveolar type 2 cells elicits a rapid epithelial-intrinsic inflammatory response. Ann Oncol. 2020;110:19–21.
5. Zhu Y, Sharma L, Chang D. Pathophysiology and clinical management of coronavirus disease (COVID-19): a mini-review. Front Immunol. 2023;14(August):1–13. doi:10.3389/fimmu.2023.1116131
6. Gupta A, Madhavan MV, Sehgal K, et al. Extrapulmonary manifestations of COVID-19. Nat Med. 2020;26(7):1017–1032. doi:10.1038/s41591-020-0968-3
7. Zhang Q, Xiang R, Huo S, et al. Molecular mechanism of interaction between SARS-CoV-2 and host cells and interventional therapy. Signal Transduct Target Ther. 2021;6(1). doi:10.1038/s41392-021-00653-w
8. Malone B, Urakova N, Snijder EJ, Campbell EA. Structures and functions of coronavirus replication–transcription complexes and their relevance for SARS-CoV-2 drug design. Nat Rev Mol Cell Biol. 2022;23(1):21–39. doi:10.1038/s41580-021-00432-z
9. V’kovski P, Kratzel A, Steiner S, Stalder H, Thiel V. Coronavirus biology and replication: implications for SARS-CoV-2. Nat Rev Microbiol. 2021;19(3):155–170. doi:10.1038/s41579-020-00468-6
10. Kumar S, Nyodu R, Maurya VK, Saxena SK. Morphology, genome organization, replication, and pathogenesis of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Coronav Dis. 2020;2:23–31. doi:10.1007/978-981-15-4814-7_3
11. BBC NEWS. Cifras de la pandemia país por país [Pandemic figures country by country]; 2021. Available from: https://www.bbc.com/mundo/noticias-51705060.
12. Farooqi T, Ahmad Malik J, Hanif Mulla A, et al. An overview of SARS-COV-2 epidemiology, mutant variants, vaccines, and management strategies. Carbohydr Polym. 2019;115800. doi:10.1016/j.jiph.2021.08.014
13. Srivastava V, Ahmad A. New perspective towards therapeutic regimen against SARS-CoV-2 infection. J Infect Public Health. 2021;14(7):852–862. doi:10.1016/j.jiph.2021.05.009
14. Muhammed Y, Yusuf A, Pius M, et al. SARS-CoV-2 spike protein and RNA dependent RNA polymerase as targets for drug and vaccine development: a review. Biosaf Health. 2021;3(5):249–263. doi:10.1016/j.bsheal.2021.07.003
15. Covid: qué son baricitinib y sotrovimab, los nuevos medicamentos que autorizó la OMS para tratar casos de la enfermedad [COVID: what are baricitinib and sotrovimab, the new drugs authorized by the WHO to treat cases of the disease]. BBC News Mundo. Available from: https://www.bbc.com/mundo/noticias-60058017.
16. Matrose NA, Obikese K, Belay ZA, Caleb OJ. Drug repurposing against coronavirus disease 2019 (COVID-19): a review. Sci Total Environ. 2021;135907. doi:10.1016/j.jpha.2021.09.001
17. Aronskyy I, Masoudi-Sobhanzadeh Y, Cappuccio A, Zaslavsky E. Advances in the computational landscape for repurposed drugs against COVID-19. Drug Discov Today. 2021;26(12):2800–2815. doi:10.1016/j.drudis.2021.07.026
18. Goyal M, Tewatia N, Vashisht H, Jain R, Kumar S. Novel Corona virus (COVID-19); Global efforts and effective investigational medicines: a review. J Infect Public Health. 2021;14(7):910–921. doi:10.1016/j.jiph.2021.04.011
19. Malek RJ, Bill CA, Vines CM. Clinical drug therapies and biologicals currently used or in clinical trial to treat COVID-19. Biomed Pharmacother. 2021;144(August):112276. doi:10.1016/j.biopha.2021.112276
20. WHO Solidarity Trial Consortium. Repurposed antiviral drugs for covid-19 — interim who solidarity trial results. N Engl J Med. 2021;384(6):497–511. doi:10.1056/nejmoa2023184
21. Kmietowicz Z. Covid-19: WHO recommends baricitinib and sotrovimab to treat patients. BMJ. 2022;o97. doi:10.1136/bmj.o97
22. Lamontagne F, Agoritsas T, Siemieniuk R, et al. A living WHO guideline on drugs to prevent covid-19. BMJ. 2021:372. doi:10.1136/bmj.n526
23. Rehman MU, Abdullah KF, Niaz K. Introduction to natural products analysis. Recent Adv Nat Prod Anal. 2020;3–15. doi:10.1016/B978-0-12-816455-6.00001-9
24. Atanasov AG, Zotchev SB, Dirsch VM, et al. Natural products in drug discovery: advances and opportunities. Nat Rev Drug Discov. 2021;20(3):200–216. doi:10.1038/s41573-020-00114-z
25. Panikar S, Shoba G, Arun M, et al. Essential oils as an effective alternative for the treatment of COVID-19: molecular interaction analysis of protease (Mpro) with pharmacokinetics and toxicological properties. J Infect Public Health. 2021;14(5):601–610. doi:10.1016/j.jiph.2020.12.037
26. Aucoin M, Cardozo V, McLaren MD, et al. A systematic review on the effects of Echinacea supplementation on cytokine levels: is there a role in COVID-19? Metab Open. 2021;11:100115. doi:10.1016/j.metop.2021.100115
27. Nagoor Meeran MF, Javed H, Sharma C, et al. Can Echinacea be a potential candidate to target immunity, inflammation, and infection - The trinity of coronavirus disease 2019. Heliyon. 2021;7(2):e05990. doi:10.1016/j.heliyon.2021.e05990
28. Das K. Herbal plants as immunity modulators against COVID-19: a primary preventive measure during home quarantine. J Herb Med. 2022;32:100501. doi:10.1016/j.hermed.2021.100501
29. Zhang D, Hamdoun S, Chen R, et al. Identification of natural compounds as SARS-CoV-2 entry inhibitors by molecular docking-based virtual screening with bio-layer interferometry. Pharmacol Res. 2021;172:105820. doi:10.1016/j.phrs.2021.105820
30. Omrani M, Keshavarz M, Nejad Ebrahimi S, et al. Potential natural products against respiratory viruses: a perspective to develop anti-COVID-19 medicines. Front Pharmacol. 2021;11. doi:10.3389/fphar.2020.586993
31. Kumar A, Choudhir G, Shukla SK, et al. Identification of phytochemical inhibitors against main protease of COVID-19 using molecular modeling approaches. J Biomol Struct Dyn. 2021;39(10):3760–3770. doi:10.1080/07391102.2020.1772112
32. Gyebi GA, Ogunro OB, Adegunloye AP, Ogunyemi OM, Afolabi SO. Potential inhibitors of coronavirus 3-chymotrypsin-like protease (3CLpro): an in silico screening of alkaloids and terpenoids from African medicinal plants. J Biomol Struct Dyn. 2021;39(9):3396–3408. doi:10.1080/07391102.2020.1764868
33. Runfeng L, Yunlong H, Jicheng H, et al. Lianhuaqingwen exerts anti-viral and anti-inflammatory activity against novel coronavirus (SARS-CoV-2). Pharmacol Res. 2020;156:104761. doi:10.1016/j.phrs.2020.104761
34. Akinlalu AO, Chamundi A, Yakumbur DT, et al. Repurposing FDA-approved drugs against multiple proteins of SARS-CoV-2: an in silico study. Sci Afr. 2021;13:e00845. doi:10.1016/j.sciaf.2021.e00845
35. Jain S, Kumar P, Vyas RK, Pandit P, Dalai AK. Occurrence and removal of antiviral drugs in environment: a review. Water Air Soil Pollut. 2013;224(2). doi:10.1007/s11270-012-1410-3
36. Srivastava K, Singh MK. Drug repurposing in COVID-19: a review with past, present and future. Metab Open. 2021;12:100121. doi:10.1016/j.metop.2021.100121
37. Tichauer JE, Soto D, Andresen M. Characterization of the modulatory effect of hydroxychloroquine on ACE2 Activity: new insights in relation to COVID-19. Biomed Res Int. 2021;2021:1–5. doi:10.1155/2021/6614000
38. Vincent MJ, Bergeron E, Benjannet S, et al. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol J. 2005;2:1–10. doi:10.1186/1743-422X-2-69
39. Alsuwaidan S, Memish ZA, Alaklobi F, Khan K, Alajami HN. The utilization of hydroxychloroquine to reduce the main signs and symptoms of COVID-19 patients, a cross-sectional study. Ann Med Surg. 2021;70:102867. doi:10.1016/j.amsu.2021.102867
40. Oriol M, Marc C-M, Maria U. Hydroxychloroquine for early treatment of adults with mild covid-19: a randomized- controlled trial. Clin Infect Dis. 2020;54(650):1–54.
41. Skipper CP, Pastick KA, Engen NW, et al. Hydroxychloroquine in Nonhospitalized Adults With Early COVID-19: a Randomized Trial. Ann Intern Med. 2020;173(8):623–631. doi:10.7326/M20-4207
42. Kamstrup P, Sivapalan P, Eklöf J, et al. Hydroxychloroquine as a primary prophylactic agent against SARS-CoV-2 infection: a cohort study. Int J Infect Dis. 2021;108:370–376. doi:10.1016/j.ijid.2021.05.076
43. Rentsch CT, DeVito NJ, MacKenna B, et al. Effect of pre-exposure use of hydroxychloroquine on COVID-19 mortality: a population-based cohort study in patients with rheumatoid arthritis or systemic lupus erythematosus using the OpenSAFELY platform. Lancet Rheumatol. 2021;3(1):e19–e27. doi:10.1016/S2665-9913(20)30378-7
44. Cavalcanti AB, Zampieri FG, Rosa RG, et al. Hydroxychloroquine with or without azithromycin in mild-to-moderate covid-19. N Engl J Med. 2020;383(21):2041–2052. doi:10.1056/nejmoa2019014
45. Lamback EB, de Oliveira MA, Haddad AF, et al. Hydroxychloroquine with azithromycin in patients hospitalized for mild and moderate COVID-19. Brazilian J Infect Dis. 2021;25(2):1–6. doi:10.1016/j.bjid.2021.101549
46. Gupta S, Dixit PK, Ghana P, et al. Open-label randomized control trial of hydroxychloroquine in patients with moderate to severe coronavirus disease 2019 infection. Med J Armed Forces India. 2021;77:S305–S311. doi:10.1016/j.mjafi.2021.02.007
47. Cadegiani FA, Goren A, Wambier CG, McCoy J. Early COVID-19 therapy with azithromycin plus nitazoxanide, ivermectin or hydroxychloroquine in outpatient settings significantly improved COVID-19 outcomes compared to known outcomes in untreated patients. New Microbes New Infect. 2021;43:100915. doi:10.1016/j.nmni.2021.100915
48. Chivese T, Musa OAH, Hindy G, et al. Efficacy of chloroquine and hydroxychloroquine in treating COVID-19 infection: a meta-review of systematic reviews and an updated meta-analysis. Travel Med Infect Dis. 2021;43(July):102135. doi:10.1016/j.tmaid.2021.102135
49. Das S, Ramachandran AK, Birangal SR, Akbar S, Ahmed B, Joseph A. The controversial therapeutic journey of chloroquine and hydroxychloroquine in the battle against SARS-CoV-2: a comprehensive review. Med Drug Discov. 2021;10:100085. doi:10.1016/j.medidd.2021.100085
50. Zapata-Cardona MI, Flórez-Álvarez L, Zapata-Builes W, et al. Atorvastatin effectively inhibits ancestral and two emerging variants of SARS-CoV-2 in vitro. Front Microbiol. 2022;13(March). doi:10.3389/fmicb.2022.721103
51. Marín-Palma D, Tabares-Guevara JH, Zapata-Cardona MI, et al. Curcumin inhibits in vitro sars-cov-2 infection in Vero E6 cells through multiple antiviral mechanisms. Molecules. 2021;26(22):1–17. doi:10.3390/molecules26226900
52. Yao X, Ye F, Zhang M, et al. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome main point: hydroxychloroquine was found to be more potent than chloroquine at inhibiting SARS-CoV-2 in vit. Clin Infect Dis. 2020;2:1–25.
53. Yepes-Perez AF, Herrera-Calderón O, Oliveros CA, et al. The Hydroalcoholic Extract of Uncaria tomentosa (Cat’s Claw) Inhibits the Infection of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) in Vitro. Evidence-Based Complement Altern Med. 2021;2021:1–11. doi:10.1155/2021/6679761
54. U.S Food and Drug Administration (FDA). Memorandum explaining basis for revocation of emergency use authorization for chloroquine phosphate and hydroxychloroquine sulfate. FDA site; 2020. Available from: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-revokes-emergency-use-authorization-chloroquine-and.
55. U.S Food and Drug Administration (FDA). FDA cautions against use of hydroxychloroquine or chloroquine for COVID-19 outside of the hospital setting or a clinical trial due to risk of heart rhythm problems; 2020. Available from: https://www.fda.gov/drugs/drug-safety-and-availability/fda-cautions-against-use-hydroxychloroquine-or-chloroquine-covid-19-outside-hospital-setting-or.
56. Kinobe RT, Owens L. A systematic review of experimental evidence for antiviral effects of ivermectin and an in silico analysis of ivermectin’s possible mode of action against SARS-CoV-2. Fundam Clin Pharmacol. 2021;35(2):260–276. doi:10.1111/fcp.12644
57. Yang SNY, Atkinson SC, Wang C, et al. The broad spectrum antiviral ivermectin targets the host nuclear transport importin α/β1 heterodimer. Antiviral Res. 2020;177:104760. doi:10.1016/J.ANTIVIRAL.2020.104760
58. Wagstaff KM, Sivakumaran H, Heaton SM, Harrich D, Jans DA. Ivermectin is a specific inhibitor of importin α / β -mediated nuclear import able to inhibit replication of HIV-1 and dengue virus. Bioch J. 2012;856:851–856. doi:10.1042/BJ20120150
59. Lehrer S, Rheinstein PH. Ivermectin docks to the SARS-CoV-2 spike receptor-binding domain attached to ACE2. In vivo. 2020;34(5):3023–3026. doi:10.21873/invivo.12134
60. Ahmed S, Mahbubul M, Ross AG, et al. A five-day course of ivermectin for the treatment of COVID-19 may reduce the duration of illness. Biomed Biotechnol Res J. 2020;21(January):1–4. doi:10.21203/rs.3.rs-317485/v1
61. Mahmud R, Rahman MM, Alam I, et al. Ivermectin in combination with doxycycline for treating COVID-19 symptoms: a randomized trial. J Int Med Res. 2021;49(5):030006052110135. doi:10.1177/03000605211013550
62. Okumuş N, Demirtürk N, Çetinkaya RA, et al. Evaluation of the effectiveness and safety of adding ivermectin to treatment in severe COVID-19 patients. BMC Infect Dis. 2021;21(1):1–11. doi:10.1186/s12879-021-06104-9
63. Zein AFMZ, Sulistiyana CS, Raffaelo WM, Pranata R. Ivermectin and mortality in patients with COVID-19: a systematic review, meta-analysis, and meta-regression of randomized controlled trials: ivermectin and COVID-19. Diabetes Metab Syndr Clin Res Rev. 2021;15(4):102186. doi:10.1016/j.dsx.2021.102186
64. Mansour SM, Shamma RN, Ahmed KA, et al. Safety of inhaled ivermectin as a repurposed direct drug for treatment of COVID-19: a preclinical tolerance study. Int Immunopharmacol. 2021;99(1):108004. doi:10.1016/j.intimp.2021.108004
65. Krolewiecki A, Lifschitz A, Moragas M, et al. Antiviral effect of high-dose ivermectin in adults with COVID-19: a proof-of-concept randomized trial. EClinicalMedicine. 2021;37. doi:10.1016/j.eclinm.2021.100959
66. López-Medina E, López P, Hurtado IC, et al. Effect of ivermectin on time to resolution of symptoms among adults with mild COVID-19: a randomized clinical trial. JAMA. 2021;325(14):1426–1435. doi:10.1001/jama.2021.3071
67. Reis G, Silva EASM, Silva DCM, et al. Effect of early treatment with ivermectin among patients with covid-19. N Engl J Med. 2022;386(18):1721–1731. doi:10.1056/NEJMoa2115869
68. Calello DP, Kazzi Z, Stolbach A. American College of Medical Toxicology (ACMT) Cautions Against Off-Label Prescribing of Ivermectin for the Prevention or Treatment of COVID-19. J Med Toxicol. 2022;18(1):69–70. doi:10.1007/s13181-021-00866-z
69. CDC Health Alert Network. CDC HEALTH ADVISORY. Rapid increase in ivermectin prescriptions and reports of severe illness associated with use of products containing ivermectin to prevent or treat COVID-19; 2021.
70. Baudou E, Lespine A, Durrieu G, et al. Serious ivermectin toxicity and human ABCB1 nonsense mutations. N Engl J Med. 2020;69(1):787–789.
71. Ivermectina: cómo la falsa ciencia inventó un fármaco “milagroso” contra la covid-19 [Ivermectin: how false science invented a “miracle” drug against COVID-19]. BBC News Mundo. Available from: https://www.bbc.com/mundo/noticias-58828993.
72. Huge study supporting ivermectin as Covid treatment withdrawn over ethical concerns | medical research | the Guardian. Available from: https://www.theguardian.com/science/2021/jul/16/huge-study-supporting-ivermectin-as-covid-treatment-withdrawn-over-ethical-concerns.
73. FDA. Why you should not use ivermectin to treat or prevent COVID-19. Available from: https://www.fda.gov/consumers/consumer-updates/why-you-should-not-use-ivermectin-treat-or-prevent-covid-19.
74. Sallard E, Lescure F-X, Burdet C, Guedj J, Yazdan Yazdanpanah NP-S. Repurposed prophylaxis strategies for COVID-19: a review. medRxiv. 2020;600:2020–2025.
75. Wang M, Cao R, Zhang L, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30(3):269–271. doi:10.1038/s41422-020-0282-0
76. Zaidi AK, Dehgani-Mobaraki P. The mechanisms of action of ivermectin against SARS-CoV-2—an extensive review. J Antibiot. 2022;75(2):60–71. doi:10.1038/s41429-021-00491-6
77. Caly L, Druce JD, Catton MG, Jans DA, Wagsta KM. The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antiviral Res. 2020;178(January):104787. doi:10.1016/j.antiviral.2020.104787
78. Yadav R, Imran M, Dhamija P, Suchal K, Handu S. Virtual screening and dynamics of potential inhibitors targeting RNA binding domain of nucleocapsid phosphoprotein from SARS-CoV-2. J Biomol Struct Dyn. 2021;39(12):4433–4448. doi:10.1080/07391102.2020.1778536
79. Dallocchio RN, Dessì A, De Vito A, Delogu G, Serra PA, Madeddu G. Early combination treatment with existing HIV antivirals: an effective treatment for COVID-19? Eur Rev Med Pharmacol Sci. 2021;25(5):2435–2448. doi:10.26355/eurrev_202103_25285
80. Taylor K, Fritz K, Parmar M. Lamivudine. Kucers use antibiot a clin rev antibacterial, antifung antiparasit antivir drugs; 2021:3729–3754. Available from: https://www.ncbi.nlm.nih.gov/books/NBK559252/.
81. Uzunova K, Filipova E, Pavlova V, Vekov T. Insights into antiviral mechanisms of remdesivir, lopinavir/ritonavir and chloroquine/hydroxychloroquine affecting the new SARS-CoV-2. Biomed Pharmacother. 2020;131:110668. doi:10.1016/j.biopha.2020.110668
82. Fadaka AO, Aruleba RT, Sibuyi NRS, Klein A, Madiehe AM, Meyer M. Inhibitory potential of repurposed drugs against the SARS-CoV-2 main protease: a computational-aided approach. J Biomol Struct Dyn. 2020;1(1):1–13. doi:10.1080/07391102.2020.1847197
83. Barreto RG, Brites C. Low frequency of hypersensitivity reactions to Abacavir in HIV infected patients in a referral center in Bahia, Brazil. Brazilian J Infect Dis. 2019;23(4):268–270. doi:10.1016/j.bjid.2019.06.003
84. Chien M, Anderson TK, Jockusch S, et al. Nucleotide Analogues as Inhibitors of SARS-CoV-2 Polymerase, a Key Drug Target for COVID-19. J Proteome Res. 2020;19(11):4690–4697. doi:10.1021/acs.jproteome.0c00392
85. Quo R, De Clercq E. Remdesivir: quo vadis? Biochem Pharmacol. 2021;193:114800.
86. Ribavirin EAA, Remdesivir S. Galidesivir, and Tenofovir against SARS-CoV-2 RNA dependent RNA polymerase (RdRp): a molecular docking study. Life Sci. 2020;253:117592. doi:10.1016/J.LFS.2020.117592
87. Beck BR, Shin B, Choi Y, Park S, Kang K. Predicting commercially available antiviral drugs that may act on the novel coronavirus (SARS-CoV-2) through a drug-target interaction deep learning model. Comput Struct Biotechnol J. 2020;18:784–790. doi:10.1016/j.csbj.2020.03.025
88. Castiglione V, Chiriacò M, Emdin M, Taddei S, Vergaro G. Statin therapy in COVID-19 infection. Eur Hear J Cardiovasc Pharmacother. 2020;6(4):258–259. doi:10.1093/EHJCVP/PVAA042
89. Gonzale SM, Aguilar-Jimenez W, Trujillo-Gil E, et al. Vitamin D treatment of peripheral blood mononuclear cells modulated immune activation and reduced susceptibility to HIV-1 infection of CD4+ T lymphocytes. PLoS One. 2019;14(9):1–15. doi:10.1371/journal.pone.0222878
90. Edwards Z, Ingold CJ, Azmat CE. Zidovudine. Kucers use antibiot a clin rev antibacterial, antifung antiparasit antivir drugs; 2021:3657–3697. Available from: https://www.ncbi.nlm.nih.gov/books/NBK554419/.
91. Ju J, Li X, Kumar S, et al. Nucleotide analogues as inhibitors of SARS-CoV Polymerase. Pharmacol Res Perspect. 2020;8(6):1–9. doi:10.1002/prp2.674
92. Zapata-Cardona MI, Florez-Alvarez L, Guerra-Sandoval AL, et al. In vitro and in silico evaluation of antiretrovirals against SARS-CoV-2: a drug repurposing approach. AIMS Microbiol. 2023;9(1):20–40. doi:10.3934/microbiol.2023002
93. García-Trejo JJ, Ortega R, Zarco-Zavala M. Putative repurposing of lamivudine, a nucleoside/nucleotide analogue and antiretroviral to improve the outcome of cancer and COVID-19 patients. Front Oncol. 2021;11:1–17. doi:10.3389/fonc.2021.664794
94. Saxena RK, Samad AA, Khan UR, et al. Assessment of prevalence of SARS-CoV-2 Infection in patients on anti- HBV (lamivudine) treatment: a questionnaire based survey. IP Int J Med Microbiol Trop Dis. 2021;7(7):10–8231. doi:10.18231/j.ijmmtd.2021.005
95. Jockusch S, Tao C, Li X, Anderson TK, Chien M. A library of nucleotide analogues terminate RNA synthesis catalyzed by polymerases of coronaviruses that cause SARS and COVID-19. Antiviral Res. 2020;180:104857.
96. Tomić D, Davidović D, Szasz A, et al. The screening and evaluation of potential clinically significant HIV drug combinations against the SARS-CoV-2 virus. Inf Med. 2021;23:100529.
97. Del Amo J, Polo R, Moreno S, et al. Incidence and severity of COVID-19 in HIV-positive persons receiving antiretroviral therapy. Ann Intern Med. 2020;173(7):536–541. doi:10.7326/M20-3689
98. Alshaeri HK, Natto ZS. A contemporary look at COVID-19 medications: available and potentially effective drugs. Eur Rev Med Pharmacol Sci. 2020;24(17):9188–9195. doi:10.26355/eurrev_202009_22870
99. Kickbusch I, Leung G, Briand S, et al. WHO R&D Blueprint Informal consultation on prioritization of candidate therapeutic agents for use in novel coronavirus 2019 infection. Euro Surveill. 2020;25(January):709–710. doi:10.1046/j.1466-7657.2000.00027
100. Hung IF, Lung KC, Tso EY, et al. Triple combination of interferon beta-1b, lopinavir–ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open-label, randomised, phase 2 trial. Lancet. 2020;395(10238):1695–1704.
101. Cao B, Wang Y, Wen D, et al. A Trial of Lopinavir–Ritonavir in Adults Hospitalized with Severe Covid-19. N Engl J Med. 2020;382(19):1787–1799. doi:10.1056/nejmoa2001282
102. Cheng CY, Lee YL, Chen CP, et al. Lopinavir/ritonavir did not shorten the duration of SARS CoV-2 shedding in patients with mild pneumonia in Taiwan. J Microbiol Immunol Infect. 2020;53(3):488–492. doi:10.1016/j.jmii.2020.03.032
103. Ader F, Peiffer-smadja N, Poissy J, et al. An open-label randomized controlled trial of the effect of lopinavir / ritonavir, lopinavir / ritonavir plus IFN- b −1a and hydroxychloroquine in hospitalized patients with COVID-19, on behalf of the DisCoVeRy study. Clin Microbiol Infect. 2021;27(12):1826–1837. doi:10.1016/j.cmi.2021.05.020
104. Arabi YM, Gordon AC, Derde LPG, et al. Lopinavir-ritonavir and hydroxychloroquine for critically ill patients with COVID-19: REMAP-CAP randomized controlled trial. Intensive Care Med. 2021;47(8):867–886. doi:10.1007/s00134-021-06448-5
105. Reis G, Moreira Silva EADS, Medeiros Silva DC, et al. Effect of early treatment with hydroxychloroquine or lopinavir and ritonavir on risk of hospitalization among patients with COVID-19: the TOGETHER randomized clinical trial. JAMA Netw Open. 2021;4(4):1–14. doi:10.1001/jamanetworkopen.2021.6468
106. Vangeel L, Chiu W, De Jonghe S, et al. Remdesivir, Molnupiravir and Nirmatrelvir remain active against SARS-CoV-2 Omicron and other variants of concern. Antiviral Res. 2022;198(January):10–12. doi:10.1016/j.antiviral.2022.105252
107. Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the Treatment of Covid-19 — final Report. N Engl J Med. 2020;383(19):1813–1826. doi:10.1056/nejmoa2007764
108. Olender SA, Perez KK, Go AS, et al. Remdesivir for severe coronavirus disease 2019 (COVID-19) versus a cohort receiving standard of care. Clin Infect Dis. 2021;73(11):E4166–E4174. doi:10.1093/cid/ciaa1041
109. Rezagholizadeh A, Khiali S, Sarbakhsh P, Entezari-Maleki T. Remdesivir for treatment of COVID-19; an updated systematic review and meta-analysis. EuropJ Pharmacol. 2021;897:173926.
110. Gottlieb RL, Vaca CE, Paredes R, et al. Early Remdesivir to Prevent Progression to Severe Covid-19 in Outpatients. N Engl J Med. 2022;386(4):305–315. doi:10.1056/nejmoa2116846
111. Parienti JJ, Prazuck T, Peyro-Saint-Paul L, et al. Effect of tenofovir disoproxil fumarate and emtricitabine on nasopharyngeal SARS-CoV-2 viral load burden amongst outpatients with COVID-19: a pilot, randomized, open-label phase 2 trial. eClinicalMedicine. 2021;38. doi:10.1016/j.eclinm.2021.100993
112. Park S, Yu K, Kim Y, et al. Antiviral Efficacies of FDA-Approved Drugs against SARS-CoV-2 Infection in Ferrets. mBIO. 2020;11(3):1–10. doi:10.1186/s13287-019-1471-y
113. Vos AG, Venter WDF Toxicidad cardiovascular de la terapia antirretroviral contemporánea [Cardiovascular toxicity of contemporary antiretroviral therapy]. Available from: https://pubmed.ncbi.nlm.nih.gov/34545036/.
114. Panche A, Diwan AD, Chandra SR. Flavonoids: an overview. J Nutr Sci. 2016;5. doi:10.1017/jns.2016.41
115. Tang N, Bai H, Chen X, Gong J, Li D, Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18(5):1094–1099. doi:10.1111/jth.14817
116. Sadeghipour P, Talasaz AH, Rashidi F, et al. Effect of intermediate-dose vs standard-dose prophylactic anticoagulation on thrombotic events, extracorporeal membrane oxygenation treatment, or mortality among patients with COVID-19 admitted to the intensive care unit: the INSPIRATION randomized clinic. JAMA. 2021;325(16):1620–1630. doi:10.1001/jama.2021.4152
117. Lopes RD, Gabriel P, De BM, et al. Therapeutic versus prophylactic anticoagulation for patients admitted to hospital with COVID-19 and elevated D-dimer concentration (ACTION): an open-label, multicentre, randomised, controlled trial. Lancet. 2021;397(10291):2253–2263. doi:10.1016/S0140-6736(21)01203-4
118. Jonmarker S, Hollenberg J, Dahlberg M, et al. Dosing of thromboprophylaxis and mortality in critically ill COVID –19 patients. Crit Care. 2020:1–10. doi:10.1186/s13054-020-03375-7
119. Barnes GD, Burnett A, Allen A, et al. Thromboembolic prevention and anticoagulant therapy during the COVID-19 pandemic: updated clinical guidance from the anticoagulation forum. J Thromb Thrombolysis. 2022;54(2):197–210. doi:10.1007/s11239-022-02643-3
120. Gabara C, Solarat B, Castro P, Fern S. Anticoagulation strategies and risk of bleeding events in critically ill COVID-19 patients. Med Inten. 2023;47(1):1–8. doi:10.1016/j.medin.2021.07.004
121. Piazza G, Campia U, Hurwitz S, et al. Registry of arterial and venous thromboembolic complications in patients with COVID-19. J Am Coll Cardiol. 2020;76(18):2060–2072. doi:10.1016/j.jacc.2020.08.070
122. Goldenberg NA, Sochet A, Albisetti M, et al. Consensus-based clinical recommendations and research priorities for anticoagulant thromboprophylaxis in children hospitalized for COVID-19–related illness. J Thromb Haemost. 2020;18(11):3099–3105. doi:10.1111/jth.15073
123. The REMAP-CAP, ACTIV-4a, and ATTACC Investigators. Therapeutic anticoagulation with heparin in critically ill patients with covid-19. N Engl J Med. 2021;385(9):777–789. doi:10.1056/nejmoa2103417
124. Rey JR, Luis Merino J, Iniesta ÁM, et al. Influencia del tratamiento con estatinas en una cohorte de pacientes ingresados por COVID-19. Med Clin. 2021. doi:10.1016/j.medcli.2021.07.003
125. Rodriguez-Nava G, Trelles-Garcia DP, Yanez-Bello MA, Chung CW, Trelles-Garcia VP, Friedman HJ. Atorvastatin associated with decreased hazard for death in COVID-19 patients admitted to an ICU: a retrospective cohort study. Crit Care. 2020;24(1):4–5. doi:10.1186/s13054-020-03154-4
126. Santosa A, Franzén S, Nåtman J, Wettermark B, Parmryd I, Nyberg F. Protective effects of statins on COVID-19 risk, severity and fatal outcome: a nationwide Swedish cohort study. Sci Rep. 2022;12(1):1–9. doi:10.1038/s41598-022-16357-2
127. Daniels LB, Ren J, Kumar K, et al. Relation of prior statin and anti-hypertensive use to severity of disease among patients hospitalized with COVID-19: findings from the American Heart Association’s COVID-19 cardiovascular disease registry. PLoS One. 2021;16(7 July):1–16. doi:10.1371/journal.pone.0254635
128. Antonazzo IC, Fornari C, Rozza D, et al. Statins use in patients with cardiovascular diseases and COVID-19 Outcomes: an Italian population-based cohort study. J Clin Med. 2022;11(24):7492. doi:10.3390/jcm11247492
129. Barge-caballero E, Marcos-rodríguez PJ, Domenech-garcía N, Mu J. Survival impact of previous statin therapy in patients hospitalized with COVID-19. Med Clín. 2023;160(1):1–9.
130. Yetmar ZA, Challener DW, Tleyjeh IM, et al. Association between chronic statin use and 30-day mortality in hospitalized patients with COVID-19. Mayo Clin Proc Innov Qual Outcomes. 2021;5(2):442–446. doi:10.1016/j.mayocpiqo.2021.02.002
131. Zapata-Cardona MI, Flórez-Álvarez L, Zapata-Builes W, Guerra-Sandoval AL, Guerra-Almonacid CM, Hincapié-García J. Atorvastatin effectively inhibits late replicative cycle steps of SARS-CoV-2 in vitro. bioRxiv. 2021;2021:1. doi:10.1101/2021.03.01.433498
132. Glorieux FH, Pettifor JM. Vitamin D/dietary calcium deficiency rickets and pseudo-vitamin D deficiency rickets. Bonekey Rep. 2014;3(March):1–6. doi:10.1038/bonekey.2014.19
133. Lung BE, Mowery ML, Calcitriol KDEE. xPharm Compr Pharmacol Ref; 2021:1–5. Available from: https://www.ncbi.nlm.nih.gov/books/NBK526025/.
134. Sadeghzadeh M, Khoshnevisasl P, Motamed N, Faghfouri L. The serum vitamin D levels in children with urinary tract infection: a case–control study. New Microbes New Infect. 2021;43:100911. doi:10.1016/j.nmni.2021.100911
135. Nimavat N, Singh S, Singh P, Singh SK, Sinha N. Vitamin D deficiency and COVID-19: a case-control study at a tertiary care hospital in India. Ann Med Surg. 2021;68:102661. doi:10.1016/j.amsu.2021.102661
136. Pereira M, Dantas Damascena A, Galvão Azevedo LM, de Almeida Oliveira T, da Mota Santana J. Vitamin D deficiency aggravates COVID-19: systematic review and meta-analysis. Crit Rev Food Sci Nutr. 2020;1–9. doi:10.1080/10408398.2020.1841090
137. Annweiler C, Beaudenon M, Simon R, et al. Vitamin D supplementation prior to or during COVID-19 associated with better 3-month survival in geriatric patients: extension phase of the GERIA-COVID study. J Steroid Biochem Mol Biol. 2021;213(May):105958. doi:10.1016/j.jsbmb.2021.105958
138. Nasiri M, Khodadadi J, Molaei S. Does vitamin D serum level affect prognosis of COVID-19 patients? Int J Infect Dis. 2021;107:264–267. doi:10.1016/j.ijid.2021.04.083
139. Elham AS, Azam K, Azam J, Mostafa L, Nasrin B, Marzieh N. Serum vitamin D, calcium, and zinc levels in patients with COVID-19. Clin Nutr ESPEN. 2021;43:276–282. doi:10.1016/j.clnesp.2021.03.040
140. Diaz-Curiel M, Cabello A, Arboiro-Pinel R, et al. The relationship between 25(OH) vitamin D levels and COVID-19 onset and disease course in Spanish patients. J Steroid Biochem Mol Biol. 2021;212(January):6–11. doi:10.1016/j.jsbmb.2021.105928
141. Entrenas M, Manuel L, Costa E, Bouillon R, Francisco J. Effect of calcifediol treatment and best available therapy versus best available therapy on intensive care unit admission and mortality among patients hospitalized for COVID-19: a pilot randomized clinical study. J Steroid Biochem Mol Biol. 2020;203:105751. doi:10.1016/j.jsbmb.2020.105751
142. Rawat D, Roy A, Maitra S, Shankar V, Khanna P, Baidya DK. Vitamin D supplementation and COVID-19 treatment: a systematic review and meta-analysis. Diabetes Metab Syndr Clin Res Rev. 2021;15(4):102189. doi:10.1016/j.dsx.2021.102189
143. Güven M, Gültekin H. The effect of high-dose parenteral vitamin D3 on COVID-19-related inhospital mortality in critical COVID-19 patients during intensive care unit admission: an observational cohort study. Eur J Clin Nutr. 2021;75(9):1383–1388. doi:10.1038/s41430-021-00984-5
144. Shah K, Saxena D, Mavalankar D. Vitamin D supplementation, COVID-19 and disease severity: a meta-analysis. Qjm. 2021;114(3):175–181. doi:10.1093/qjmed/hcab009
145. Lin LT, Hsu WC, Lin CC. Antiviral natural products and herbal medicines. J Tradit Complement Med. 2014;4(1):24–35. doi:10.4103/2225-4110.124335
146. Silveira D, Prieto-Garcia JM, Boylan F, et al. COVID-19: is there evidence for the use of herbal medicines as adjuvant symptomatic therapy? Front Pharmacol. 2020;11:1–44. doi:10.3389/fphar.2020.581840
147. Marutescu L, Popa M, Saviuc C, Lazar V, Chifiriuc MC. Botanical pesticides with virucidal, bactericidal, and fungicidal activity. New Pestic Soil Sensors. 2017;311–335. doi:10.1016/B978-0-12-804299-1.00009-6
148. Humayun F, Khan A, Ahmad S, et al. Abrogation of SARS-CoV-2 interaction with host (NRP1) neuropilin-1 receptor through high-affinity marine natural compounds to curtail the infectivity: a structural-dynamics data. Comput Biol Med. 2021:104714. doi:10.1016/j.compbiomed.2021.104714
149. Abebe EC, Ayele TM, Muche ZT, Dejenie TA. Neuropilin 1: a novel entry factor for sars-cov-2 infection and a potential therapeutic target. Biol Targets Ther. 2021;15:143–152. doi:10.2147/BTT.S307352
150. Alhadrami HA, Burgio G, Thissera B, et al. Neoechinulin A as a Promising SARS-CoV-2 M pro Inhibitor: in vitro and in silico study showing the ability of simulations in discerning active from inactive enzyme inhibitors. Mar Drugs Artic. 2022;20(3):163. doi:10.3390/md20030163
151. Mahmoud DB, Ismail WM, Moatasim Y, et al. Delineating a potent antiviral activity of Cuphea ignea extract loaded nano-formulation against SARS-CoV-2: in silico and in vitro studies. J Drug Deliv Sci Technol. 2021;66:102845. doi:10.1016/j.jddst.2021.102845
152. Yang M, Wei J, Huang T, et al. Resveratrol inhibits the replication of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in cultured Vero cells. Phyther Res. 2021;35(3):1127–1129. doi:10.1002/ptr.6916
153. Ter Ellen BM, Kumar ND, Bouma EM, et al. Resveratrol and pterostilbene inhibit sars-cov-2 replication in air–liquid interface cultured human primary bronchial epithelial cells. Viruses. 2021;13(7):1335. doi:10.3390/v13071335
154. Salminen W, Agbaje-williams M, Ajayi FO. A unique formulation of cardioprotective bio-actives: an overview of their safety profile. Medicines. 2019;6(4):107. doi:10.3390/medicines6040107
155. Kemper C, Behnam D, Brothers S, et al. Safety and pharmacokinetics of a highly bioavailable resveratrol preparation (JOTROL TM). AAPS open. 2022;8(1):11. doi:10.1186/s41120-022-00058-1
156. Suručić R, Maja T, Petković M, et al. Pomegranate peel extract polyphenols attenuate the SARS-CoV-2 S-glycoprotein binding ability to ACE2 Receptor: in silico and in vitro studies. Mol Cell Biochem. 2021;476(2):1179–1193. doi:10.1007/s11010-020-03981-7
157. Kandeil A, Mostafa A, Kutkat O, et al. Bioactive polyphenolic compounds showing strong antiviral activities against severe acute respiratory syndrome coronavirus 2. Pathogens. 2021;10(6):758. doi:10.3390/pathogens10060758
158. Tirelli C, De Amici M, Albrici C, et al. Exploring the role of immune system and inflammatory cytokines in sars-cov-2 induced lung disease: a narrative review. Biology. 2023;12(2):177.
159. Food E, Authority S. Refined exposure assessment for curcumin (E 100). EFSA J. 2014;12(10):1–43. doi:10.2903/j.efsa.2014.3876
160. Lao CD, Ruffin MT, Normolle D, et al. Dose escalation of a curcuminoid formulation. BMC Complement Altern Med. 2006;6:4–7. doi:10.1186/1472-6882-6-10
161. Sharma RA, Euden SA, Platton SL, et al. Phase I clinical trial of oral curcumin: biomarkers of systemic activity and compliance. Clin Cancer Res. 2004;10(20):6847–6854. doi:10.1158/1078-0432.CCR-04-0744
162. Pancholi V, Smina TP, Kunnumakkara AB, Maliakel B, Krishnakumar IM. Safety assessment of a highly bioavailable curcumin-galactomannoside complex (CurQfen) in healthy volunteers, with a special reference to the recent hepatotoxic reports of curcumin supplements: a 90-days prospective study. Toxicol Rep. 2021;8(May):1255–1264. doi:10.1016/j.toxrep.2021.06.008
163. Abian O, Ortega-alarcon D, Jimenez-alesanco A, Ceballos-laita L. Structural stability of SARS-CoV-2 3CLpro and identification of quercetin as an inhibitor by experimental screening. Int J Biol Macromol. 2020;164:1693–1703. doi:10.1016/j.ijbiomac.2020.07.235
164. Bhowmik D, Nandi R, Prakash A, Kumar D. Evaluation of flavonoids as 2019-nCoV cell entry inhibitor through molecular docking and pharmacological analysis. Heliyon. 2021;7:e06515. doi:10.1016/j.heliyon.2021.e06515
165. Saeedi-boroujeni A. Anti-inflammatory potential of Quercetin in COVID-19 treatment. J Inflamm. 2021;6:1–9.
166. Miyamoto N, Izumi H, Miyamoto R, et al. Quercetin induces the expression of peroxiredoxins 3 and 5 via the Nrf2/NRF1 transcription pathway. Investig Ophthalmol Vis Sci. 2011;52(2):1055–1063. doi:10.1167/iovs.10-5777
167. Olagnier D, Farahani E, Thyrsted J, et al. SARS-CoV2-mediated suppression of NRF2-signaling reveals potent antiviral and anti-inflammatory activity of 4-octyl-itaconate and dimethyl fumarate. Nat Commun. 2020;11(1):1–12. doi:10.1038/s41467-020-18764-3
168. Carfora V, Spiniello G, Ricciolino R, et al. Anticoagulant treatment in COVID ‑ 19: a narrative review. J Thromb Thrombolysis. 2021;51(3):642–648. doi:10.1007/s11239-020-02242-0
169. Shohan M, Nashibi R, Mahmoudian-sani M. The therapeutic efficacy of quercetin in combination with antiviral drugs in hospitalized COVID-19 patients: a randomized controlled trial. Eur. J. Pharmacol. 2022;914:174615.
170. Belhassan A, Zaki H, Chtita S, et al. Camphor, artemisinin and sumac phytochemicals as inhibitors against COVID-19: computational approach. Comput Biol Med. 2021;136:104758. doi:10.1016/j.compbiomed.2021.104758
171. Li L, Ma L, Hu Y, et al. Natural biflavones are potent inhibitors against SARS-CoV-2 papain-like protease. Phytochemistry. 2022;193(July 2021):112984. doi:10.1016/j.phytochem.2021.112984
172. Xiao T, Cui M, Zheng C, et al. Myricetin Inhibits SARS-CoV-2 viral replication by targeting mpro and ameliorates pulmonary inflammation. Front Pharmacol. 2021;12:1–9. doi:10.3389/fphar.2021.669642
173. Wen CC, Kuo YH, Jan JT, et al. Specific plant terpenoids and lignoids possess potent antiviral activities against severe acute respiratory syndrome coronavirus. J Med Chem. 2007;50(17):4087–4095. doi:10.1021/jm070295s
174. Alhadrami HA, Sayed AM, Sharif AM, Azhar EI, Rateb ME. Olive-derived triterpenes suppress SARS COV-2 main protease: a promising scaffold for future therapeutics. Molecules. 2021;26(9):2654.
175. Carino A, Moraca F, Fiorillo B, et al. Hijacking SARS-CoV-2/ACE2 receptor interaction by natural and semi-synthetic steroidal agents acting on functional pockets on the receptor binding domain. Front Chem. 2020;8(October):1–15. doi:10.3389/fchem.2020.572885
176. Pasquereau S, Nehme Z, Haidar Ahmad S, et al. Resveratrol inhibits hcov-229e and sars-cov-2 coronavirus replication in vitro. Viruses. 2021;13(2):1–11. doi:10.3390/v13020354
177. Zhang B, Swamy S, Balijepalli S, et al. Direct pulmonary delivery of solubilized curcumin reduces severity of lethal pneumonia. FASEB J. 2019;33(12):13294–13309. doi:10.1096/fj.201901047RR
178. Cheng F-J, Huynh T-K, Yang C-S, et al. Hesperidin Is a Potential Inhibitor against. Nutrients. 2021:13. doi:10.3390/nu14010013
179. Sofowora A, Ogunbodede E, Onayade A. The role and place of medicinal plants in the strategies for disease prevention. Afr J Tradit Complement Altern Med. 2013;10(5):210–229. doi:10.4314/ajtcam.v10i5.2
180. Luo L, Jiang J, Wang C, et al. Analysis on herbal medicines utilized for treatment of COVID-19. Acta Pharm Sin B. 2020;10(7):1192–1204. doi:10.1016/j.apsb.2020.05.007
181. Valoyes DC, Palacios Palacios L. Patrones de uso de las plantas medicinales en el Chocó y Cauca (Colombia) [Patterns of use of medicinal plants in Chocó and Cauca (Colombia)]. Cienc en Desarro. 2020;11(2):85–96 Spanish . doi:10.19053/01217488.v11.n2.2020.10583
182. Flórez-álvarez L, Martínez-Moreno J, Zapata-Cardona MI, Galeano E, Alzate-Guarin F, Zapata W. In vitro antiviral activity against SARS-CoV-2 of plant extracts used in Colombian traditional medicine. Vitae. 2022;29(1):1–11. doi:10.17533/udea.vitae.v29n1a347854
183. Jaimes-Gualdron T, Florez-Alvarez L, Zapata-Cardona MI, Rojano BA, Rugeles MT, Zapata-Builes W. Corozo (Bactris guineensis) fruit extract has antiviral activity in vitro against SARS-CoV-2. Funct Foods Health Dis. 2022;12(9):534–546. doi:10.31989/ffhd.v12i9.918
184. Urueña C, Ballesteros-Ramírez R, Gomez-Cadena A, et al. Randomized double-blind clinical study in patients with COVID-19 to evaluate the safety and efficacy of a phytomedicine (P2Et). Front Med. 2022;9:991873. doi:10.3389/fmed.2022.991873
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.