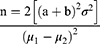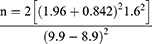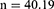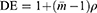Back to Journals » Open Access Journal of Clinical Trials » Volume 13
Effectiveness of a Structured Nutrition Education Course for Caregivers of Children and Adolescents with Type 1 Diabetes in Improving Glycemic and Dietary Outcomes: A Cluster-Randomized Controlled Trial Protocol
Authors Ndahura NB , Munga J, Kimiywe J , Mupere E
Received 28 January 2021
Accepted for publication 16 March 2021
Published 19 April 2021 Volume 2021:13 Pages 1—10
DOI https://doi.org/10.2147/OAJCT.S304290
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Arthur E. Frankel
Nicholas Bari Ndahura,1,2 Judith Munga,1 Judith Kimiywe,1 Ezekiel Mupere3
1Department of Food, Nutrition, and Dietetics, Kenyatta University, Nairobi, Kenya; 2Department of Human Nutrition and Home Economics, Kyambogo University, Kampala, Uganda; 3Department of Pediatrics and Child Health, Makerere University, Kampala, Uganda
Correspondence: Nicholas Bari Ndahura
Department of Human Nutrition and Home Economics, Kyambogo University, P.O. Box 1 Kyambogo, Kampala, Uganda
Tel +256 772636271
Email [email protected]
Purpose: This study will aim to evaluate whether the provision of a structured nutrition education course to caregivers of children and adolescents with type 1 diabetes mellitus (T1DM) will help improve their children’s glycemic control, dietary intake, and diversity.
Research Design and Methods: The study will be a cluster randomized controlled trial conducted at 10 health facilities with established T1DM clinics in Uganda. The facilities will include: Mulago National Referral Hospital, St. Francis Hospital, Lubaga Hospital, Mbale Regional Referral Hospital, Soroti Regional Referral Hospital, Holy innocents’ Hospital, Virika Hospital, Kagando Hospital, Nyakibale Hospital, and Wakiso Health Centre IV. The facilities will be randomized to control or intervention at a ratio of 1:1. A total of 100 caregiver-child pairs will be recruited. The participants in the control group will continue to receive routine medical care, while those in the intervention group will receive routine medical care and attend a structured group nutrition education course. The course will be delivered over 3 months, it will consist of a total of 8 face-to-face sessions lasting 45 minutes each. A two-member team of a diabetes specialist nurse and dietician will conduct the sessions. Each session will be conducted once a week and a question-and-answer session held every after 2 sessions. The primary outcome which is a change in glycated hemoglobin (HbA1c) and secondary outcomes (caregivers’ level of knowledge on general and diabetes-specific nutrition knowledge, children’s dietary diversity score, and children’s mean intake of energy, protein, and fat) will be assessed at baseline, 3, and 6 months. Intention-to-treat analysis will be conducted. Data will be reported according to the Consolidated Standards of Reporting Trials (CONSORT) statement for cluster-randomized trials. The trial is registered with the Pan African Clinical Trials Registry (PACTR201902548129842).
Keywords: glycated hemoglobin, diabetes, glycemic control, caregivers, Uganda
Introduction
Type 1 diabetes mellitus (T1DM) is one of the major forms of diabetes that affects children worldwide.1 T1DM is caused by the body’s autoimmune response leading to the destruction of the insulin-producing cells, therefore individuals with T1DM produce very little or no insulin. The reason why this happens is not yet fully understood but it has been attributed to several factors.2,3 Dietary components, viral infections and toxins have been proposed as possible environmental factors contributing to the pathogenesis of T1DM.4
Prevalence and incidence of T1DM vary markedly among countries but globally it is estimated that 600,900 children and adolescents (aged 0–14 years) have T1DM, with 98,200 new cases each year. The largest number of children and adolescents with new and existing T1DM are in India, the United States of America, and Brazil. However, in terms of incidence per 100,000 population per year, Scandinavian populations such as Finland (62.3) and Sweden (43.2) have the highest incidence rates of T1DM among children and adolescents aged 0 to 14 years. In Africa, it is estimated that 25,800 children and adolescents (aged 0–19 years) have T1DM, with 10,300 newly diagnosed cases each year.2 T1DM is the most predominant form of diabetes in African children,5 several studies in Africa reported the prevalence of T1DM as 0.33 per 1000 in Nigerian children and 0.95 per 1000 in Sudanese school children.6,7 In Tanzania and Sudan, the incidence was reported at 1.5 per 100,000 per year and 10.1 per 100,000 per year respectively.8,9 A recent survey of TIDM prevalence and incidence in Rwanda reported that the prevalence of TIDM at 16.4 per 100,000 in those less than 26 years, and 4.8 per 100,000 in those less than 15 years. Incidence figures were 2.7 per 100,000 per year for those less than 26 years and 1.2 per 100,000 per year for those less than 15 years.10 In Uganda, there have not been any published studies that document the incidence or prevalence of T1DM. However, recent data from 32 T1DM clinics indicates enrolment of 1187 children, this is on the increase from about 150 enrolled children in 2009.11,12 Despite the improvements in pediatric T1DM care and management in Uganda over the years, several barriers to standard care of pediatric diabetic patients in Uganda still exist such as lack of adequately trained health professionals, limited availability of insulin, injection devices and self-monitoring consumables such as test strips and glucometers and high cost of management and care relative to household income. Furthermore, T1DM places a profound emotional burden on families with children and adolescents with T1DM.5,13
A health facility-based study conducted in Uganda in 2018 reported more than 80% of the children having poorly controlled blood glucose levels with a mean HbA1c level of 9.7%. It was further noted that adherence to dietary recommendations was low, and most likely characterized of a high intake of saturated fat and low fruit and vegetable intake. However, it was suggested that reinforcing caregiver involvement in the children’s diet could help improve adherence to dietary recommendations.14,15 In Tanzania, a study reported a significant association between caregivers’ knowledge of diabetes and HbA1c levels.16 A parent-based nutrition educational intervention targeting mealtime behaviors reported a decrease in mean daily blood glucose levels among children with type 1 diabetes.17 However, in the United Kingdom, a structured education course for 11 to 16 year olds with T1DM reported no change in HbA1c among the participants.18
Patient and parent or caregiver-centered nutrition education is little explored in sub-Saharan Africa, despite being a fundamental component of diabetes education. When made easy to understand, knowledge-based, patient and parent or caregiver centered, nutrition education improves glycaemic control and helps prevent the development of complications.19,20 It is therefore vital that children diagnosed with T1DM and their caregivers be educated and trained with adequate nutritional management skills and knowledge to enable them to manage and survive the onset of T1DM safely and successfully.21–23 The nutritional goal for individuals with diabetes is to attain and sustain near-normal blood glucose levels by ensuring proper management of insulin therapy, physical activity, and diet. However, in children, it is important that the diet also provides for their other macro and micro-nutritional needs to ensure normal growth and development. Therefore, caregivers empowered with updated nutrition knowledge through nutrition education can serve as change agents and therefore bring about an improvement in their children’s feeding behaviour.24
In Uganda, there is no documented information of any study conducted to establish if nutrition education of caregivers of type 1 diabetic children affects their nutrition knowledge and their children’s glycemic control and dietary practices. Furthermore, the nutrition education module in the current diabetes education curriculum is not contextualized for the Ugandan pediatric T1DM patients and yet structured nutrition education is vital in the management of diabetes in children as it strengthens their ability to successfully manage their condition using available resources.25,26 Therefore, the developed structured nutrition education course will be the first Ugandan contextualized nutrition education course specifically developed for caregivers of children and adolescents with T1DM.
The primary objective of the study is to evaluate whether the provision of a structured nutrition education course to caregivers of children and adolescents with T1DM will help improve their children’s glycemic control, dietary intake and diversity compared with the usual health education.
Research Design and Methods
Study Setting
The study will be conducted at 10 T1DM clinics in Uganda. These clinics where purposively selected because they have established pediatric type 1 diabetes clinics.12 The clinics will be randomly allocated to the control or intervention group at a ratio of 1:1.
Study Design
The study design as indicated in the CONSORT flow diagram (Figure 1) will be a cluster randomized controlled trial that is CONSORT guidelines compliant.27 T1DM clinics (clusters) rather than individuals will be randomly allocated to the intervention and control groups.28 Outcomes will be assessed at three-time points: baseline, 3, and 6 months. The control group will also receive the structured nutrition education course after the post-intervention data collection.
 |
Figure 1 CONSORT diagram showing flow of study.Notes: Adapted from Schulz KF, Altman DG, Moher D, CONSORT Group. CONSORT 2010 statement: updated guidelines for reporting parallel group randomized trials. Annals of Internal Medicine. 2010;152(11):726-732. Copyright © 2010, The American College of Physicians.27 |
The participants in the control group will continue to receive routine medical care and the usual health education. The intervention group will also continue to receive routine medical care and health education; however, they will also attend structured group nutrition education sessions. The nutrition education sessions will be conducted using food demonstrations and audio-visual aids. In addition, they will receive nutrition education materials (posters and brochures) for further reference.
Study Duration
The total study duration is 36 months, with the intervention (structured nutrition education course) being delivered over 3 months with 3 months post-intervention follow-up. Data collection started in 2019.29
Sampling
A mixed-methods sampling technique will be used.30 10 T1DM clinics will be purposively selected. Simple random sampling will be used to distribute the clusters into the intervention and control groups. Consecutive sampling will be used to select the study participants in each cluster due to the low number of participants in the age group (3–14 years).31
Randomization
The study will comprise two study groups; a control group and an intervention group. 10 T1DM clinics will be randomly assigned using a formula generated in Microsoft Office Excel 2016 to either intervention or control group at a ratio of 1:1. The biostatistician will be blinded to control for bias during data analysis. However, due to the nature of the intervention, the study participants will not be blinded.
Sample Size Calculation
To have 80% power to detect a 1.0% difference in HbA1c; the sample size (n) was calculated using the following formula.32,33
Considerations
n = the sample size in each of the groups
μ1= population mean in intervention group (HbA1c = 9.9)34
μ2 = population mean in control group (HbA1c = 8.9)
μ1-μ2 = the difference the investigator wishes to detect
σ2 = standard deviation (1.6)34
The power of the test was set at 80% and significance at 5%
a = 1.96 (conventional multiplier for alpha = 0.05)
b = 0.842 (conventional multiplier for power = 0.80, (beta = 0.20)
To adjust for the clustering effect the sample size was inflated by a design effect (DE) to get the adjusted sample size.35
Where 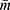
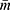 is the average cluster size as the clusters vary in size and ρ is the intra-cluster correlation coefficient (ICC). Since there is no previous study documenting the ICC, an ICC of 0.01 was considered and an average cluster size of 12 was considered.
is the average cluster size as the clusters vary in size and ρ is the intra-cluster correlation coefficient (ICC). Since there is no previous study documenting the ICC, an ICC of 0.01 was considered and an average cluster size of 12 was considered.
The calculated sample size is 40.19 * DE (1.11) = 44.61. The sample size will be increased by 10% to 49.07 participants per study group to cater for loss to follow-up. This was rounded off to 50 respondents in each study group, therefore a total of 100 study participants will be recruited.
Recruitment of Participants
Caregivers of/and children with T1DM at the diabetes clinics will be informed about the objectives of the study and the inclusion criteria. Those caregiver-child pairs that accept to participate in the study and meet the inclusion criteria will be asked to give their consent and assent respectively. The diabetes clinics are currently not aware of whether they are in the intervention or control group.
Inclusion Criteria
Caregivers of/and children diagnosed with T1DM aged between 3–14 years. The patients should have attended the diabetic clinic for a minimum of six months. In addition, the caregivers should have consented, and the children assented to taking part in the study.
Exclusion Criteria
Children diagnosed with and receiving treatment for acute infections such as urinary tract infections, skin infections, respiratory infections, and chronic complications such as diabetic retinopathy, nephropathy, and diabetic neuropathy as illness is associated with hyperglycemia which will impact the primary outcome of the study (HbA1c). Children who attend boarding schools will be excluded as the feeding practices are predetermined by the school management and this would introduce bias to the study. Furthermore, caregivers or children that have attended prior nutrition education courses will be excluded.
Intervention
Design
The structured nutrition education course will consist of a total of 8 sessions lasting 45 minutes each conducted in the intervention centers at different times. The topics to be covered and a brief description of each of the sessions are detailed in Table 1. The sessions will be interactive and include problem-solving exercises. Each session will be conducted once a week and a question-and-answer session held every after 2 sessions. The question-and-answer sessions are intended to help participants reflect and consolidate their understanding.
 |
Table 1 Structured Nutrition Education Course Content |
Delivery
A two-member team of experienced health educators (a diabetes specialist nurse and a specialist diabetes dietician) will conduct the sessions using food demonstrations and audio-visual aids. In addition, the participants will receive nutrition education materials (posters and brochures). To promote adherence and completion of the course, Individual follow-up sessions will be conducted for those individuals that may need further or any clarification on any component of the session. Also, the study participants will be sent a reminder every week via the mobile phone of the next session and encouraged to complete all sessions.36 Written and verbal feedback will be sought from the participants after each session. Furthermore, venues will be selected for adequate space and ease of access.
Treatment Fidelity
Treatment fidelity will be ensured by using expert content validated facilitator and participant guides and the same set of health educators to deliver the intervention.
Primary Outcome Measure
The primary outcome for the study is the percentage change in mean HbA1c levels of the children. We hypothesize that children and adolescents with T1DM whose caregivers are randomized to the intervention group and attend the structured nutrition education course will demonstrate significantly lower HbA1c values than those receiving routine health education.
HbA1c testing will be done at the different study sites using a portable point-of-care system for hemoglobin A1c testing, using the HemoCue® HbA1c 501 system analyzer37 (Ängelholm, Sweden). It will be calibrated as per manufacturer instructions at the time of the study. The reference levels will be < 7.5% (good control) and ≥ 7.5% (poor control). A blood sample will be obtained by pricking each child using a sterile single-use lancet. A drop of blood will then be placed at the tip of the HemoCue® HbA1c 501 patient test cartridge and the HbAlc results determined at three-time points (baseline, 3 and 6 months). A 1.0% difference in HbA1c will be deemed to be of clinical significance. However, Iron deficiency anemia (IDA) can lead to falsely high HbA1c values, the reason as to why this happens remains unclear.38 However, blood samples with extremely high HbA1c values (> 15%) will be repeated using a different analytical method.39 Furthermore, a clinical assessment for IDA will be conducted and if confirmed, those HbA1c results will not be considered in the final data analysis.
Secondary Outcomes Measures
Secondary outcomes will be caregivers’ level of knowledge of general and diabetes-specific nutrition knowledge, children’s mean intake of energy, protein, and fat, and children’s dietary diversity score, these outcomes will be assessed at three-time points (baseline, 3 and 6 months). We hypothesize that children with T1DM whose caregivers are randomized to the intervention group and attend the structured nutrition education course will have higher dietary diversity scores and their caregivers will demonstrate significantly improved general knowledge on nutrition in diabetes, carbohydrate counting, and food label interpretation than those receiving the routine health education.
A validated, brief questionnaire will be Ugandan contextualized and used to collect information on general knowledge on nutrition in diabetes, carbohydrate counting, and food label interpretation.40 The repeated 24-hour dietary recall will be used to collect quantitative information on the intake of energy, protein, and fat. Caregivers will be asked to recall all the foods and fluids consumed by their children 24 hours preceding the interview in terms of quantities of household measures. Food photographs and volumetric vessels will be used to help the participants correctly identify and quantify the foods and drinks consumed. To reduce the random error that may arise out of the day-to-day food intake variation, two 24-hour dietary recalls will be conducted on a random sub-sample of 40% of the sampled caregiver-child pairs on non-consecutive days. Respondent bias will be minimized by conducting interviews on randomly selected non-consecutive days both weekdays and weekend days.41,42
The dietary diversity questionnaire (DDQ) will be used to collect information on the variety of foods consumed by the study participants.43 The DDQ will comprise a list of different food groups from which the consumed foods from each food group will be selected based on the information provided by the respondent. Ingredients in mixed dishes in any quantity will be matched to a food group for a score of 1, each food group will only be counted once, and the total number of food groups tallied to give the dietary diversity score.43–45
Other Measurements
A structured questionnaire will be used to collect information on socio-demographic information (sex, age, marital status, occupation, education, religion, income, family size), and the children’s medical history (insulin type, number of injections, and years since diagnosis).
Statistical Analysis
All statistical analyses will be by intention-to-treat with a p-value of <0.05 being considered as statistically significant. The data will be analyzed using IBM SPSS Statistics for Macintosh, Version 26.46 Kolmogorov–Smirnov statistic will be used to check the normality of the variables before analysis. Descriptive summary statistics will be used to describe the characteristics of the study population. Inferential statistics such as the chi-square test will be used to examine the differences between categorical variables and t-test for continuous variables. The difference-in-differences (DiD) statistical technique will be used to identify changes in outcomes associated with the intervention.47 The intervention effect will be estimated after controlling for baseline differences between the two groups by comparing the difference in primary and secondary outcomes between the study groups at the start of the intervention and end of the intervention.
Consent and Participation
The study will compile with the Helsinki Declaration. To ensure that study participants are as fully informed as possible about the nature of their involvement, a participant information leaflet detailing the purpose of the study, what and how the information will be obtained (procedures), withdrawal privilege, voluntary participation, and confidentiality will be developed for the children, adolescents and their caregivers. Signed informed consent will be requested from the caregivers. Children and adolescents will also be asked for assent in taking part in the study after explaining to them what the study entails using language that is appropriate for their age and mental capacity. Data of participants who withdraw will not be included in the final analysis. However, their information will be analyzed separately to determine the characteristics, reasons for drop out, and lessons learned.
Adverse Events
Caregivers shall be trained on the risks and how to recognize the early warning signs of hypoglycemia and hyperglycemia. And any cases of hyper or hypoglycemia will immediately be referred to the doctor/diabetes specialist nurse for standard operating procedures for the management of hypoglycemia or hyperglycemia. In, addition, a diabetes emergency kit/hypo box will be available at all diabetes clinics in the intervention group. The intervention will not alter the participant’s medication routine but rather will support compliance to recommendations. The contacts of the principal investigator, medical doctor, and diabetes specialist nurse will be provided to all participants, and they will be informed to contact them in case of an adverse event.
Data Monitoring and Withdrawal
The formulation of the data monitoring team was not deemed necessary as the intervention will not alter the participant’s medication routine but rather will monitor compliance to recommendations that already form part of the diabetes education package. In addition, periodic reports will be submitted by the researcher to the REC regarding the progress of the trial and any adverse events. The attending clinician of the study participants will have the obligation to withdraw the participant from the study should he or she anticipate that the intervention may put the participant at risk.
Data Storage
All computers with data related to the study will have the latest antivirus, anti-malware software installed, they will be password protected and kept in a secure place at all times and best practice recommendation of secure retention of data for 5 years will be adhered to. The principal investigator and biostatistician will have access to all trial data.
Dissemination
Results will be disseminated via peer-reviewed publications and to the health management teams at the selected health facilities. Copies will be deposited at the libraries of the participating health facilities and representative bodies for people with diabetes mellitus.
Ethics Approval
Ethics approval has been granted by the St. Francis Hospital Nsambya Review and Ethics Committee (SFHN/REC/83) and a research permit was obtained from the Uganda National Council of Science and Technology (HS186ES). Any protocol amendments will be reported to the REC and trial participants. The trial is registered with The Pan African Clinical Trials Registry (PACTR201902548129842). The trial will be conducted in accordance with the Declaration of Helsinki.
Discussion
The nutritional goal for individuals with type 1 diabetes is to attain and sustain near-normal blood glucose levels by ensuring proper management of insulin therapy, physical activity, and dietary practices like ensuring a balance between carbohydrate intake and insulin administration. However, in children, it is vital that the diet also provides for their nutritional needs to ensure normal growth and development. A caregiver’s level of knowledge of general and diabetes-specific nutrition knowledge; in particular its nutritional management and their active involvement in their child’s diabetes management are crucial tools to achieving the above-mentioned goal.22 A study by Noorani, Ramaiya, and Manji16 conducted in Tanzania reported a significant association between diabetes knowledge of caregivers with HbA1c levels. Chege and Kuria48 also established a significant association between dietary practices and level of nutritional knowledge among caregivers and recommended the implementation of interventions to educate caregivers on good nutritional practices. Another study conducted in Ghana also showed the tremendous effect of caregiver feeding behaviors on child nutritional outcomes.49 It should be noted that, most of the studies done look at the relationship between caregiver’s nutrition knowledge and its effect on a child’s nutritional status. Studies about nutrition management of T1DM among children with type 1 diabetes are limited especially in Uganda.
This study may be faced with some impediments. First of all, caregivers may over or underestimate dietary intake due to recall bias. This will be minimized by using household measures during the quantification of food consumed and conducting the 24-hour dietary recall on two different days of the week to cater for variability in foods and drinks consumed. Secondly, study participants may find it difficult to consistently attend the intervention sessions for a period of 3 months, however, to ensure completion of the course, study participants will be sent a reminder every week via the mobile phone of the next session and encouraged to complete all sessions, in addition, their transport will be reimbursed. Thirdly, socioeconomic differences may exist between study participants. This may cause a variation in the application of the nutrition knowledge obtained during the intervention although this will be adjusted for during multivariate analysis.
Major strengths of the study will be the randomized design, the structured nutrition education course will also include Ugandan contextualized illustrations and instructional methods that make learning very simple and interesting. In addition, some of the topics such as carbohydrate counting will refer to already exiting Ugandan contextualized materials such as the carbohydrate counting guide for Uganda which have not been included in the current diabetes education curriculum used in the health facilities. Additionally, the findings will promote the utilization of a contextualized structured nutrition education guide tailored to the needs of caregivers of pediatric type 1 diabetes mellitus patients and enable them to use foods within their reach in a way that helps their child maintain good glycaemic control and ensure adequate dietary intake.
Acknowledgment
The authors would like to thank all the staff at the diabetes center, St. Francis Hospital, Nsambya, Kampala, Uganda.
Author Contributions
All authors made a significant contribution towards the conceptualization and design of the work reported; took part in drafting, revising, or critically reviewing the article; have agreed on the journal to which the article has been submitted; read and approved the final version that was published; and agree to be accountable for all aspects of the work.
Funding
This trial is supported by a grant from the Kyambogo University African Development Bank Higher Education Science and Technology Project (Uganda). The design, analysis, and reporting of this trial are independent of the funder.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Ronald C, Ma W, Juliana C, Chan N. Incidence of childhood type 1 diabetes: a worrying trend. Nat Rev Endocrinol. 2009;5(2009):529–530. doi:10.1038/nrendo.2009.180
2. International Diabetes Federation. IDF Diabetes Atlas.
3. American Diabetes Association. Classification and diagnosis of diabetes: standards of medical care in diabetes-2018. Diabetes Care. 2018;41(Suppl1):S13–s27. doi:10.2337/dc18-S002
4. Cooke DW, Plotnick L. Type 1 diabetes mellitus in pediatrics. Pediatrics Rev. 2008;29(11):374–385. doi:10.1542/pir.29-11-374
5. Piloya-Were T, Sunni M, Ogle GD, Moran A. Childhood diabetes in africa. Curr Opin Endocrinol Diabetes Obes. 2016;23(4):306–311. doi:10.1097/med.0000000000000262
6. Afoke AO, Ejeh NM, Nwonu EN, Okafor CO, Udeh NJ, Ludvigsson J. Prevalence and clinical picture of IDDM in nigerian igbo schoolchildren. Diabetes Care. 1992;15(10):1310–1312. doi:10.2337/diacare.15.10.1310
7. Elamin A, Eltayeb B, Tuvemo T, Tuvemo T. High incidence of type i diabetes mellitus in sudanese children. Ann Saudi Med. 1997;17:17. doi:10.5144/0256-4947.1997.478
8. Elamin A, Omer MI, Zein K, Tuvemo T. Epidemiology of childhood type i diabetes in sudan, 1987–1990. Diabetes Care. 1992;15(11):1556–1559. doi:10.2337/diacare.15.11.1556
9. Swai AB, Lutale JL, McLarty DG. Prospective study of incidence of juvenile diabetes mellitus over 10 years in dar es salaam, tanzania. BMJ. 1993;306(6892):306. doi:10.1136/bmj.306.6892.1570
10. Marshall SL, Edidin D, Arena VC, et al. Prevalence and incidence of clinically recognized cases of type 1 diabetes in children and adolescents in rwanda, africa. Diabet Med. 2015;32(9):1186–1192. doi:10.1111/dme.12701
11. Bahendeka SK. Diabetes in sub-saharan africa: let us not forget type 1. Lancet Diabetes Endocrinol. 2017;5(8):575–577. doi:10.1016/S2213-8587(17)30223-1
12. Bahendeka S, Mutungi G, Tugumisirize F, et al. Healthcare delivery for paediatric and adolescent diabetes in low resource settings: type 1 diabetes clinics in uganda. Glob Public Health. 2019;1–15.
13. International Diabetes Federation. IDF Diabetes Atlas.
14. Kyokunzire C, Matovu N. Factors associated with adherence to diabetes care recommendations among children and adolescents with type 1 diabetes: a facility-based study in two urban diabetes clinics in uganda. Diabetes Metab Syndr Obesity. 2018;11:93–104. doi:10.2147/DMSO.S156858
15. Ochola S, Masibo PK. Dietary intake of schoolchildren and adolescents in developing countries. Ann Nutr Metab. 2014;64(Suppl. 2):24–40. doi:10.1159/000365125
16. Noorani M, Ramaiya K, Manji K. Glycaemic control in type 1 diabetes mellitus among children and adolescents in a resource limited setting in dar es salaam - tanzania. BMC Endocr Disord. 2016;16(1):29. doi:10.1186/s12902-016-0113-y
17. Patton SR, Odar C, Midyett LK, Clements MA. Pilot study results for a novel behavior plus nutrition intervention for caregivers of young children with type 1 diabetes. J Nutr Educ Behav. 2014;46(5):429–433. doi:10.1016/j.jneb.2013.11.007
18. Price K, Knowles J, Fox M, et al. Effectiveness of the kids in control of food (kic k–off) structured education course for 11–16 year olds with type 1 diabetes. Diabetic Med. 2016;33(2):192–203. doi:10.1111/dme.12881
19. Chiang JL, Maahs DM, Garvey KC, et al. Type 1 diabetes in children and adolescents: a position statement by the american diabetes association. Diabetes Care. 2018;41(9):2026–2044. doi:10.2337/dci18-0023
20. Muchiri JW, Gericke GJ, Rheeder P. Effect of a nutrition education programme on clinical status and dietary behaviours of adults with type 2 diabetes in a resource-limited setting in south africa: a randomised controlled trial. Public Health Nutr. 2016;19(1):142–155. doi:10.1017/S1368980015000956
21. Lange K, Swift P, Pankowska E, Danne T. ISPAD clinical practice consensus guidelines 2014. Diabetes education in children and adolescents. Pediatr Diabetes. 2014;15(Suppl 20):77–85. doi:10.1111/pedi.12187
22. Ogle G, Middlehurt A, Silink M, Hanas R. Pocketbook for Management of Diabetes in Childhood and Adolescence in Under-Resourced Countries.
23. Nansel TR, Laffel LMB, Haynie DL, et al. Improving dietary quality in youth with type 1 diabetes: randomized clinical trial of a family-based behavioral intervention. Int J Behav Nutr Phys Act. 2015;12:58. doi:10.1186/s12966-015-0214-4
24. Sunguya BF, Poudel KC, Mlunde LB, et al. Effectiveness of nutrition training of health workers toward improving caregivers’ feeding practices for children aged six months to two years: a systematic review. Nutr J. 2013;12(1):66. doi:10.1186/1475-2891-12-66
25. Stallwood L. Relationship between caregiver knowledge and socioeconomic factors on glycemic outcomes of young children with diabetes. J Spec Pediatr Nurs. 2006;11(3):158–165. doi:10.1111/j.1744-6155.2006.00062.x
26. Dehayem MY, Takogue R, Choukem SP, et al. Impact of a pioneer diabetes camp experience on glycemic control among children and adolescents living with type 1 diabetes in sub-saharan africa. BMC Endocr Disord. 2016;16:5. doi:10.1186/s12902-016-0086-x
27. Schulz KF, Altman DG, Moher D. Consort 2010 statement: updated guidelines for reporting parallel group randomized trials. Ann Intern Med. 2010;152(11):726–732. doi:10.7326/0003-4819-152-11-201006010-00232
28. Hemming K, Eldridge S, Forbes G, Weijer C, Taljaard M. How to design efficient cluster randomised trials. BMJ. 2017;358:358. doi:10.1136/bmj.j3064
29. Ndahura NB, Munga J, Kimiywe J, Mupere E. Caregivers’ nutrition knowledge and dietary intake of type 1 diabetic children aged 3–14 years in uganda. Diabetes Metab Syndr Obesity. 2021;Volume 14:127–137. doi:10.2147/DMSO.S285979
30. Teddlie C, Yu F. Mixed methods sampling: a typology with examples. J Mix Methods Res. 2007;1(1):77. doi:10.1177/1558689806292430
31. Martínez-Mesa J, González-Chica DA, Duquia RP, Bonamigo RR, Bastos JL. Sampling: how to select participants in my research study? An Bras Dermatol. 2016;91(3):326–330. doi:10.1590/abd1806-4841.20165254
32. Florey CD. Sample size for beginners. BMJ. 1993;306(6886):1181–1184. doi:10.1136/bmj.306.6886.1181
33. Noordzij M, Tripepi G, Dekker FW, Zoccali C, Tanck MW, Jager KJ. Sample size calculations: basic principles and common pitfalls. Nephrol Dialysis Transplant. 2010;25(5):1388–1393. doi:10.1093/ndt/gfp732
34. Krishnavathana H, Loar R, Anderson BJ, Heptulla RA. The role of socioeconomic status, depression, quality of life, and glycemic control in type 1 diabetes mellitus. J Pediatr. 2006;149(4):526–531. doi:10.1016/j.jpeds.2006.05.039
35. Campbell MJ, Walters SJ. How to Design, Analyse and Report Cluster Randomised Trials in Medicine and Health Related Research. Chichester, West Sussex: John Wiley & Sons Ltd; 2014.
36. Desroches S, Lapointe A, Ratté S, Gravel K, Légaré F, Turcotte S. Interventions to enhance adherence to dietary advice for preventing and managing chronic diseases in adults. Cochrane Database Syst Rev. 2013;(2):CD008722–CD008722. doi:10.1002/14651858.CD008722.pub2
37. Andersson A, Lindh J, Eriksson A. Evaluation of the hemocue hba1c 501 system in primary care settings. Point Care. 2017;16(3):128–130. doi:10.1097/POC.0000000000000140
38. Guo W, Zhou Q, Jia Y, Xu J. Increased levels of glycated hemoglobin a1c and iron deficiency anemia: a review. Med Sci Monitor. 2019;25:8371. doi:10.12659/MSM.916719
39. Weykamp C. Hba1c: a review of analytical and clinical aspects. Ann Lab Med. 2013;33(6):393. doi:10.3343/alm.2013.33.6.393
40. Rovner AJ, Nansel TR, Mehta SN, Higgins LA, Haynie DL, Laffel LM. Development and validation of the type 1 diabetes nutrition knowledge survey. Diabetes Care. 2012;35(8):1643–1647. doi:10.2337/dc11-2371
41. Gibson RS, Charrondiere UR, Bell W. Measurement errors in dietary assessment using self-reported 24-hour recalls in low-income countries and strategies for their prevention. Adv Nutrition. 2017;8(6):980–991. doi:10.3945/an.117.016980
42. Kyamuhangire W, Lubowa A, Kaaya A, et al. The importance of using food and nutrient intake data to identify appropriate vehicles and estimate potential benefits of food fortification in uganda. Food Nutr Bull. 2013;34(2):131–142. doi:10.1177/156482651303400202
43. FAO. Guidelines for Measuring Household and Individual Dietary Diversity. Rome: FAO; 2013.
44. Steyn NP, Nel J, Labadarios D, Maunder EMW, Kruger HS. Which dietary diversity indicator is best to assess micronutrient adequacy in children 1 to 9 y? Nutrition. 2014;30(1):55–60. doi:10.1016/j.nut.2013.06.002
45. Caswell BL, Talegawkar SA, Siamusantu W, West KP
46. IBM. IBM SPSS Statistics for Macintosh, Version 26.0 [Computer Program]. Armonk, NY: IBM Corp; 2019.
47. Wooldridge JM Econometric analysis of cross section and panel data; 2010. http://site.ebrary.com/id/10453042.
48. Chege PM, Kuria EN. Relationship between nutrition knowledge of caregivers and dietary practices of children under five in kajiado county, kenya. Womens Health Bulletin. 2017;4(3). doi:10.5812/whb.43820
49. Nti CA, Lartey A. Effect of caregiver feeding behaviours on child nutritional status in rural ghana. Int J Consum Stud. 2007;31(3):303–309. doi:10.1111/j.1470-6431.2006.00553.x
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.