Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 16
Effectiveness and Safety of Upadacitinib in Combination with Topical Corticosteroids in Adolescent Patients with Moderate-to-Severe Atopic Dermatitis
Authors Hagino T , Hamada R, Yoshida M, Fujimoto E, Saeki H , Kanda N
Received 7 September 2023
Accepted for publication 24 October 2023
Published 7 November 2023 Volume 2023:16 Pages 3201—3212
DOI https://doi.org/10.2147/CCID.S439053
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Teppei Hagino,1 Risa Hamada,2 Mai Yoshida,2 Eita Fujimoto,3 Hidehisa Saeki,2 Naoko Kanda1
1Department of Dermatology, Nippon Medical School Chiba Hokusoh Hospital, Inzai, Japan; 2Department of Dermatology, Nippon Medical School, Tokyo, Japan; 3Fujimoto Dermatology Clinic, Funabashi, Japan
Correspondence: Teppei Hagino, Department of Dermatology, Nippon Medical School Chiba Hokusoh Hospital, Inzai, Japan, Tel +81 476 99 1111, Fax +81 476 99 1911, Email [email protected]
Purpose: To investigate the therapeutic effectiveness and safety of Janus kinase 1 inhibitor upadacitinib in adolescent patients with atopic dermatitis (AD).
Patients and Methods: This study examined therapeutic effectiveness and safety of upadacitinib for 39 Japanese adolescent patients (aged 12– 17 years) diagnosed with moderate-to-severe AD from August 2021 to January 2023. The patients were treated with upadacitinib 15 mg/day plus twice daily topical corticosteroids. Total eczema area and severity index (EASI) or EASI on head and neck, upper limbs, lower limbs, and trunk or for erythema, edema/papulation, excoriation, or lichenification, atopic dermatitis control tool (ADCT), peak pruritus-numerical rating scale (PP-NRS), and laboratory indexes were assessed at weeks 0, 4, and 12 of treatment. Treatment-emergent adverse events were recorded.
Results: Total EASI or EASI on 4 anatomical sites or for 4 rash types, ADCT, and PP-NRS were significantly reduced at week 4 and 12 compared to week 0. The achievement rates at weeks 4 or 12 were 64.1% or 62.5% for EASI 75, 93.5% or 73.1% for ADCT < 7-point, and 80.6% or 60% for PP-NRS ≥ 4-point improvement, respectively, indicating their peak at week 4 and slight decrease at week 12. The percent reduction of EASI for excoriation was higher than that for lichenification or edema/papulation at week 4 or week 12, respectively. The percent reductions of EASI for erythema and edema/papulation on head and neck were lower than those on lower limbs at week 12. Total eosinophil counts (TEC) and IgE reduced at week 4 compared to week 0 while TARC, IgE, TEC, and LDH increased at week 12 compared to week 4.
Conclusion: These results suggest therapeutic effectiveness and tolerability of upadacitinib and support its therapeutic usefulness for adolescent AD patients.
Keywords: atopic dermatitis, upadacitinib, adolescent, effectiveness, safety
Introduction
Atopic dermatitis (AD) is a chronic inflammatory skin disease characterized by recalcitrant eczema, type 2-skewed immune responses, disrupted skin barrier, and severe pruritus.1,2 AD is associated with disease burden, and lack of disease control induces persistent itch, sleep disturbance, depression, anxiety, impairment of daily activities, and reduces quality of life (QOL) of patients.3–5 In the skin lesions with AD, the levels of cytokines transducing intracellular Janus kinase (JAK)/signal transducers and activators of transcription (STAT) signals are increased, such as interleukin (IL)-4, IL-5, IL-13, IL-31, thymic stromal lymphopoietin (TSLP) or IL-22, which contribute to the development and exacerbation of AD.6–9 Recently in Japan, the approval of the IL-4Rα antibody, dupilumab, for patients with AD post six months of age has expanded the therapeutic options especially for pediatric AD patients, including those aged 12 and above. Dupilumab is a well-balanced antibody preparation with substantial evidence supporting its safety and effectiveness.10 JAK inhibitors including upadacitinib can suppress the effects of above cytokines and are approved as therapeutic options for AD.11 Previous real-world clinical studies have revealed the therapeutic effectiveness and safety and resultant good disease control by upadacitinib 15 mg/day plus topical corticosteroids for patients with AD, mainly in adult patients.12–15 However, there are few studies specifically examining the effectiveness and safety of this treatment in adolescent patients with moderate-to-severe AD. Adolescents face challenges in managing AD that are distinct from adults, such as heightened sensitivity to skin irritation, increased social and emotional pressures by appearance, and reduced compliance with treatment regimens.16 Therefore, it is necessary to establish treatment options for adolescent AD patients. This study aims to evaluate the therapeutic effectiveness and safety of upadacitinib 15 mg/day plus topical corticosteroids for adolescent patients with moderate-to-severe AD in real-world clinical practice.
Materials and Methods
Study Design and Data Collection
A retrospective study was conducted on 39 Japanese patients (aged 12 to 17 years) diagnosed with moderate-to-severe AD between August 2021 and January 2023. The patients were treated with oral upadacitinib 15 mg/day plus twice daily topical corticosteroids of moderate to strongest classes. The diagnosis of moderate-to-severe AD (eczema area and severity index [EASI] ≥16 or EASI of head and neck ≥2.4) was done based on the Japanese Atopic Dermatitis Guidelines 2021.17 This study was conducted in accordance with the Declaration of Helsinki (2004) and was approved by the Ethics Committee of Chiba Hokusoh Hospital. Patients were recruited from the dermatology department of Chiba Hokusoh Hospital based on medical record reviews, ensuring that none had been part of any clinical trials. Given the retrospective nature of this study, a control group was not included. The 12-week duration was chosen as an initial evaluation period to discern the immediate effects and safety of the treatments, understanding the chronic nature of AD. Prior to this treatment, all patients had been treated with topical corticosteroids alone and did not respond adequately. Lastly, this study was not registered on any clinical trial site due to its retrospective design. The written informed consent was obtained from the patients and their parents. The demographic information of the patients, such as sex, age, body mass index, duration of AD, and history of allergic diseases, was recorded.
Assessment of Therapeutic Effectiveness
We analyzed eczema area and severity index (EASI), investigator’s global assessment (IGA), IgE, thymus and activation-regulated chemokine (TARC), lactate dehydrogenase (LDH), and total eosinophil count (TEC) at weeks 0, 4, and 12 of treatment. The reference values are as follows: for IgE, the typical range is 0–170 (IU/mL); for TARC, the range varies with age, being less than 450 pg/mL for adults and under 743 (pg/mL) for children aged 2 years and above; LDH falls within the range of 124–222 (IU/mL); and for TEC, the standard value lies between 70 and 450 (/mL). Patients reported the atopic dermatitis control tool (ADCT) and peak pruritus-numerical rating score (PP-NRS) simultaneously.18,19 We analyzed the EASI scores on head and neck, upper limbs, lower limbs, and trunk, or for erythema, edema/papulation, excoriation, and lichenification at weeks 0, 4, and 12 of treatment. We calculated the proportions of patients whose EASI decreased by at least 75%, 90%, or 100% from baseline (EASI 75, EASI 90, or EASI 100, respectively). We examined the proportion of patients whose PP-NRS was reduced by ≥4-point from baseline (PP-NRS 4) among the patients with baseline PP-NRS ≥4. We investigated the proportion of patients who achieved an ADCT score <7-point (ADCT 7), which is the threshold for control of AD, among patients with baseline ADCT >7-point. We investigated the proportion of patients who achieved IGA = 0 (clear) or 1 (almost clear) with ≥2 grades of reduction from baseline (IGA 0/1).
Assessment of Safety
Treatment-emergent adverse events (TEAEs) were defined as adverse events (AEs) that emerged or worsened during the follow-up survey period (from the start of upadacitinib treatment until 30 days after the final administration).
Statistical Analysis
For variables with a normal distribution, results were shown as mean ± standard deviation (SD), and for variables with a nonparametric distribution, the results are shown as median [interquartile range]. The differences between measurements at weeks 0, 4, and 12 were analyzed using Friedman test with Bonferroni post-hoc test for variables with a nonparametric distribution. Differences in frequencies were analyzed by Fisher’s exact test. Statistical significance was set at p < 0.05. All statistical analyses were performed using Easy R (EZR) (Saitama Medical Center, Jichi Medical University).
Results
Patient Demographics and Baseline Characteristics
We enrolled 39 adolescent patients (aged 12 to 17 years) with moderate-to-severe AD (26 males and 13 females). Table 1 shows the comorbidities, baseline total EASI score, EASI scores on head and neck, upper limbs, lower limbs, and trunk, or for erythema, edema/papulation, excoriation or lichenification, IGA, ADCT, PP-NRS, and baseline serum IgE, TARC, LDH, and TEC.
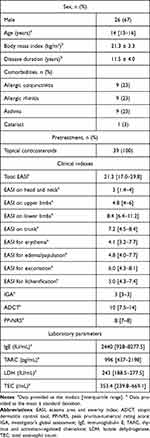 |
Table 1 Demographics and Baseline Characteristics of Adolescent Patients with Atopic Dermatitis (n = 39) |
Improvement of EASI, IGA, ADCT, and PP-NRS at Weeks 4 and 12 of Treatment with 15 mg/Day Upadacitinib Plus Topical Corticosteroids
All clinical indexes, EASI, IGA, ADCT, and PP-NRS, significantly decreased at weeks 4 and 12 compared to week 0 (Table 2). There were no differences in EASI, IGA, ADCT, and PP-NRS between week 12 versus week 4. The percent reductions from baseline at week 4 or 12 were median [interquartile] 77.3 [72.7–83.5] % or 77.7 [62.7–87.5] % for EASI, 76 [47.8–84.7] % or 65.5 [34.8–90] % for ADCT, and 62.5 [50–75] % or 75 [42.9–87.5] % for PP-NRS, respectively. There were no significant differences in the percent reductions of EASI, ADCT, and PP-NRS between week 4 versus 12. The achievement rates at week 4 or 12 were 64.1% or 62.5% for EASI 75, 93.5% or 73.1% for ADCT 7, and 80.6% or 60% for PP-NRS 4, respectively, indicating the peak at week 4 and modest decrease at week 12 without significant differences (Figure 1), while those were 12.8% or 15.6% for EASI 90, 12.8% or 15.6% for EASI 100, and 11.6% or 18.6% for IGA 0/1, indicating their gradual increase until week 12 without significant differences from those at week 4 (Figure 1). The transitions of EASI 90, EASI 100 and IGA0/1 were very close to one another.
 |
Table 2 The Clinical Indexes and Laboratory Parameters at Week 0, 4 and 12 of Upadacitinib Treatment, and the Significance of Differences Between the Stages (n = 39) |
Improvement of EASI on Different Anatomical Sites at Weeks 4 and 12 of Treatment
The EASI scores on all four anatomical sites decreased at weeks 4 and 12 compared to week 0 (Table 2). There were no significant differences in EASI scores on 4 anatomical sites at week 12 compared to week 4.
The median percent reductions of EASI from baseline at week 4 or 12 were 70% or 68.8% on head and neck, 80% or 80.6% on upper limbs, 78.6% or 87.5% on lower limbs, and 81.3% or 83.3% on trunk, respectively (Figure 2a). The percent reduction of EASI on head and neck was lower than that on upper limbs at week 4, and that on head and neck was lower than that on lower limbs at week 12, indicating lower treatment responsiveness on head and neck compared to upper or lower limbs.
Improvement of EASI for Individual Clinical Components on the Whole Body at Weeks 4 and 12 of Treatment
The EASI scores for erythema, edema/papulation, excoriation or lichenification decreased at weeks 4 and 12 compared to week 0 (Table 2). There were no significant differences in EASI scores for all four clinical components at week 12 compared to week 4.
The median percent reductions of EASI from baseline at weeks 4 or 12 were 74.2% or 69.5% for erythema, 62.7% or 54.1% for edema/papulation, 77.7% or 73.4% for excoriation, and 68.6% or 71.4% for lichenification, respectively (Figure 2b). The percent reduction of EASI for excoriation was significantly higher than that for lichenification at week 4 and was higher than that for edema/papulation at week 12.
Improvement of EASI for Individual Clinical Components on Different Anatomical Sites at Weeks 4 and 12 of Treatment
We then assessed if the percent reduction of EASI for erythema, edema/papulation, excoriation, or lichenification might differ with different anatomical sites. The percent reduction of EASI for erythema on head and neck at week 4 (48.9%) was lower than that on upper limbs (83.3%) and on lower limbs (76.8%), and that on head and neck at week 12 (57.4%) was lower than that on lower limbs (80.6%) (Figure 3a). The percent reduction of EASI for edema/papulation on head and neck at week 12 (46.7%) was lower than that on lower limbs (73.3%) (Figure 3b). There were no site-dependent differences in percent reduction of EASI for excoriation or lichenification (Figure 3c and d). These results indicate that the therapeutic effectiveness of upadacitinib for erythema and for edema/papulation on head and neck might be lower than those on lower limbs in adolescent patients.
The Alteration of Laboratory Parameters During Upadacitinib Treatment
TEC and IgE significantly decreased at week 4 compared to week 0, while TARC and LDH appeared to decrease at week 4 compared to week 0; however, the differences were not significant (Table 2). IgE, TARC, LDH, and TEC significantly increased at week 12 compared to week 4 though the median TEC at week 12 appeared unchanged.
Safety Profile
The safety profile of upadacitinib in this real-world study (Table 3) was mostly equivalent to that observed in Phase 3 clinical trials (Measure Up 1, Measure Up 2, AD Up, and Rising Up).20–23 No novel AEs were identified in this study. Adverse events occurred in 25 adolescent patients (64.1%). Neither serious AEs nor AEs leading to death were observed. Kaposi’s varicelliform eruption occurred in 1 patient (2.6%), herpes labialis occurred in 3 patients (7.7%), and herpes zoster occurred in 2 patients (5.1%). Acne occurred in 8 patients (20.5%). These AEs were mild in severity and improved with appropriate treatment. AEs leading to discontinuation of upadacitinib were worsening of AD in 2 patients (5.1%); one patient improved with increasing upadacitinib dose up to 30 mg/day while in the other patient, treatment was switched to dupilumab and topical corticosteroids. The elevation of serum creatine phosphokinase values was observed in 7 patients (17.9%), which was mild in severity and improved without treatment.
 |
Table 3 Treatment-Emergent Adverse Events (TEAEs) Through Week 12 After Treatment with Upadacitinib Plus Topical Corticosteroids for Adolescent Patients with Atopic Dermatitis (n = 39) |
Discussion
In this study, total EASI of adolescent AD patients remarkably reduced at week 4 and did not further reduce at week 12. The achievement rate of EASI 75 was also peaked at week 4 and was plateaued at week 12. The results were slightly different from those of our previous study mainly for adult AD patients, whose EASI score and achievement rate of EASI 75 continued to improve until week 12.12 The faster improvement of EASI by upadacitinib in adolescent patients than in adults may be attributable to the smaller body weight of the former, leading to higher per kg dosage of upadacitinib, which might provide higher local concentrations of upadacitinib at sites of inflammation, and provide longer time above half maximum inhibitory concentration for STAT phosphorylation by JAK-linked cytokines,24 and higher average daily percent STAT inhibition in adolescent patients compared to adult patients. Another possible reason for the faster improvement of EASI in adolescent patients may be altered immunological profiles; fold increase of expression in lesional versus normal skin for type 2 inflammation-related (IL13, CCL17, CCL22) or epidermal hyperplasia-related genes (IL22, IL20), whose expression or function can be suppressed by JAK inhibitors, was lower in adolescent AD patients compared to adult AD patients.25–27 Thus type 2 inflammation or epidermal hyperplasia in adolescent AD patients, at least in the context of these genes, may be more susceptible to the suppression by upadacitinib treatment compared to adult AD patients.
Upadacitinib improved all four individual clinical components of the EASI score in adolescent AD patients; however, there were some differences in its effectiveness between these components. The therapeutic effectiveness for excoriation was higher than that for edema/papulation or lichenification. This trend was also revealed in our previous study for mainly adult patients.28
The higher responsiveness of excoriation to upadacitinib treatment might be caused by its rapid and direct suppression on IL-4, IL-13, IL-31, and TSLP-induced pruritus5,29 and on IL-31-induced elongation of sensory nerves30 through JAK1/STAT pathway. On the other hand, the lower responsiveness of lichenification might be due to the extremely high levels of molecules promoting epidermal growth such as keratin 6B, keratin 16, or IL-36γ and of molecules promoting remodeling and fibrosis such as IL-33, whose effects are resistant to JAK inhibitors, in chronic phase of AD.31 The lower responsiveness of edema/papulation may be its promotion by cytokines resistant to JAK inhibitors such as IL-33 or vascular endothelial growth factor (VEGF), which suppresses the expression32 or alignment of vascular endothelial-cadherin,33 respectively, damaging endothelial barrier and increasing vascular permeability and efflux of plasma components.
The present results indicate that the treatment responsiveness to upadacitinib on head and neck was lower than that on upper or lower limbs in adolescent AD patients. The lower treatment responsiveness of head-and-neck clinical components was similarly observed in our previous study of upadacitinib treatment for mainly adult patients,14 and also in JAK1/2 inhibitor baricitinib treatment for mainly adult patients13 or previous study of dupilumab treatment for adult patients.34 These results indicate that the lower treatment responsiveness of head-and-neck clinical components compared to the other body sites might be independent of age or treatment modalities. Further, the lower responsiveness of erythema or edema/papulation on head and neck compared to lower limbs (Figure 3a and b) indicate that the lower responsiveness of head-and-neck clinical components to upadacitinib treatment in adolescent patients might be attributable to the lower responsiveness of erythema and/or edema/papulation on this site. The treatment resistance of head-and-neck erythema or edema/papulation is possibly because the skin of head-and-neck is more exposed to external stimuli such as aeroallergens, sunlight, or cosmetics, and is more likely to be affected by specific pathogens, such as Malassezia35 or Demodex.36,37 Especially severity scores of erythema and edema/papulation are known to correlate with stratum corneum VEGF levels.38 Both Malassezia35 and Demodex39,40 induce the production of VEGF in keratinocytes or mast cells, which increases dermal vasculature and blood flow, exacerbating erythema, and disrupts the alignment of vascular endothelial-cadherin around the endothelial cell borders33,38,41 and promotes the leakage of plasma proteins and leukocytes, exacerbating edema/papulation.
In this study, all the laboratory parameters, IgE, TARC, TEC, and LDH, increased at week 12 compared to week 4 in adolescent AD patients (Table 2) though the levels of clinical indexes at week 12 were similar to those at week 4. The reason for the reversal of laboratory parameters after clinical improvement should further be elucidated. It is hypothesized that the reversal of TARC, TEC, and LDH levels after clinical improvement might be mediated by upadacitinib-induced decrease of endogenous IL-17A level since JAK1 siRNA or JAK inhibitors suppress IL-17A secretion in anti-CD3/28-stimulated human CD4+T cells in the presence of IL-2, IL-1β, IL-6, and IL-23, indicating that suppression of JAK1 might reduce the production of IL-17A in T cells.42 IL-17A suppresses TARC production in dendritic cells and allergen-stimulated production of IL-5, which induces eosinophilia via promoting survival of eosinophils, in mediastinal lymph node cells of asthma model mice.43 Thus, the possible upadacitinib-induced reduction of endogenous IL-17A may diminish the IL-17A-mediated suppression of TARC and IL-5 production, leading to the increase of TARC and TEC levels irrespective of clinical improvement of AD.
The leakage of LDH from keratinocytes may be induced by the intracellular accumulation of reactive oxygen species (ROS) associated with increased mitochondrial activity.44,45 Since IL-17A induces nuclear translocation of transcription factor NF-E2-related factor 2 (Nrf2) and Nrf2-dependent expression of heme oxygenase-1 (HO-1)46 which suppresses accumulation of ROS in keratinocytes, upadacitinib-induced decrease of endogenous IL-17A might lead to the down-regulation of Nrf2/HO-1 pathway, inducing accumulation of ROS, which activates inflammasome cascade and resultant release of cytoplasmic LDH.47,48 The reversal of IgE level after clinical improvement by upadacitinib treatment may be mediated by upadacitinib-induced inhibition of IL-21-mediated JAK1/JAK3/STAT3 pathway24 since IL-21 suppresses IgE class switch recombination in human B cells through this pathway.49
We should analyze the transition of these laboratory parameters for further long-term treatment with upadacitinib in adolescent AD patients. The reversal of levels of these parameters after clinical improvement by upadacitinib was not prominent in our previous study whose participants are mainly adult patients.12 Whether the reversal is specific to adolescent patients should further be studied by comparing the results between adolescent versus adult patients strictly.
The safety profile of upadacitinib for adolescent AD patients in this real-world study was mostly similar to that of previous phase 3 clinical trials23 and also to that of our previous real-world study mainly for adult AD patients.12 The incidence of acne was slightly higher in this study for adolescent patients versus that in our previous study mainly for adult patients (20.5% versus 16.1%), and the elevation of serum creatine phosphokinase values also more frequently occurred in adolescent patients (17.9% versus 9.7%).12 The results may possibly be because adolescence is peak age of onset of acne and because adolescents tend to exercise harder compared to adults. Neither novel nor serious AEs occurred in the present study. The safety profile supports the tolerability of upadacitinib in adolescent AD patients.
In the broader context of systemic treatments available for AD in Japan, particularly for patients aged 12 and above, the choices remain limited. Alongside the JAK1 inhibitor upadacitinib, other therapies have recently been approved, including the JAK1 inhibitor abrocitinib, the IL-4Rα antibody dupilumab, and the IL-31R antibody nemolizumab. Each drug exhibits unique safety characteristics. Specifically, abrocitinib is often linked with headaches and nausea, while conjunctivitis is a commonly observed side effect with dupilumab.10 As our study highlights, for upadacitinib, clinicians should be vigilant for occurrences of acne and herpes zoster.15 It is imperative that clinicians meticulously evaluate the risk-benefit profile of these therapies, tailoring their choice to the individual needs of the patient.
Our study has several limitations. Firstly, the patients’ population was limited to Asians. The results for adolescent patients of the other races should further be studied. Secondly, our study was done for a relatively short duration (≤12 weeks), and effectiveness and safety of longer-term treatment should further be evaluated. Thirdly, the effectiveness and safety of upadacitinib were assessed in combination with topical corticosteroids. The comparison between combination therapy versus monotherapy should further be conducted.
Conclusion
In conclusion, upadacitinib treatment significantly reduced EASI, ADCT, and PP-NRS at weeks 4 and 12 compared to week 0, with mild manageable AEs in adolescent patients with moderate-to-severe AD. The improvement of EASI was peaked at early point of time (week 4). Though TEC and IgE decreased at week 4 compared to week 0, TEC, IgE, LDH, and TARC increased at week 12 compared to week 4. The therapeutic effectiveness for erythema or edema/papulation on head and neck was lower than that on lower limbs. These findings suggest the therapeutic effectiveness and tolerability of upadacitinib in adolescent patients with AD and support its potentiality as a promising treatment option for adolescent AD patients. However, further long-term studies are necessary to fully dissect its effectiveness and efficacy in adolescent AD patients.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Ethics Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of Nippon Medical School Chiba Hokusoh Hospital (protocol code H-2022-945 and February 10, 2022 of approval).
Acknowledgments
This research was partially supported by the grant, Initiative for Realizing Diversity in the Research Environment from MEXT, Japan.
Disclosure
H. S. received a lecture fee and research cost from AbbVie GK. T. H. and N. K. received lecture fees from AbbVie GK. The authors report no other conflicts of interest in this work.
References
1. Carroll CL, Balkrishnan R, Feldman SR, Fleischer AB Jr, Manuel JC. The burden of atopic dermatitis: impact on the patient, family, and society. Pediatr Dermatol. 2005;22(3):192–199. doi:10.1111/j.1525-1470.2005.22303.x
2. Eichenfield LF, Tom WL, Chamlin SL, et al. Guidelines of care for the management of atopic dermatitis: section 1. Diagnosis and assessment of atopic dermatitis. J Am Acad Dermatol. 2014;70(2):338–351.
3. Rønnstad ATM, Halling-Overgaard AS, Hamann CR, Skov L, Egeberg A, Thyssen JP. Association of atopic dermatitis with depression, anxiety, and suicidal ideation in children and adults: a systematic review and meta-analysis. J Am Acad Dermatol. 2018;79(3):448–456.e430.
4. Silverberg JI, Hanifin JM. Adult eczema prevalence and associations with asthma and other health and demographic factors: a US population-based study. J Allergy Clin Immunol. 2013;132(5):1132–1138.
5. Williams H, Robertson C, Stewart A, et al. Worldwide variations in the prevalence of symptoms of atopic eczema in the International Study of Asthma and Allergies in Childhood. J Allergy Clin Immunol. 1999;103(1 Pt 1):125–138.
6. Howell MD, Kuo FI, Smith PA. Targeting the Janus Kinase Family in Autoimmune Skin Diseases. Front Immunol. 2019;10:2342.
7. Ferreira S, Guttman-Yassky E, Torres T. Selective JAK1 Inhibitors for the Treatment of Atopic Dermatitis: focus on Upadacitinib and Abrocitinib. Am J Clin Dermatol. 2020;21(6):783–798.
8. Weidinger S, Beck LA, Bieber T, Kabashima K, Irvine AD. Atopic dermatitis. Nat Rev Dis Primers. 2018;4(1):1.
9. Brunner PM, Guttman-Yassky E, Leung DY. The immunology of atopic dermatitis and its reversibility with broad-spectrum and targeted therapies. J Allergy Clin Immunol. 2017;139(4s):567.
10. Kamata M, Tada Y. Optimal Use of Jak Inhibitors and Biologics for Atopic Dermatitis on the Basis of the Current Evidence. JID Innov. 2023;3(3):100195.
11. He H, Guttman-Yassky E. JAK Inhibitors for Atopic Dermatitis: an Update. Am J Clin Dermatol. 2019;20(2):181–192.
12. Hagino T, Saeki H, Kanda N. The efficacy and safety of upadacitinib treatment for moderate to severe atopic dermatitis in real-world practice in Japan. J Dermatol. 2022;49(11):1158–1167.
13. Hagino T, Saeki H, Fujimoto E, Kanda N. Efficacy and safety of baricitinib treatment for moderate to severe atopic dermatitis in real-world practice in Japan. J Dermatol. 2023;50(7):869–879.
14. Hagino T, Saeki H, Fujimoto E, Kanda N. The differential effects of upadacitinib treatment on skin rashes of four anatomical sites in patients with atopic dermatitis. J Dermatolog Treat. 2023;34(1):2212095.
15. Hagino T, Saeki H, Fujimoto E, Kanda N. Background factors predicting the occurrence of herpes zoster in atopic dermatitis patients treated with upadacitinib. J Dermatol. 2023.
16. Ricci G, Bellini F, Dondi A, Patrizi A, Pession A. Atopic dermatitis in adolescence. Dermatol Reports. 2012;4(1):45.
17. Saeki H, Ohya Y, Furuta J, et al. Executive summary: Japanese guidelines for atopic dermatitis (ADGL) 2021. Allergol Int. 2022;71(4):448–458.
18. Yosipovitch G, Reaney M, Mastey V, et al. Peak Pruritus Numerical Rating Scale: psychometric validation and responder definition for assessing itch in moderate-to-severe atopic dermatitis. Br J Dermatol. 2019;181(4):761–769.
19. Simpson E, Eckert L, Gadkari A, et al. Validation of the Atopic Dermatitis Control Tool (ADCT©) using a longitudinal survey of biologic-treated patients with atopic dermatitis. BMC Dermatol. 2019;19(1):15.
20. Guttman-Yassky E, Teixeira HD, Simpson EL, et al. Once-daily upadacitinib versus placebo in adolescents and adults with moderate-to-severe atopic dermatitis (Measure Up 1 and Measure Up 2): results from two replicate double-blind, randomised controlled phase 3 trials. Lancet. 2021;397(10290):2151–2168.
21. Reich K, Teixeira HD, de Bruin-Weller M, et al. Safety and efficacy of upadacitinib in combination with topical corticosteroids in adolescents and adults with moderate-to-severe atopic dermatitis (AD Up): results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2021;397(10290):2169–2181.
22. Katoh N, Ohya Y, Murota H, et al. A phase 3 randomized, multicenter, double-blind study to evaluate the safety of upadacitinib in combination with topical corticosteroids in adolescent and adult patients with moderate-to-severe atopic dermatitis in Japan (Rising Up): an interim 24-week analysis. JAAD Int. 2022;6:27–36.
23. Paller AS, Ladizinski B, Mendes-Bastos P, et al. Efficacy and Safety of Upadacitinib Treatment in Adolescents With Moderate-to-Severe Atopic Dermatitis: analysis of the Measure Up 1, Measure Up 2, and AD Up Randomized Clinical Trials. JAMA Dermatol. 2023;159(5):526–535.
24. McInnes IB, Byers NL, Higgs RE, et al. Comparison of baricitinib, upadacitinib, and tofacitinib mediated regulation of cytokine signaling in human leukocyte subpopulations. Arthritis Res Ther. 2019;21(1):183.
25. Renert-Yuval Y, Del Duca E, Pavel AB, et al. The molecular features of normal and atopic dermatitis skin in infants, children, adolescents, and adults. J Allergy Clin Immunol. 2021;148(1):148–163.
26. Yano C, Saeki H, Komine M, et al. Mechanism of Macrophage-Derived Chemokine/CCL22 Production by HaCaT Keratinocytes. Ann Dermatol. 2015;27(2):152–156.
27. Dao TTP, Song K, Kim JY, Kim YS. Igalan from Inula helenium (L.) suppresses the atopic dermatitis-like response in stimulated HaCaT keratinocytes via JAK/STAT3 signaling. Inflamm Res. 2020;69(3):309–319.
28. Hagino T, Yoshida M, Hamada R, Fujimoto E, Saeki H, Kanda N. Therapeutic effectiveness of upadacitinib on individual types of rash in Japanese patients with moderate-to-severe atopic dermatitis. J Dermatol. 2023.
29. Misery L, Brenaut E, Pierre O, et al. Chronic itch: emerging treatments following new research concepts. Br J Pharmacol. 2021;178(24):4775–4791.
30. Nakashima C, Otsuka A, Kabashima K. Interleukin-31 and interleukin-31 receptor: new therapeutic targets for atopic dermatitis. Exp Dermatol. 2018;27(4):327–331.
31. Tsoi LC, Rodriguez E, Stölzl D, et al. Progression of acute-to-chronic atopic dermatitis is associated with quantitative rather than qualitative changes in cytokine responses. J Allergy Clin Immunol. 2020;145(5):1406–1415.
32. Chalubinski M, Wojdan K, Luczak E, et al. IL-33 and IL-4 impair barrier functions of human vascular endothelium via different mechanisms. Vascul Pharmacol. 2015;73:57–63.
33. Ashina K, Tsubosaka Y, Kobayashi K, Omori K, Murata T. VEGF-induced blood flow increase causes vascular hyper-permeability in vivo. Biochem Biophys Res Commun. 2015;464(2):590–595.
34. Vittrup I, Krogh NS, Larsen HHP, et al. A nationwide 104 weeks real-world study of dupilumab in adults with atopic dermatitis: ineffectiveness in head-and-neck dermatitis. J Eur Acad Dermatol Venereol. 2023;37(5):1046–1055.
35. Darabi K, Hostetler SG, Bechtel MA, Zirwas M. The role of Malassezia in atopic dermatitis affecting the head and neck of adults. J Am Acad Dermatol. 2009;60(1):125–136.
36. Uchida H, Kamata M, Egawa S, et al. Newly developed erythema and red papules in the face and neck with detection of demodex during dupilumab treatment for atopic dermatitis improved by discontinuation of dupilumab, switching to upadacitinib or treatment with oral ivermectin: a report of two cases. J Eur Acad Dermatol Venereol. 2023;37(3):e300–e302.
37. Edslev SM, Andersen PS, Agner T, et al. Identification of cutaneous fungi and mites in adult atopic dermatitis: analysis by targeted 18S rRNA amplicon sequencing. BMC Microbiol. 2021;21(1):72.
38. Amarbayasgalan T, Takahashi H, Dekio I, Morita E. Content of vascular endothelial growth factor in stratum corneum well correlates to local severity of acute inflammation in patients with atopic dermatitis. Int Arch Allergy Immunol. 2012;157(3):251–258.
39. Casas C, Paul C, Lahfa M, et al. Quantification of Demodex folliculorum by PCR in rosacea and its relationship to skin innate immune activation. Exp Dermatol. 2012;21(12):906–910.
40. Forton FMN. The Pathogenic Role of Demodex Mites in Rosacea: a Potential Therapeutic Target Already in Erythematotelangiectatic Rosacea? Dermatol Ther (Heidelb). 2020;10(6):1229–1253.
41. Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9(6):669–676.
42. Hammitzsch A, Chen L, de Wit J, et al. Inhibiting ex-vivo Th17 responses in Ankylosing Spondylitis by targeting Janus kinases. Sci Rep. 2018;8(1):15645.
43. Schnyder-Candrian S, Togbe D, Couillin I, et al. Interleukin-17 is a negative regulator of established allergic asthma. J Exp Med. 2006;203(12):2715–2725.
44. Swindell WR, Bojanowski K, Chaudhuri RK. Isosorbide Fatty Acid Diesters Have Synergistic Anti-Inflammatory Effects in Cytokine-Induced Tissue Culture Models of Atopic Dermatitis. Int J Mol Sci. 2022;23(22):7.
45. Leman G, Pavel P, Hermann M, et al. Mitochondrial Activity Is Upregulated in Nonlesional Atopic Dermatitis and Amenable to Therapeutic Intervention. J Invest Dermatol. 2022;142(10):2623–2634.e2612.
46. Numata I, Okuyama R, Memezawa A, et al. Functional expression of heme oxygenase-1 in human differentiated epidermis and its regulation by cytokines. J Invest Dermatol. 2009;129(11):2594–2603.
47. Chen Z, Zhong H, Wei J, et al. Inhibition of Nrf2/HO-1 signaling leads to increased activation of the NLRP3 inflammasome in osteoarthritis. Arthritis Res Ther. 2019;21(1):300.
48. Zou Y, Luo X, Feng Y, et al. Luteolin prevents THP-1 macrophage pyroptosis by suppressing ROS production via Nrf2 activation. Chem Biol Interact. 2021;345:109573.
49. Yang Z, Wu CM, Targ S, Allen CDC. IL-21 is a broad negative regulator of IgE class switch recombination in mouse and human B cells. J Exp Med. 2020;217(5):568.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



