Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 16
Effectiveness of Biologics, Patient-Reported Outcomes, and Clinical Photography in a Subset of Patients with Moderate-to-Severe Psoriasis: Week 12 Results from the Psoriasis Study of Health Outcomes (PSoHO)
Authors Travaglini M , Maul JT, Kors C, Zaheri S, Gerwien J, Müller M, Brnabic A, Sabatino S, Schuster C, Tsai TF
Received 21 June 2023
Accepted for publication 27 September 2023
Published 20 October 2023 Volume 2023:16 Pages 2971—2983
DOI https://doi.org/10.2147/CCID.S426972
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Jeffrey Weinberg
Massimo Travaglini,1 Julia-Tatjana Maul,2,3 Christian Kors,4 Shirin Zaheri,5 Jens Gerwien,6 Michaela Müller,6 Alan Brnabic,6 Silvia Sabatino,6 Christopher Schuster,6,7 Tsen-Fang Tsai8
1Centre for the Treatment of Psoriasis, Di Summa-Perrino Hospital, Brindisi, Italy; 2Department of Dermatology and Venereology, University Hospital of Zurich, Zurich, Switzerland; 3Faculty of Medicine, University of Zurich, Zurich, Switzerland; 4Private Practice Dermatology, Berlin, Germany; 5Department of Dermatology, The Harley Street Clinic, HCA Healthcare UK, London, UK; 6Eli Lilly and Company, Indianapolis, IN, USA; 7Department of Dermatology, Medical University of Vienna, Vienna, Austria; 8Department of Dermatology, National Taiwan University Hospital and National Taiwan University College of Medicine, Taipei City, Taiwan
Correspondence: Tsen-Fang Tsai, Department of Dermatology, National Taiwan University Hospital and National Taiwan University College of Medicine, 7, Chung-Shan South Road, Taipei City, 100225, Taiwan, Tel +886 223123456#65734, Email [email protected]
Purpose: Since skin is highly accessible, clinical photography is a useful tool to visually substantiate the real-world effectiveness outcomes of biologic-treated adults with moderate-to-severe psoriasis (PsO). We report the effectiveness and patient-reported outcomes at Week 12 between anti-interleukin (IL)-17A biologics and other biologics as well as ixekizumab and guselkumab in patients with available clinical photography at baseline and Week 12.
Patients and Methods: The Psoriasis Study of Health Outcomes (PSoHO) is an international, non-interventional, cohort study investigating the effectiveness of biologics in adults with moderate-to-severe psoriasis at Week 12. Outcomes included the proportion of patients who achieved 90% improvement in Psoriasis Area and Severity Index (PASI90) and/or static Physician Global Assessment (sPGA) 0/1 (primary endpoint), PASI100, PASI90, Dermatology Life Quality Index (DLQI), and Itch Numeric Rating Scale (NRS) (secondary endpoints) at Week 12. Data are reported descriptively.
Results: This analysis included 59 biologic-treated (23 anti-IL-17A; 36 other biologics) patients with available clinical photographs from the overall PSoHO study (n=1981). At baseline, the mean (standard deviation [SD]) age was 45.7 (11.1) years, 71.2% were male, 52.5% were bio-experienced and the median (interquartile range) duration of disease was 10.5 (12.4) years. Mean (SD) PASI was 16.9 (9.3) and sPGA was 3.5 (0.8). At Week 12, 65.2%/47.2% of the anti-IL-17A/other biologics cohort achieved the primary outcome. Response rates for PASI90/100 were numerically higher with anti-IL-17A than with other biologics. Patients receiving anti-IL-17A had numerically better outcomes for DLQI 0/1 and Itch NRS than those receiving other biologics at Week 12. Clinical photographs confirmed skin improvements in ixekizumab- and guselkumab-treated patients.
Conclusion: This subgroup analysis showed that anti-IL-17A biologics are effective at rapidly improving signs and symptoms of PsO and improving quality of life. Additionally, serial photography provided visual evidence of biologic treatment response over time.
Keywords: psoriasis, ixekizumab, guselkumab, real-world, clinical photography
Introduction
Plaque psoriasis (PsO) is an immune-mediated chronic inflammatory skin condition for which patients report both physical and psychological symptoms that negatively impact their quality of life.1–4
When considering treatment options, randomized controlled trials (RCTs) are considered the gold standard for providing evidence to inform therapeutic decision-making. However, the stringently controlled RCT environment can be a limiting factor for the extrapolation of the results to a broader population. Real-world studies gather evidence from the heterogeneous patient population encountered in everyday clinical practice. Factors such as prior treatment, comorbidities, age, and polypharmacy are factored into clinical decision-making in this setting, which increases the applicability of the results to a broader population.5–7
Speed of effect, skin clearance, and itch reduction are among the main drivers of patient satisfaction8,9 and are linked to improved quality of life10,11 with differences between male and female patients.12 A recent RCT, the IXORA-R study, reported rapid improvements in skin clearance and quality of life in patients receiving ixekizumab (IXE) versus those receiving guselkumab (GUS).13 An indirect comparison shows that these findings align with other RCTs, which also report favorable outcomes for IXE-treated patients with moderate-to-severe PsO.11,14
An ongoing real-world study, the Psoriasis Study of Health Outcomes (PSoHO), is investigating the effectiveness of anti-interleukin (IL)-17A biologics versus other biologics in adult patients with moderate-to-severe PsO over 3 years.15 The primary endpoint is a 90% improvement in Psoriasis Area and Severity Index (PASI90) and/or static Physician Global Assessment (sPGA) 0/1 at Week 12, and the secondary outcomes include the proportion of patients with PASI75/90/100, absolute PASI scores ≤5, ≤2, and ≤1, and Dermatology Life Quality Index (DLQI) score of 0/1. At Week 12, 71.4% of anti-IL-17A-treated patients had achieved the primary endpoint versus 58.6% of patients treated with other biologics, and when individual biologics were analyzed, IXE recipients had the highest response rate for this endpoint versus the other biologics investigated (74.2% vs 52.8–67.2%); 57.1% of patients receiving GUS achieved the primary endpoint.15 Similar results were seen with the secondary endpoints.15 These findings are consistent with the IXORA-R results, which showed the superiority of IXE versus GUS at Week 12.13 Together these studies provide robust evidence of the efficacy of IXE in both RCT and real-world settings.
This post-hoc analysis focuses on a heterogeneous group of patients from PSoHO with available clinical photography and investigates the improvement in disease scores and patient-reported outcomes (PROs) between two prespecified cohorts as well as those receiving IXE or GUS in a real-world setting, visually substantiated by clinical photography at baseline and Week 12.
Materials and Methods
Study Design
The PSoHO study is an ongoing 36-month, international, prospective, multicenter, non-interventional, cohort observational study, investigating the effectiveness of biologics in patients with moderate-to-severe PsO in the real-world setting. Prescribed biologics were grouped into anti-IL-17A (IXE and secukinumab) and other biologics (brodalumab, adalimumab, certolizumab, etanercept, infliximab, ustekinumab, GUS, risankizumab, and tildrakizumab). The full study design details have been previously published.15 Briefly, patients included were adults (age ≥18 years) with an established diagnosis (≥6 months prior to baseline) of moderate-to-severe PsO who initiated or switched biologic treatment during routine medical care. Exclusion criteria were treatment initiation contraindicated due to country-specific approved indication; changes in dose or dosing intervals of an existing biologic treatment; restart of biologic treatment previously received any time during the patient’s treatment history; prior completion or withdrawal from PSoHO; and current participation in any other PsO study with investigational products.
Clinical Photography
This post-hoc analysis focused on visually comparing the effectiveness of anti-IL-17A versus other biologics, and IXE versus GUS specifically, in patients participating in PSoHO who had available clinical photographs. IXE and GUS were chosen for individual analysis because their treatment groups had more than 10 patients.
Only sites with available, pre-existing standardized camera equipment and procedures in place for serial clinical photography are invited to participate in the photography portion of the study during the baseline visit. Patients who provided consent are undergoing sequential photography of selected lesions or involved areas at baseline, 12 ± 4 weeks, 6 months, and then every 6 months until study completion, as part of their routine care if possible. The same lesion or area is photographed at each visit, under similar lighting conditions and magnifications to enable comparison of serial photographs. Patients with photographs of the same location available at both baseline and Week 12 were included in the subset analysis, while patients with photographs taken in poor lighting or who had extensive tanning or post-inflammatory hyperpigmentation precluding visual assessment were excluded.
Ethics
This study is being conducted in accordance with the ethical principles of the Declaration of Helsinki. Written informed consent to participate in the study and for the publication of clinical photography was obtained from all patients prior to study enrolment. The protocol, amendments and consent documentation were reviewed and approved by local ethical review boards in all participating countries (Table S1). The trial has been registered at the European Network of Centres for Pharmacoepidemiology and Pharmacovigilance (ENCEPP) EUPAS2420716 and was conducted according to Good Pharmacoepidemiology Practices guidelines and the Declaration of Helsinki.
Outcomes
The primary endpoint of the PSoHO study is to compare the proportion of patients treated with anti-IL-17A biologics versus all other biologics who achieved a PASI90 and/or sPGA 0/1 at Week 12 ± 4. Secondary outcomes include several patient-reported measures,15 including the DLQI,17 the Itch Numeric Rating Scale (NRS),18 and the Psoriasis Signs and Symptoms Diary (PSSD),19,20 and physician-reported measures including PASI100, PASI90, and absolute PASI scores ≤5, ≤2, and ≤1. For patients receiving IXE or GUS, baseline photographs were compared with the Week 12 photographs as an additional qualitative assessment to support the PASI and sPGA results.
Statistical Analysis
Statistical analysis of baseline characteristics was descriptive only. Data are reported by drug class and by drug for the individual analysis. Continuous variables are described using mean and standard deviation (SD) or median and interquartile range (IQR). Categorical variables are described using n and percentages. Unadjusted efficacy outcome results were reported descriptively with 95% confidence intervals (CI) calculated using the normal approximation. Missing data were imputed using non-responder imputation.
Results
This post-hoc analysis included a subset of 59 biologic-treated patients with moderate-to-severe PsO from the overall PSoHO cohort (n=1981)15 who had available clinical photography at baseline and Week 12. Included patients were enrolled from six countries (Argentina, n=2; Colombia, n=1; Germany, n=5; Italy, n=17; Mexico, n=4; Poland, n=1; and Taiwan, n=29).
Baseline Characteristics
At baseline, 23 (39.0%) patients with available clinical photography initiated anti-IL-17A therapy (IXE, n=19; secukinumab, n=4) and 36 (61.0%) patients with available clinical photography initiated other biologics (adalimumab, n=7; brodalumab, n=3; etanercept, n=4; GUS, n=18; infliximab, n=1; risankizumab, n=2; ustekinumab, n=1). Overall, the majority of patients were male (71.2%) with a median (IQR) disease duration of 10.5 (12.4) years; 52.5% were bio-experienced and 23.7% had comorbid psoriatic arthritis (PsA). For the overall study cohort, baseline results for mean (SD) sPGA, PASI, DLQI, and Itch NRS are summarized in Table 1. Baseline characteristics were generally similar between individual treatment groups (Table 1). There were some differences in characteristics between the anti-IL-17A and other biologics cohorts, including disease duration, body surface area and previous biologic use. For the IXE and GUS cohorts, numerical between-group differences included age, PSSD signs score, and PSSD symptoms score.
 |
Table 1 Baseline Characteristics of Patients with PsO and Clinical Photography |
Physician-Reported Outcomes
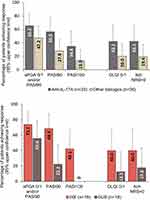
Generally, the physician-reported outcomes favored anti-IL-17A versus other biologics and IXE versus GUS but the results were not statistically significant. The primary endpoint of PASI90 and/or sPGA 0/1 was achieved by a higher proportion of patients receiving anti-IL-17A than in those receiving other biologics, and more patients receiving anti-IL-17A achieved PASI90 and PASI100 than patients receiving other biologics (Figure 1). Considering individual treatments, higher proportions of patients receiving IXE achieved PASI90 and/or sPGA 0/1, and PASI90, than those receiving GUS; 42.1% of patients receiving IXE achieved PASI100 while no patients receiving GUS achieved PASI100 (Figure 1).
Patient-Reported Outcomes
Generally, PROs were numerically higher for patients receiving anti-IL-17A than those receiving other biologics (Figures 1 and 2). Nearly double the proportion of patients receiving anti-IL-17A achieved a DLQI score of 0/1 than those receiving other biologics, and a similar outcome was observed for Itch NRS (Figure 1). Again, patients receiving IXE had numerically better outcomes for both DLQI score 0/1 and Itch NRS than those receiving GUS. Mean PSSD signs and symptoms scores for anti-IL-17A and other biologics followed similar trends, with a reduction from baseline to Week 12 (Figure 2); at Week 12 patients receiving anti-IL-17A had numerically greater improvements in mean scores than those receiving other biologics. Similarly, patients receiving IXE had numerically greater improvements in mean scores at Week 12 than those receiving GUS.
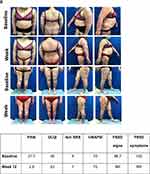
Clinical photographs generally showed a visual improvement in skin appearance following treatment with IXE (Figure 3) or GUS (Figure 4) and select examples are shown in Figures S1 and S2.
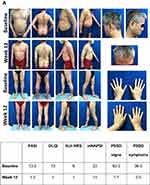 |
Figure 4 Continued. |
Discussion
In this post-hoc analysis, patients with moderate-to-severe PsO and available clinical photography receiving anti-IL-17A biologics demonstrated numerically higher scores for improvement in skin and quality of life than those receiving other biologics. Considering the individual treatment groups, numerically higher scores for improvement in skin and quality of life were observed in patients receiving IXE than GUS. Since PSoHO is a real-world study, a more heterogeneous patient population was included than the populations included in RCTs, making it more representative of patients encountered in the real-world setting. In addition, we were able to use clinical photography obtained during routine clinic visits to visually substantiate the effectiveness outcomes.
Patients receiving IXE and GUS in this study were a similar age to those in the IXORA-R study,13 but the duration of disease and previous biologic use was numerically longer and higher in this study, respectively; however, in a subgroup analysis of the PSoHO population by Lynde et al,21 patients grouped by age and disease duration had similar response rates to the overall population, suggesting that these baseline characteristics are not likely to substantially influence the effectiveness of the biologics investigated. Of note, patients with PsA in the anti-IL-17A cohort had significantly higher odds of high-level skin clearance than the other biologics cohort. Likewise, the odds of achieving clearance in difficult-to-treat body areas like the scalp, nails, or genital region was significantly higher among patients receiving anti-IL-17A biologics compared to other biologics. These findings underscore the importance of considering the presence of comorbidities such as PsA or involvement in special body areas when determining the most appropriate treatment options.21,22
Physician-assessed skin improvements (sPGA and/or PASI90, PASI90, and PASI100) were numerically higher for anti-IL-17A-treated patients than the other biologics cohort, findings that are consistent with the overall PSoHO study.15 On the other hand, results of a network meta-analysis that classed biologics as IL-17, IL-12/-23 and tumor necrosis factor inhibitors, reported overlap between individual treatments within the IL-17 class (including brodalumab) and IL-12/-23 class for rapid skin clearance (PASI90 at Week 12). The authors concluded that individually, IXE and brodalumab provided the most rapid skin clearance versus other biologics.11,23 Similarly, the numerically greater effectiveness of IXE versus GUS for skin improvements at Week 12 found in this analysis reflects the results observed in the IXORA-R trial.13 The IXORA-R trial assessed efficacy, safety, and speed of response for IXE-treated patients versus GUS-treated patients with moderate-to-severe PsO. At the 12-week primary endpoint, significantly more IXE-treated patients achieved rapid skin clearance than GUS-treated patients, and IXE was found to be non-inferior to GUS with respect to complete skin clearance at Week 24.13,24 Of note, in IXORA-R, IXE cleared skin more rapidly and with a greater cumulative benefit than GUS,13 and further analyses of the PSSD data from PSoHO has shown that patients receiving IXE had a significantly larger percentage change from baseline than GUS in PSSD symptoms scores over Weeks 1–12 and signs scores over Weeks 2–12,20 giving further weight to the supportive nature of the PSoHO results when compared with those from IXORA-R. Another real-world study of patients registered in the Austrian Psoriasis Registry (PSoRA) found that IXE had the greatest PASI improvement at 3 months and the highest long-term PASI improvement of all the biologics tested.25 Furthermore, van Muijen et al26 investigated a direct comparison of the real-world effectiveness of biologics in patients from a Dutch psoriasis registry. Patients receiving IXE and those receiving GUS experienced better results for both absolute and relative PASI outcomes than those receiving other biologics; however, the proportion of patients achieving PASI90 was relatively lower in comparison with RCTs. Regarding the long-term effectiveness of IXE in the real world, drug survival was investigated over a 3-year period in an Italian population with chronic plaque psoriasis.27 Drug survival for IXE remained above 80% during the study period without significant impact from age, gender, body mass index or comorbid PsA or bio-experience. Skin clearance is an important treatment goal for patients with PsO; patients achieving total skin clearance (PASI100) report significant improvements in health-related quality of life.28 Outcomes from RCT and real-world observational studies report proportions of GUS-treated patients achieving PASI100;13,29,30 these studies included more participants (n=33–507) than the present study (n=18) and could explain why no GUS-treated patients in this study achieved PASI100. Of note, GUS-treated patients in our study appeared to be more bio-experienced and consequently more challenging to treat—a finding that was also reported by Pinter et al15 and Torres et al.31 While not conclusive, it was suggested time from approval may have contributed to this finding and further investigation is warranted.31 The GUS group also had a higher proportion of patients with comorbid PsA than the IXE group (38.9% vs 26.3%), and the previous subgroup analysis of the PSoHO results reported that patients with comorbid PsA had considerably higher odds of achieving PASI100 with IXE versus GUS,21 which could be another reason why PASI100 was not achievable for patients receiving GUS in this cohort. Furthermore, pivotal studies investigating IL-23 inhibitors such as GUS report the primary efficacy endpoint at Week 16 and a plateau in efficacy that occurs around Week 20 for both IXE and GUS;32–34 it is possible that GUS-treated patients in this study did not achieve PASI100 as the maximal response with GUS was not reached at the Week 12 primary endpoint.
Results for PROs (DLQI, Itch NRS, and PSSD signs and symptoms) were numerically better for patients receiving anti-IL-17A biologics than for patients in the other biologics cohort. Again, these findings are consistent with the overall PSoHO study and additional studies for DLQI and Itch NRS/PSSD, respectively.15,20,35 Considering individual treatments, patients receiving IXE achieved numerically better PROs than patients receiving GUS. The IXORA-R study also assessed DLQI and Itch NRS at the 12-week preliminary endpoint and reported significantly more patients receiving IXE reached a DLQI score of 0 and/or an Itch NRS score of 0 (indicating no impact of disease on quality of life and/or complete resolution of itch) than patients receiving GUS.13
While the IXORA-R and PSoHO studies have included PROs—such as PSSD—a limited number of other IXE studies have done the same. A review by Reich et al36 found that mostly non-comparative studies had assessed PROs with IXE. Baseline data for age and bio-experience of the non-comparative studies were similar to the IXE cohort in our study, although the prevalence of PsA differed with a higher prevalence across the non-comparative studies than our IXE cohort. The findings of the review suggested that IXE-treated patients had better or similar treatment adherence and persistence than other biologics, and that further studies investigating PROs are indicated for a better understanding of the real-world health-related quality of life. In contrast, a pooled study of the Phase 3 VOYAGE 1 and VOYAGE 2 trials reported favorable PROs for patients receiving GUS versus adalimumab.32
Sequential clinical photography is a useful tool for qualitatively assessing response to treatment and can be used to confirm investigator-reported outcomes. In the present analysis, improvements in physician- and patient-assessed outcomes were supported by clinical photography that visualized the skin improvements. To the best of our knowledge, only one other study has investigated efficacy outcomes of IXE alongside clinical photography results in patients with moderate-to-severe PsO.37 The single-arm study reported rapid and visible improvements in PsO with IXE in as little as 1 week of treatment.
PSoHO is an observational study and is subject to various forms of bias, including selection bias, participation bias, or measurement error. Different countries have various levels of access to treatment and not all treatments are registered or reimbursed, potentially increasing the variability within some treatment groups. This was a descriptive study only; no statistical comparisons were made between groups at Week 12, and no adjustments were made for differences in patient and disease characteristics or treatments between groups at baseline. The analysis had a small sample size; only individual treatment groups with more than ten patients with available clinical photography at baseline and Week 12 were included, and as a result this cohort might not be representative of the total study population. In addition, patients included in the analysis were sourced only from those centers with available camera equipment, which could also have introduced bias. Not including brodalumab in the anti-IL-17A group is also a potential limitation of the analysis; to investigate the impact of including brodalumab in the anti-IL-17A group, we are conducting another analysis for a future publication. This manuscript focuses on improvements for patients between baseline and Week 12, and thus lacks a longer-term view of the disease course in patients receiving these treatments. Analyses are underway utilizing longer-term data from PSoHO, to be published in due course.
Conclusions
This PSoHO subgroup analysis showed that anti-IL-17A biologics are effective at rapidly improving the signs and symptoms of PsO and improving patient quality of life, and that serial clinical photography can provide visual evidence of biologic treatment response over time that supports the more quantitative assessment of disease improvements provided by PASI and sPGA.
Abbreviations
BMI, body mass index; BSA, body surface area; CI, confidence intervals; DLQI, Dermatology Life Quality Index; ENCEPP, European Network of Centres for Pharmacoepidemiology and Pharmacovigilance; IL-17A, anti-interleukin 17A; IQR, interquartile range; IXE, ixekizumab; GUS, guselkumab; mNAPSI, modified Nail Psoriasis Severity Index; NRS, Numeric Rating Scale; PASI90, 90% improvement in Psoriasis Area and Severity Index; PASI100, 100% improvement in Psoriasis Area and Severity Index; PROs, patient-reported outcomes; PSoHO, Psoriasis Study of Health Outcomes; PsO, psoriasis; PSoRA, Austrian Psoriasis Registry; PSSD, Psoriasis Signs and Symptoms Diary; PsA, psoriatic arthritis; RCTs, randomized controlled trials; SD, standard deviation; sPGA, static Physician Global Assessment.
Data Sharing Statement
Data available from the corresponding author upon request.
Consent for Publication
All authors consent to the publication of the manuscript.
Acknowledgments
The authors would like to acknowledge Jane Snowball and Sheridan Henness, PhD (Rx Communications, Mold, UK) for medical writing assistance with the preparation of this manuscript, funded by Eli Lilly and Company. Photographs in Figures 3 and 4 are subject to Copyright © 2023 Eli Lilly and Company. All rights reserved.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was funded by Eli Lilly and Company.
Disclosure
With no relation to the present manuscript Julia-Tatjana Maul has served as advisor and/or received speaking fees and/or participated in clinical trials sponsored by AbbVie, Almirall, Amgen, BMS, Celgene, Eli Lilly, LEO Pharma, Janssen-Cilag, MSD, Novartis, Pfizer, Pierre Fabre, Roche, Sanofi, and UCB. Jens Gerwien, Michaela Müller, Alan Brnabic, and Christopher Schuster are employees and minor share holders of Eli Lilly. Tsen-Fang Tsai is an investigator and/or consultant for Boehringer Ingelheim, AbbVie, Bristol-Myers Squibb, Celgene, Eli-Lilly, Janssen-Cilag, Novartis, Pfizer, and Sanofi, outside the submitted work.
References
1. Sondermann W, Schreiber A, Körber A, et al. Psychosocial burden and body mass index are associated with dermatology-related quality of life in psoriasis patients. Eur J Dermatol. 2020;30(2):140–147. doi:10.1684/ejd.2020.3755
2. Sahin E, Hawro M, Weller K, et al. Prevalence and factors associated with sleep disturbance in adult patients with psoriasis. J Eur Acad Dermatol Venereol. 2022;36(5):688–697. doi:10.1111/jdv.17917
3. Schadler ED, Ortel B, Mehlis SL. Biologics for the primary care physician: review and treatment of psoriasis. Disease-A-Month. 2019;65(3):51–90. doi:10.1016/j.disamonth.2018.06.001
4. Nazik H, Nazik S, Gul FC. Body image, self-esteem, and quality of life in patients with psoriasis. Indian Derm. 2017;8(5):343.
5. LoCasale RJ, Pashos CL, Gutierrez B, et al. Bridging the gap between RCTs and RWE through endpoint selection. Ther Innov Regul Sci. 2021;55(1):90–96. doi:10.1007/s43441-020-00193-5
6. Nast A, Smith C, Spuls PI, et al. EuroGuiDerm Guideline on the systemic treatment of Psoriasis vulgaris–Part 1: treatment and monitoring recommendations. J Eur Acad Derm Venereol. 2020;34(11):2461–2498. doi:10.1111/jdv.16915
7. Nast A, Smith C, Spuls PI, et al. EuroGuiDerm Guideline on the systemic treatment of Psoriasis vulgaris–Part 2: specific clinical and comorbid situations. J Eur Acad Derm Venereol. 2021;35(2):281–317. doi:10.1111/jdv.16926
8. Gorelick J, Shrom D, Sikand K, et al. Understanding treatment preferences in patients with moderate to severe plaque psoriasis in the USA: results from a cross-sectional patient survey. Dermatol Ther. 2019;9(4):785–797. doi:10.1007/s13555-019-00334-1
9. Maul JT, Navarini AA, Sommer R, et al. Gender and age significantly determine patient needs and treatment goals in psoriasis–a lesson for practice. J Eur Acad Derm Venereol. 2019;33(4):700–708. doi:10.1111/jdv.15324
10. Papp KA, Lebwohl MG. Onset of action of biologics in patients with moderate-to-severe psoriasis. J Drugs Dermatol. 2018;17(3):247–250.
11. Warren RB, See K, Burge R, et al. Rapid response of biologic treatments of moderate-to-severe plaque psoriasis: a comprehensive investigation using Bayesian and frequentist network meta-analyses. Dermatol Ther. 2020;10(1):73–86. doi:10.1007/s13555-019-00337-y
12. Maul JT, Augustin M, Sorbe C, et al. Association of sex and systemic therapy treatment outcomes in psoriasis: a two‐country, multicentre, prospective, noninterventional registry study. Br J Dermatol. 2021;185(6):1160–1168. doi:10.1111/bjd.20387
13. Blauvelt A, Papp K, Gottlieb A, et al. A head‐to‐head comparison of ixekizumab vs. guselkumab in patients with moderate‐to‐severe plaque psoriasis: 12‐week efficacy, safety and speed of response from a randomized, double‐blinded trial. Br J Dermatol. 2020;182(6):1348–1358. doi:10.1111/bjd.18851
14. Armstrong AW, Soliman AM, Betts KA, et al. Comparative efficacy and relative ranking of biologics and oral therapies for moderate-to-severe plaque psoriasis: a network meta-analysis. Dermatol Ther. 2021;11(3):885–905. doi:10.1007/s13555-021-00511-1
15. Pinter A, Puig L, Schäkel K, et al. Comparative effectiveness of biologics in clinical practice: week 12 primary outcomes from an international observational psoriasis study of health outcomes (PSoHO). J Eur Acad Dermatol Venereol. 2022;36(11):2087–2100. doi:10.1111/jdv.18376
16. European Network of Centres for Pharmacoepidemiology and Pharmacovigilance (ENCEPP); 2018. Available from: https://www.encepp.eu/encepp/viewResource.htm?id=25115.
17. Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI)--A simple practical measure for routine clinical use. Clin Exp Dermatol. 1994;19(3):210–216. doi:10.1111/j.1365-2230.1994.tb01167.x
18. Kimball AB, Naegeli AN, Edson‐Heredia E, et al. Psychometric properties of the Itch Numeric Rating Scale in patients with moderate‐to‐severe plaque psoriasis. Br J Dermatol. 2016;175(1):157–162. doi:10.1111/bjd.14464
19. Mathias SD, Feldman SR, Crosby RD, Colwell HH, McQuarrie K, Han C. Measurement properties of a patient-reported outcome measure assessing psoriasis severity: the psoriasis symptoms and signs diary. J Dermatol Treat. 2016;27(4):322–327. doi:10.3109/09546634.2015.1114567
20. Reich A, Pinter A, Maul J-T, et al. Speed of clinical improvement in the real-world setting from Patient-Reported Psoriasis Symptoms and Signs Diary (PSSD): secondary outcomes from the Psoriasis Study of Health Outcomes (PSoHO) through 12 weeks. J Eur Acad Dermatol Venereol. 2023;37(9):1825–1840. doi:10.1111/jdv.19161
21. Lynde C, Riedl E, Maul JT, et al. Comparative effectiveness of biologics across subgroups of patients with moderate-to-severe plaque psoriasis: results at Week 12 from the PSoHO Study in a real-world setting. Adv Ther. 2023;40(3):869–886. doi:10.1007/s12325-022-02379-9
22. Piaserico S, Riedl E, Pavlovsky L, et al. Comparative effectiveness of biologics for patients with moderate-to-severe psoriasis and special area involvement: week 12 results from the observational Psoriasis Study of Health Outcomes (PSoHO). Front Med. 2023;10:1257. doi:10.3389/fmed.2023.1185523
23. Augustin M, Schuster C, Mert C, Nast A. The value of indirect comparisons of systemic biologics for psoriasis: interpretation of efficacy findings. Dermatol Ther. 2022;12(8):1711–1727. doi:10.1007/s13555-022-00765-3
24. Blauvelt A, Leonardi C, Elewski B, et al. A head-to-head comparison of ixekizumab vs. guselkumab in patients with moderate-to-severe plaque psoriasis: 24-week efficacy and safety results from a randomized, double-blinded trial. Br J Dermatol. 2021;184(6):1047–1058. doi:10.1111/bjd.19509
25. Graier T, Salmhofer W, Jonak C, et al. Biologic drug survival rates in the era of anti-interleukin-17 antibodies: a time-period-adjusted registry analysis. Br J Dermatol. 2021;184(6):1094–1105. doi:10.1111/bjd.19701
26. van Muijen ME, Thomas SE, Groenewoud H, et al. Direct comparison of real-world effectiveness of biologics for psoriasis using Absolute and Relative Psoriasis Area and Severity Index Scores in a prospective multicentre cohort. Acta Derm Venereol. 2022;102:adv00711. doi:10.2340/actadv.v102.206
27. Caldarola G, Chiricozzi A, Megna M, et al. Real-life experience with ixekizumab in plaque psoriasis: a multi-center, retrospective, 3-year study. Expert Opin Bio Ther. 2023;23(4):365–370. doi:10.1080/14712598.2023.2193288
28. Viswanathan HN, Chau D, Milmont CE, et al. Total skin clearance results in improvements in health-related quality of life and reduced symptom severity among patients with moderate to severe psoriasis. J Dermatol Treat. 2015;26(3):235–239. doi:10.3109/09546634.2014.943687
29. Galluzzo M, Tofani L, Lombardo P, et al. Use of guselkumab for the treatment of moderate-to-severe plaque psoriasis: a 1 year real-life study. J Clin Med. 2020;9(7):2170. doi:10.3390/jcm9072170
30. Snast I, Sherman S, Holzman R, Hodak E, Pavlovsky L. Real‐life experience of guselkumab in patients with psoriasis. Dermatol Ther. 2020;33(6):e13964. doi:10.1111/dth.13964
31. Torres T, Puig L, Vender R, et al. Drug survival of interleukin (IL)‑17 and IL‑23 inhibitors for the treatment of psoriasis: a retrospective multi‑country, multicentric cohort study. Am J Clin Dermatol. 2022;23(6):891–904. doi:10.1007/s40257-022-00722-y
32. Armstrong AW, Reich K, Foley P, et al. Improvement in patient-reported outcomes (dermatology life quality index and the psoriasis symptoms and signs diary) with guselkumab in moderate-to-severe plaque psoriasis: results from the Phase III VOYAGE 1 and VOYAGE 2 studies. Am J Clin Dermatol. 2019;20(1):155–164. doi:10.1007/s40257-018-0396-z
33. Langley RG, Tsai TF, Flavin S, et al. Efficacy and safety of guselkumab in patients with psoriasis who have an inadequate response to ustekinumab: results of the randomized, double‐blind, phase III NAVIGATE trial. Br J Dermatol. 2018;178(1):114–123. doi:10.1111/bjd.15750
34. Reich K, Armstrong AW, Foley P, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with Adalimumab for the treatment of patients with moderate to severe psoriasis with randomized withdrawal and retreatment: results from the Phase III, double-blind, placebo-and active comparator–controlled VOYAGE 2 trial. J Am Acad Dermatol. 2017;76(3):418–431. doi:10.1016/j.jaad.2016.11.042
35. Proietti I, Bernardini N, Skroza N, et al. PSSD and biologic therapy: real-life data in 417 patients with moderate to severe psoriasis. Rev Recent Clin Trials. 2022;17(3):171–176. doi:10.2174/1574887117666220623161751
36. Reich A, Reed C, Schuster C, Robert C, Treuer T, Lubrano E. Real-world evidence for ixekizumab in the treatment of psoriasis and psoriatic arthritis: literature review 2016–2021. J Dermatol Treat. 2023;34(1):2160196. doi:10.1080/09546634.2022.2160196
37. Khattri S, Goldblum O, Solotkin K, et al. Early onset of clinical improvement with ixekizumab in a randomized, open-label study of patients with moderate-to-severe plaque psoriasis. J Clin Aesthet Dermatol. 2018;11(5):33–37.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.