Back to Journals » International Journal of Women's Health » Volume 15
Effective Treatment for Recurrent Ovarian Cancer Guided by Drug Sensitivity from Ascites-Derived Organoid: A Case Report
Authors Chen W , Fang PH, Zheng B, Liang Y, Mao Y, Jiang X, Tang Q
Received 16 January 2023
Accepted for publication 27 May 2023
Published 13 July 2023 Volume 2023:15 Pages 1047—1057
DOI https://doi.org/10.2147/IJWH.S405010
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Marleen van Gelder
Wanyi Chen,1,* Po-Han Fang,2,* Bin Zheng,3 Yue Liang,1 Yiwen Mao,1 Xuefeng Jiang,1 Qionglan Tang4
1Department of Obstetrics and Gynecology, the First Affiliated Hospital of Jinan University, Guangzhou, People’s Republic of China; 2International School, Jinan University, Guangzhou, People’s Republic of China; 3Guangdong Research Center for Organoid Engineering and Technology, Guangzhou, People’s Republic of China; 4Department of Pathology, Sun Yat-sen Memorial Hospital, Sun Yat-sen University, Guangzhou, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Qionglan Tang, Email [email protected]
Abstract: So far, ovarian cancer has still been the most lethal gynecological malignancy. The chemotherapy and targeted medication are the mainstay for the recurrent ovarian cancer treatment. About 70% of the advanced-stage cases will relapse. Ascites-derived organoid is a pre-clinical model for the precise prediction of the therapeutic effectiveness for the ovarian cancer: it can be used to assess the drug sensitivity, to guide individualized precise treatment, and to improve advanced stage as well as recurrent ovarian cancer patient’ survival and prognosis. Until now, there has been no report concerning the establishment of the organoid out of the patient’s ascites and the concurrent usage of drug sensitivity test to guide the individualized precise treatment for the ovarian cancer. Here, we report a case of recurrent ovarian cancer of a 59-year-old female patient whose CA125 at its peak increased to 4523.4 U/mL. Then, patient’s own ovarian cancer organoid was constructed from the ascites by the abdominocentesis; concurrently, medication sensitivity test was performed on the organoid to guide individualized precise treatment. After the treatment, CA125 decreased to 33.7 U/mL, and the patient’s condition relieved effectively. This is the first published case report using ascites-derived organoid and the drug sensitivity test thereof to guide the precise treatment of recurrent ovarian cancer.
Keywords: ascites-derived organoid, ovarian cancer organoid, recurrent ovarian cancer, drug sensitivity test, precise treatment
Introduction
Ovarian cancer is a common gynecologic cancer worldwide, and ranks second in cancer death of female reproductive malignancies, and 90% of ovarian cancer are of an epithelial cell type and comprise multiple histologic types, with various specific molecular changes, clinical behaviours, and treatment outcomes. The remaining 10% are non-epithelial ovarian cancers – mainly germ cell tumours and sex cord-stromal tumours – with much more favourable prognosis.1,2 Ovarian cancer is a heterogeneous disease, of which the epithelial ovarian cancer (EOC) is the most common form. EOC develops according to two different carcinogenic pathways. Type I EOCs are suggested to be relatively indolent and genetically stable tumors that typically arise from recognizable precursor lesions, such as endometriosis or borderline tumors with low malignant potential. In contrast, type II EOCs are proposed to be high-grade, biologically aggressive tumors from their outset, with a propensity for metastasis from small-volume primary lesions.3 The outcomes of EOC patients vary, depending on the stage at diagnosis. Although the 5-year survival rate of about 70% could be reached for early-stage detection, the vast majority of patients are diagnosed at advanced stage with only 5-year survival rate of 34%.4 Recurrence is the major factor resulting in the low survival rate of advanced EOC, for the recurrence rate is higher in the advanced stage than the early stage; nowaday proteomics analysis of ovarian cancer, as well as their adaptive responses to therapy, can uncover new therapeutic choices, which can reduce the emergence of drug resistance and potentially improve patient outcomes, but there has been no satisfactory treatment until now.5–7 The platinum-based chemotherapy is currently used as first-line therapeutic regimen after primary surgery and still recommended for the platinum-sensitive recurrent ECO that relapses after 12 months.8 In contrast, in recurrent platinum-resistant ECO, though studies had shown results preferring single-agent due to the drug toxicity, there was no evidence for a preferred sequence, neither for a preferred therapeutic agent.8,9 In addition, the increased heterogeneity of the disease and individual heterogeneity could lead to diverse clinical response to the same drug. Therefore, a pre-clinical model, which could predict the clinical efficiency of a pharmacologic agent, could assist physician to plan the therapeutic regimen.
Patient-derived tumor organoid (PDTO) emerges as an in vitro 3-dimensional cell model, which exhibits the genetic and histopathology feature of the parental tumor tissue.10–14 It has been presented as an in vitro pre-clinical model, providing valuable data concerning the clinical efficiency of a drug.15–24 A recent study from the lab of Clevers has generated organoid models with all the features of diverse EOC subtypes,25 indicating the potential clinical value of the PDTO model for EOC to predict the clinical efficiency of therapeutic agency before application, especially in the case of advanced recurrent EOC. Here, we present a case of recurrent EOC patient with malignant ascites, whose relapse was resistant to platinum-based chemotherapy. An organoid model was generated using the malignant ascites and the PDTO drug sensitivity results were used to guide the individualized treatment.
In this study, we report a case of 59-year-old female patient diagnosed of recurrent high-grade serous ovarian cancer with the initial manifestation being the ascites. Based on the ovarian cancer organoid generated from patient’s ascites, together with the analysis of the drug sensitivity test, patient had significant remission for her disease and symptoms after receiving the treatment of topotecan.
Case Presentation
A 59-year-old female patient, who had entered menopause for 10 years, was hospitalized for abdominal pain and distention on December 6, 2018. The patient had been diagnosed of FIGO stage II ovarian cancer and undergone the abdominal surgery on January 16, 2019, after two cycles neoadjuvant treatment including paclitaxel. During the debulking surgical procedure, total hysterectomy with bilateral salpingo-oophorectomy, abdominal aortic and pelvic lymphadenectomy, together with appendectomy and omentectomy were conducted. The post-operative pathology biopsy indicated a high-grade serous ovarian cancer, and the pathological stage was classified as pM0, IIIB. Post-operative immunohistochemistry results demonstrated CK7 (+), CA125 (+), WT-1 (+), PAX-8 (+), ER (10%+), P53 (95%+), ki-67 (60%+), P16 (-), PR (-), CEA (-), HCG (-) molecular subtype. Post-operative chemotherapies of six cycles of paclitaxel and carboplatin, as post-operative chemotherapy, were given (paclitaxel 210mg, day 1 + carboplatin 300mg, 1 day, every 21 days) according to guidance of Chinese Society of Oncology (CSCO). The serum CA125 level decreased to normal range, and until October of 2020, neither the review color Doppler nor trunk PET-CT found any signs of tumor recurrence.
After re-admission in June 2021, the pelvic MRI images found a large amount of peritoneal fluid, and PET-CT images indicated new-onset metastases in the pelvic and left inguinal lymph nodes, as well as in the muscle of psoas major, iliacus, and iliopsoas. The serum CA125 level rose above the normal range to 4523.48U/mL. Cytology analysis of the ascites discovered a large amount of glandular tumor cells arrayed as papillae, and the immunohistochemistry results were CK7 (+), ER (+), PAX-8 (+), P53 (+), CK20 (-), CDX-2 (-) (Figure 1). Considering the medical history, the patient was diagnosed as post-operative recurrence of the ovarian serous carcinoma.
Based on the recognized platinum sensitivity/resistance definition, a combination therapy of paclitaxel, cisplatin, and bevacizumab (paclitaxel 180mg, intravenous chemotherapy, 1 day + cisplatin 70mg, intra-peritoneal injection, 1 day + bevacizumab 300mg, intravenous chemotherapy, 1 day, every 21 days) was given. After four cycles of the combination therapy, blood cancer biomarker assay indicated that the patient was resistant to combined therapeutic regime including platin. The serum CA125 level rose from 212.7U/mL during the third round of treatment to 225.8U/mL during fourth round of treatment. At the same time, PDTO was established from the collected ascites.
To facilitate precise medicine for the patient, patient ascites-derived tumor organoids (PDTOs) were generated and PTDO drug sensitivity assay was conducted (Figure 2). Maximal tumor cell inhibitory rate and relative half-maximal inhibitory concentrations (IC50) were determined (Table 1). The relative IC50 values could reflect the clinical responses of the anti-tumor drugs, as drug sensitive: IC50 ≤ 0.5; undefined area: 0.5 > IC50 ≤ 1; drug resistant: IC50 ≥1. The drug sensitivity results suggested that chemotherapy drugs topotecan, gemcitabine and targeted therapeutic drugs niraparib and Fluzoparib may exhibit clinical anti-cancer effect as the IC50 values of these drugs were 0.56, 0.81, 0.5 and 0.60, respectively (Figure 3). The chemotherapy agent topotecan and the target therapy agent niraparib exhibited stronger tumor cell inhibitory potency. The tumor cell inhibitory rates and IC for topotecan and niraparib were 94.29% and 99.39%, and 0.56 and 0.50, respectively (Figure 4).
 |
Table 1 Maximal Tumor Cell Inhibitory Rate and Relative Half-Maximal Inhibitory Concentrations (IC50) |
 |
Figure 4 Comparison of tumor inhibitory rate of ascites organoid from recurrent ovarian cancer. |
The patient claimed that she had irregularly taken olaparib orally for 3 months since October 2020. Yet she still suffered of the EOC relapse, suggesting that oral olaparib treatment may be ineffective against the recurrent EOC, corresponding to PDTO drug sensitivity test. It is well known that nilaparil is one of the PARP inhibitors, and PARP inhibitors work mainly by inhibiting catalytic activity and “trapping” ability. On the one hand, PARP inhibitors compete with nicotinamide adenine dinucleotide (niacinamide dinucleotide, NAD) to bind PARP, preventing the formation of polyadenosine diphosphate ribose (PAR) chains and thus making DNA single-strand damage irreparable. On the other hand, PARP inhibitors can bind to the NAD+ binding site of PARP to form a stable conformation that leads to irreversible dissociation of DNA-PARP, thus making double-strand damage irreparable and eventually leading to apoptosis. Based on current research evidence, PARP inhibitors for PSROC maintenance therapy have become the standard treatment recognized in various guidelines. In addition, although the organoid inhibitory rates of platinum + taxol can achieve 90% at the highest drug concentration. But the concentration of IC50 (which can reach half of the drug concentration of tumor cell inhibition) is higher than the effective concentration, in the drug-resistant region. This is consistent with patient’s lack of response to Taxol + Cisplatin + bevacizumab. Therefore, topotecan was selected as one of the candidates of the therapeutic regimen. Integrating NCCN guideline for recurrent ovarian cancer treatment, the final therapeutic regimen was composed of carboplatin, topotecan, and bevacizumab. After 3 cycles of combination therapy (topotecan 200mg, intravenous chemotherapy, 1 day + carboplatin 300mg, intravenous chemotherapy, 1 day + bevacizumab 400mg, intravenous chemotherapy, 1 day, every 21 days), the serum CA125 level decreased from 225.8 to 33.7 U/mL (Figure 5).
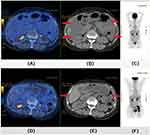
The amount of peritoneal effusion can be divided into small amount, medium amount and large amount. In clinical practice, ultrasound is commonly used to judge the classification of peritoneal effusion. For small amount of peritoneal effusion, the peritoneal effusion can only be detected through ultrasound examination. The peritoneal effusion under ultrasound is located in various spaces, and the depth is <3 cm. For medium amount of abdominal effusion, the abdominal effusion floods the bowel under ultrasound, but not crosses the mid abdomen, and the depth is 3–10 cm. For large amount of abdominal fluid, the abdominal effusion floods the whole abdominal cavity under ultrasound, and the middle abdomen is filled with abdominal fluid, the depth is >10 cm. In addition, PET-CT image was used to perform enhanced scanning of the patient’s pelvic cavity. After all the scanning, the image was processed. Combined with the results of PET-CT images examination this time and last time, the size changes of abdominal tumors in patients were compared, and the difference between the two was statistically significant. The transabdominal gynecological ultrasound and PET-CT images indicated a clear reduction of the peritoneal effusion. In addition, a partially shrink of the metastatic tumor sites within the pelvic cavity was observed (Figure 6).
Progression of the patient’s condition and accompanying interventions are illustrated in Figure 7.
 |
Figure 7 Case timeline. |
Discussion
Ovarian cancer, being one of the most common gynecological malignancies, with surgery in conjunction with the chemotherapy as its standard method of treatment, still has its relapse, ensued by the poor prognosis among the majority of patients that has undergone the standard initial treatment.26 Current research shows that when mutations occur within DNA repair pathways, there is an increased risk of chemotherapy resistance. The synthetic lethality of PARP inhibitors is directed against BRCA mutations, which are emerging as novel targets for the treatment of epithelial ovarian cancer. Among PARP inhibitors, olaparib, rucaparib, and niraparib have been approved by the FDA and/or the EMA in EOC in different settings. Olaparib, rucaparib, and niraparib trap PARP approximately 100-fold more efficiently than veliparib.27 The more specifically pathophysiological mechanism is still unclear, and there was no significant improvement in neither the therapeutic effect nor the patient’s survival rate. For the recent years, the crux, being essentially the hotspot as well, for the research and therapy of the ovarian cancer is to find and develop new treatment plan. Lately, molecular therapy for the ovarian cancer has shown great potentials. However, target medications only work for some of the patients, and sensitivity to the chemotherapy medications varies among patients. It is worthy to report that BRCA1/2 germline mutations are the strongest known genetic risk factors for epithelial ovarian cancer and are found in 6–15% of the women with epithelial ovarian cancer. The BRCA1/2 status can be used for patients’ counselling regarding expected survival, as BRCA1/2 carriers with epithelial ovarian cancer respond better than non-carriers to platinum-based chemotherapies. This yields greater survival, even though the disease is generally diagnosed at a later stage and higher grade.28 As the front field of the precise medication develops, tumor organoid has shown great value as a new pre-clinical disease model for the selection of the treatment regimen. Known for being able to maintain tumor’s heterogeneity, organoid is formed in vitro by induced differentiation of the stem cell or the organ’s progenitor cell. By using stem cell or organ’s progenitor cell as the raw material with the addition of extracellular basement membrane substitution, matrigel, as the three-dimensional scaffold, results in the eventual formation of a three-dimensional cell cluster, namely, the organoid with the structures and functions alike the tissue in situ.29 Until now, organoid models for the bladder cancer, stomach cancer, prostate cancer, pancreatic cancer, liver cancer, breast cancer, endometrial cancer, colon cancer, etc have been reported. Heterogeneity is an important feature of the malignant tumor and the main cause of the anti-neoplastic medication treatment failure. Ovarian cancer has a rather greater heterogeneity, and its oncogene type, prognosis, and other factors vary quite differently. Organoid is capable of fostering various kinds of the ovarian cancer; in contrast with other culture systems, organoid can maintain the ovarian cancer’s heterogeneity.30
Tumor organoid bio-bank can be used for the medication development and high-throughput method for the medication screening.31 Researches have confirmed that patient tissue-fostered organoid has a certain predictive ability for the clinical prognosis and a great potential to be used for the individualized treatment. Among the related studies concerning the selection of the therapeutic medications, Maenhoudt et al, through assessing commonly used medications for chemotherapy (paclitaxel, carboplatin, doxorubicin, gemcitabine) by the reaction of the high-grade serous ovarian cancer (HGSOC) organoid, have found that ovarian cancer organoid displaying the specific sensitivity to different medications; various medications have distinctive effects on the organoid from the same patient, indicating that ovarian cancer organoid is potentially feasible to be used for the medication selection.32 Nanki et al have used 23 types of FDA-approved medications to conduct sensitivity tests on the organoid derived from a patient with primary ovarian cancer, showing that those organoids can be used to effectively select the individualized medication.33 Also, Nanki et al have established 7 cases of ovarian cancer organoids, including 3 cases of HGSOC, 1 case of clear cell carcinoma, and 3 cases of endometrioid carcinoma, all of which have undergone the drug sensitivity tests. It is found that ovarian cancer organoid with the BRCA1 mutant is sensitive to olaparib, clear-cell-derived ovarian cancer organoid is resistant to the routine chemo-medication, namely, platinum and paclitaxel, corresponding to the clinical findings of clear cell ovarian cancer’s resistance to the platinum. McCorkle et al likewise have proved the theory that lapatinib and bozitinib could directly inhibit the ABCB1 transporter’s activity induced by the paclitaxel, via the establishment of paclitaxel-resistant cell line and ovarian cancer organoid model.34 Lapatinib and bozitinib in combination with paclitaxel in vitro collaboratively inhibit the over-expressed ABCB1 ovarian cancer cell’s reproduction. Adding the FDA-approved lapatinib into the second-line paclitaxel treatment for the recurrent ovarian cancer is a promising strategy.
In the related studies for genetic analysis and function, Hill et al have assessed the defects in the protection of homologous reorganization and replication forks in 33 cases of HGSOC organoid constructed from 22 patients’ tumor tissues, and have found that no matter which DNA repair gene mutation has occurred, the homologous reorganization defect within the organoid always has something to do with the sensitivity to the inhibitor of PARP (poly(ADP-ribose) polymerase), thus implying that PARP inhibitor could be the potential therapeutic medication for those patients.35 Even more, the defect in the replication fork protection is associated with the sensitivity to the carboplatin, CHK1 (Checkpoint Kinase 1) and ATR (Ataxia Telangiectasia and Rad3 related) inhibitors. Comparing with the genome analysis, function assessment based on the organoid model can predict more precisely the therapeutic effect in the clinical practice. By integrating the genome analysis and function assessment of the ovarian cancer organoid, targets including DNA damage and repair defect can be better identified.
In the related researches for immune function, Gorski et al have found that the tumor organoids, based on the patient’s tissues from the first-time or intermittent debulking surgeries, can be used to predict the clinical platinum sensitivity status and study the driving factors for the resistance to the carboplatin.36 Wan et al established the unique bi-specific PD-1/PD-L1 to handle the new model of the ovarian cancer organoid, and then immune function and single-cell RNA sequencing transcriptional analysis were carried out on such organoid model.37 They found that in the process of treating ovarian cancer via the immunotherapy, NK cells and a small amount of CD8+ T cell could enhance the therapeutic effects by participating in the cellular activity and cytotoxic process. Besides, the clinical models have demonstrated that PARP inhibition inactivates GSK3 and upregulates PD-L1 in a dose-dependent manner. Consequently, T-cell activation is being suppressed, resulting in enhanced cancer cell apoptosis.38
To sum it up, organoid is a novel and reliable type of pre-clinical models that can be used in the ovarian cancer research. Ovarian cancer organoid derived from the patient’s tissue is an in vitro tumor model that is highly homologous to the primary tumor in its histological and genetic characteristics. Organoid is capable of precisely replicating the complicated structures, functional characteristics, and response to the medications of the in -vivo tumor of the patient; therefore, organoid is a pre-clinical model for the individualized treatment planning, and it promotes greatly the development of both translational and precision medicine for ovarian cancer. Organoid model for ovarian cancer can be constructed within a short amount of time and then be assessed for medication sensitivity and resistance; by cutting down not only the cost but also the preparation time needed to start the therapy, organoid is going to play a key role in the selection of anti-ovarian cancer medications and guidance of individualized treatment.
For this report, some limitations exist. In the course of our research, Bevacizumab is a humanized anti-VEGF monoclonal antibody that binds to VEGF with high affinity and specificity, thus blocking the binding of VEGF to its receptor. It can block the growth and metastasis of tumor by inhibiting the angiogenesis of tumor and its metastatic site. But the organoid model does not contain endothelial cells, which cannot form blood vessels for the time being, so it is not possible to detect drugs that target blood vessels. Although organoids have a wide range of potential applications, the current organoid model still has limitations. First, organoid models have no natural microenvironment, including surrounding mesenchymal, immune cells, nervous system, or muscle layers. The solution to this limitation is to further improve the systematic construction of organotypic culture, such as supplementing related immune cells, co-culture cells, stromal cells or nerve cells, etc. Secondly, it is still difficult to establish a model of the immune microenvironment around the tumor. The tumor immune microenvironment is in the process of dynamic change. Meanwhile, different tumor types and individual patients have different immune microenvironments. Finally, regulatory factors or other small chemical molecules in the medium may have significant effects on gene expression and signaling pathways in organoids and may influence drug sensitivity. This problem needs our attention and further solution.31,39,40 One such limitation is more studies need to be done for clarification concerning the integration of ovarian cancer organoid models together with the genetic and immunologic function and the mechanism behind such application in the target therapy and immunotherapy. The other limitation is that the loss of contact with the patient after following up for a while. Therefore, whether and how the organoid application in the ovarian cancer treatment affects the progression-free survival is not clear.
Conclusion
In this current case, ovarian cancer organoid model was established out of the patient’s ascites; the model was assessed for medication selection to shorten the time for treatment and lower the cost. The organoid model played an important role in this patient’s individualized treatment for the ovarian cancer. This case provides the clear clinical evidences for the ovarian cancer model establishment and individualized treatment for the recurrent ovarian cancer under the guidance of the drug sensitivity test.
Abbreviations
EOC, epithelial ovarian cancer; PDTO, patient-derived tumor organoid; FIGO, International Federation of Gynecology and Obstetrics; FDG, fluorodeoxyglucose; PET-CT, positron emission tomography-computed tomography; MRI, magnetic resonance imaging; HGSOC, high-grade serous Ovarian cancer; PARP, poly(ADP-ribose) polymerase; CHK1, Checkpoint Kinase 1; ATR, Ataxia Telangiectasia and Rad3 related.
Data Sharing Statement
This case report contains clinical data from the medical records in the First Affiliated Hospital of Jinan University. Additional information is available from the corresponding author upon reasonable request.
Ethics Approval
The study was conducted in accordance with the Declaration of Helsinki and was approved by the Medical Ethics Committee of First Affiliated Hospital of Jinan University.
Consent Statement
Written informed consent for publication of details was obtained from the patient.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was partly supported by Guangdong Basic and Applied Basic Research Foundation (2019A1515012091).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
2. Cheung A, Shah S, Parker J, et al. Non-epithelial ovarian cancers: how much do we really know? Int J Environ Res Public Health. 2022;19(3):1106. doi:10.3390/ijerph19031106
3. Pavlidis N, Rassy E, Vermorken JB, et al. The outcome of patients with serous papillary peritoneal cancer, fallopian tube cancer, and epithelial ovarian cancer by treatment eras: 27 years data from the SEER registry. Cancer Epidemiol. 2021;75:102045. doi:10.1016/j.canep.2021.102045
4. Torre LA, Trabert B, DeSantis CE, et al. Ovarian Cancer Statistics, 2018. Ca-Cancer J Clin. 2018;68(4):284–296. doi:10.3322/caac.21456
5. Maiorano BA, Maiorano M, Lorusso D, Maiello E. Ovarian cancer in the era of immune checkpoint inhibitors: state of the art and future perspectives. Cancers. 2021;13(17):4438. doi:10.3390/cancers13174438
6. Oronsky B, Ray CM, Spira AI, Trepel JB, Carter CA, Cottrill HM. A brief review of the management of platinum-resistant-platinum-refractory ovarian cancer. Med Oncol. 2017;34(6). doi:10.1007/s12032-017-0960-z
7. Ghose A, Gullapalli S, Chohan N, et al. Applications of proteomics in ovarian cancer: dawn of a new era. Proteomes. 2022;10(2):16. doi:10.3390/proteomes10020016
8. Armstrong DK, Alvarez RD, Bakkum-Gamez JN, et al. NCCN guidelines insights: ovarian cancer, version 1.2019. J Natl Compr Canc Ne. 2019;17(8):896. doi:10.6004/jnccn.2019.0039
9. Liu SR, Kasherman L, Fazelzad R, et al. The use of bevacizumab in the modern era of targeted therapy for ovarian cancer: a systematic review and meta-analysis. Gynecol Oncol. 2021;161(2):601–612. doi:10.1016/j.ygyno.2021.01.028
10. Drost J, Clevers H. Organoids in cancer research. Nat Rev Cancer. 2018;18(7):407–418. doi:10.1038/s41568-018-0007-6
11. Jurj A, Pasca S, Braicu C, Rusu I, Korban SS, Berindan-Neagoe I. Focus on organoids: cooperation and interconnection with extracellular vesicles - Is this the future of in vitro modeling? Semin Cancer Biol. 2021;86:367–381. doi:10.1016/j.semcancer.2021.12.002
12. Krieger TG, Le Blanc S, Jabs J, et al. Single-cell analysis of patient-derived PDAC organoids reveals cell state heterogeneity and a conserved developmental hierarchy. Nat Commun. 2021;12(1). doi:10.1038/s41467-021-26059-4
13. Rahmanian M, Seyfoori A, Ghasemi M, et al. In-vitro tumor microenvironment models containing physical and biological barriers for modelling multidrug resistance mechanisms and multidrug delivery strategies. J Control Release. 2021;334:164–177. doi:10.1016/j.jconrel.2021.04.024
14. Wu YS, Li KY, Li YQ, et al. Grouped-seq for integrated phenotypic and transcriptomic screening of patient-derived tumor organoids. Nucleic Acids Res. 2022;50(5):e28–e28. doi:10.1093/nar/gkab1201
15. Lee SH, Hu WH, Matulay JT, et al. Tumor evolution and drug response in patient-derived organoid models of bladder cancer. Cell. 2018;173(2):515. doi:10.1016/j.cell.2018.03.017
16. Yang H, Siu HC, Law S, et al. A comprehensive human gastric cancer organoid biobank captures tumor subtype heterogeneity and enables therapeutic screening. Cell Stem Cell. 2018;23(6):882. doi:10.1016/j.stem.2018.09.016
17. Gao D, Vela I, Sboner A, et al. Organoid cultures derived from patients with advanced prostate cancer. Cell. 2014;159(1):176–187. doi:10.1016/j.cell.2014.08.016
18. Boj SF, Hwang CI, Baker LA, et al. Organoid models of human and mouse ductal pancreatic cancer. Cell. 2015;160(1–2):324–338. doi:10.1016/j.cell.2014.12.021
19. Broutier L, Mastrogiovanni G, Verstegen M, et al. Human primary liver cancer-derived organoid cultures for disease modeling and drug screening. Nat Med. 2017;23(12):1424. doi:10.1038/nm.4438
20. Sachs N, de Ligt J, Kopper O, et al. A living biobank of breast cancer organoids captures disease heterogeneity. Cell. 2018;172(1–2):373. doi:10.1016/j.cell.2017.11.010
21. Boretto M, Maenhoudt N, Luo XL, et al. Patient-derived organoids from endometrial disease capture clinical heterogeneity and are amenable to drug screening. Nat Cell Biol. 2019;21(8):1041. doi:10.1038/s41556-019-0360-z
22. de Witte CJ, Valle-Inclan JE, Hami N, et al. Patient-derived ovarian cancer organoids mimic clinical response and exhibit heterogeneous inter- and intrapatient drug responses. Cell Rep. 2020;31(11):107762. doi:10.1016/j.celrep.2020.107762
23. Gunther C, Winner B, Neurath MF, Stappenbeck TS. Organoids in gastrointestinal diseases: from experimental models to clinical translation. Gut. 2022;71(9):1892–1908. doi:10.1136/gutjnl-2021-326560
24. Li ZC, Xu HB, Gong YQ, et al. Patient-derived upper tract urothelial carcinoma organoids as a platform for drug screening. Adv Sci. 2022;9(4:654).
25. Kopper O, de Witte CJ, Lohmussaar K, et al. An organoid platform for ovarian cancer captures intra- and interpatient heterogeneity. Nat Med. 2019;25(5):838. doi:10.1038/s41591-019-0422-6
26. Pujade-Lauraine E, Banerjee S, Pignata S. Management of platinum-resistant, relapsed epithelial ovarian cancer and new drug perspectives. J Clin Oncol. 2019;37(27):2437–2448. doi:10.1200/JCO.19.00194
27. Boussios S, Rassy E, Moschetta M, et al. BRCA mutations in ovarian and prostate cancer: bench to bedside. Cancers. 2022;14(16):3888. doi:10.3390/cancers14163888
28. Shah S, Cheung A, Kutka M, Sheriff M, Boussios S. Epithelial ovarian cancer: providing evidence of predisposition genes. Int J Environ Res Public Health. 2022;19(13):8113. doi:10.3390/ijerph19138113
29. Lancaster MA, Knoblich JA. Organogenesis in a dish: modeling development and disease using organoid technologies. Science. 2014;345(6194). doi:10.1126/science.1247125
30. Koshiyama M, Matsumura N, Konishi I. Recent concepts of ovarian carcinogenesis: type I and type II. Biomed Res Int. 2014;2014(2014):1–11. doi:10.1155/2014/934261
31. Jabs J, Zickgraf FM, Park J, et al. Screening drug effects in patient-derived cancer cells links organoid responses to genome alterations. Mol Syst Biol. 2017;13(11). doi:10.15252/msb.20177697
32. Maenhoudt N, Defraye C, Boretto M, et al. Developing organoids from ovarian cancer as experimental and preclinical models. Stem Cell Rep. 2020;14(4):717–729. doi:10.1016/j.stemcr.2020.03.004
33. Nanki Y, Chiyoda T, Hirasawa A, et al. Patient-derived ovarian cancer organoids capture the genomic profiles of primary tumours applicable for drug sensitivity and resistance testing. Sci Rep. 2020;10(1):12581. doi:10.1038/s41598-020-69488-9
34. McCorkle JR, Gorski JW, Liu J, et al. Lapatinib and poziotinib overcome ABCB1-mediated paclitaxel resistance in ovarian cancer. PLoS One. 2021;16(8):e254205. doi:10.1371/journal.pone.0254205
35. Hill SJ, Decker B, Roberts EA, et al. Prediction of DNA repair inhibitor response in short-term patient-derived ovarian cancer organoids. Cancer Discov. 2018;8(11):1404–1421. doi:10.1158/2159-8290.CD-18-0474
36. Gorski JW, Zhang Z, McCorkle JR, et al. Utilizing patient-derived epithelial ovarian cancer tumor organoids to predict carboplatin resistance. Biomedicines. 2021;9(8):1021. doi:10.3390/biomedicines9081021
37. Wan CX, Keany MP, Dong H, et al. Enhanced efficacy of simultaneous PD-1 and PD-L1 immune checkpoint blockade in high-grade serous ovarian cancer. Cancer Res. 2021;81(1):158–173. doi:10.1158/0008-5472.CAN-20-1674
38. Revythis A, Limbu A, Mikropoulos C, et al. Recent insights into PARP and immuno-checkpoint inhibitors in epithelial ovarian cancer. Int J Environ Res Public Health. 2022;19(14):8577. doi:10.3390/ijerph19148577
39. Workman MJ, Mahe MM, Trisno S, et al. Engineered human pluripotent-stem-cell-derived intestinal tissues with a functional enteric nervous system. Nat Med. 2017;23(1):49–59. doi:10.1038/nm.4233
40. Ohlund D, Handly-Santana A, Biffi G, et al. Distinct populations of inflammatory fibroblasts and myofibroblasts in pancreatic cancer. J Exp Med. 2017;214(3):579–596. doi:10.1084/jem.20162024
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.