Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 16
Effect of Yak Meat to the Daily Ration of Scalded Rats for Wound Healing
Authors Wang HJ, Feng YP, Tian XX, Wu XH, Hao LZ, Li Y, Mei SJ
Received 11 October 2022
Accepted for publication 8 March 2023
Published 29 March 2023 Volume 2023:16 Pages 751—767
DOI https://doi.org/10.2147/CCID.S393066
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Jeffrey Weinberg
Hong-Jin Wang,1,* Yan-Ping Feng,1,* Xiao-Xia Tian,2 Xiao-Han Wu,1 Li-Zhuang Hao,2 Yi Li,1 Shi-Juan Mei3
1Department of Burns and Plastic Surgery, Affiliated Hospital of Qinghai University, Xining, Qinghai, 810012, People’s Republic of China; 2Qinghai University, Academy of Animal Science and Veterinary Medicine, Key Laboratory of Plateau Grazing Animal Nutrition and Feed Science of Qinghai Province, Xining, Qinghai, 810016, People’s Republic of China; 3Department of Oncology, affiliated Hospital of Qinghai University, Xining, Qinghai, 810010, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yi Li, Department of Burns and Plastic Surgery, Affiliated Hospital of Qinghai University, No. 29 Tongren Road, Xining, Qinghai province, 810010, People’s Republic of China, Tel +86-13709760887, Email [email protected] Shi-Juan Mei, Department of Oncology, Affiliated Hospital of Qinghai University, No. 29 Tongren Road, Xining, Qinghai province, 810010, People’s Republic of China, Tel +86-15009715352, Email [email protected]
Objective: Treatment of burn wound healing involves infection, nutrition, psychology and rehabilitation, and proper nutritional support can promote wound healing, enhance immune function and reduce the incidence of complications. This study aimed to investigate the effects of feed containing yak meat on scalded rats’ body condition and wound healing.
Methods: Adopting a two-factor factorial design, the growth performance, food intake, body weight, and Lee’s index of rats were measured. The wound conditions of scalded rats with different feeds (basic, basic + yak meat, and basic + yellow beef) were observed at different periods, and their wounds’ hematoxylin and eosin (H&E) staining states were detected. The proliferating cell nuclear antigen (PCNA)-positive cells and apoptosis were analyzed to evaluate the effects of feed on the wound healing of scalded rats.
Results: The feed intake was the highest in the yellow beef feed group and the lowest in the yak meat feed group. The body weight was the highest in the yak meat feed group and the lowest in the yellow beef feed group. Furthermore, 45 days after scalding, the obesity index in the yak beef feed group was the closest to that of the rats before scalding. The wound recovery of the rats in the yak meat feed group was the best at 30 days, and the H&E staining results also proved that the recovery effect of the scalded rats in the yak meat feed group was better than other two groups. According to the results of PCNA and apoptosis, the yak meat feed group had lower positive cell rate and faster wound healing.
Conclusion: The rats in the yak meat feed group recovered better than those in the other groups, and the yak beef feed had the best effect on the wound healing of the scalded rats.
Keywords: scalded rats, yak meat feed, yellow beef feed, basic feed, wound healing
Introduction
Yak meat has always been famous for its high protein, dry matter content, and low fat. Yak meat’s potassium, calcium, iron, zinc, and magnesium contents are relatively high. Yak meat’s amino acid content is rich, its saturated fatty acid content is low, and its unsaturated fatty acid content is high. All these quantities indicate that yak meat is very beneficial to human health.1–4 Li et al5 detected 17 amino acids from Haibei and Datong yak meats. The essential amino acid contents and the amino acids contained in the ideal protein makeup, as proposed by the Food and Agriculture Organization of the United Nations/World Health Organization, were evaluated and found to be higher than those in the ideal model. Both kinds of yak meat had a reasonable amino acid ratio, comprehensive nutrition, and high-quality protein sources. Ya et al6 compared the nutritional composition of Western Sichuan yak meat with that of ordinary yellow beef. The results revealed that the protein content of Western Sichuan yak meat was significantly higher than that of ordinary yellow beef (P < 0.05), the fat content was significantly lower than that of ordinary yellow beef (P < 0.05), and the essential amino acid content of yak meat were significantly higher than that of common yellow beef (P < 0.05). The calcium (Ca), iron, zinc (Zn), magnesium (Mg), and Phosphorus (P) content in yak meat were significantly higher than those in ordinary yellow beef (P < 0.05), and Zn and Mg were abundant.6 In addition, the nutritional composition of adult yak meat in different regions was also different. There were no significant differences in crude protein (CP), ash, and dry matter contents of the longissimus dorsi muscle of the approximately 7-year-old yaks in Jiulong County, Sichuan Province; Diqing Prefecture, Yunnan Province; and Bazhou, Xinjiang Autonomous Region (P > 0.05), but there were statistically significant differences in the ether extract, cholesterol, amino acids, and fatty acids contents (P < 0.05).7
Burns accelerate the body’s nutrition consumption, cause emaciation, and increase metabolism by two to three times the normal rate, lasting for several weeks. The healing process of burn wound involves a comprehensive cascade of precisely regulated steps and events, including hemostasis, inflammation, fibroplasia, angiogenesis, epithelialization, matrix formation, and remodeling.8 After severe burns, an organism will undergo a high-intensity metabolic reaction, which increases the possibility of infection and makes tissue repair difficult.9 A daily ration with good nutrition can prevent the body from suffering from several symptoms, such as decreased immunity, slow wound healing, and systemic infection due to burns. The ration can also provide energy for the body, improve cell activity, maintain positive nitrogen balance, and regulate immunity, gastrointestinal function, and structure to promote wound healing.10 Burn treatment involves managing an organism’s infection, nutrition, psychology, and rehabilitation. Proper nutritional support can promote wound healing, enhance immune function, and reduce the incidence rate of complications.11
To investigate the effects of feed containing yak meat on scalded rats’ body condition and wound healing, in this study, scalded rats are fed with 4:6 yak beef feed, 4:6 yellow beef feed, and basic feed. Their daily food intake, body weight, Lee’s index, wound healing, proliferating cell nuclear antigen (PCNA), and cell apoptosis rate of the wound tissue are measured. Finally, the effects of the three kinds of feed on the body condition and wound healing of scalded rats are comprehensively evaluated and compared to fully understand each food type’s impact.
Materials and Methods
Animals and Materials
The experimental Sprague–Dawley (SD) rats, their feed, and padding materials were purchased from Shaanxi Xi’an Keao Biotechnology Co., Ltd., China. The weight of the rats was 300 ± 50 g and with license No.: SCXK (Shaanxi) 2018–001. The yak meat and yellow beef (Simmental cattle) were purchased from Datong Fengju Agriculture and Animal Husbandry Technology Co., Ltd, China.
The G1003 hematoxylin and eosin (H&E) staining set was purchased from Servicebio, Wuhan, China. An upright white light photographing microscope (Nikon [Japan] Eclipse Ci-L) was used. The G1501 Tunel kit was purchased from Servicebio. The primary antibody, A01040, was purchased from Abbkine, USA. The secondary antibody, KIT-53, was purchased from Fuzhou Maixin Biotech Co., Ltd., China. The histochemical kit was a 3.3-diaminobenzidine (DAB) (DAB0031) kit purchased from Beijing Zhongshan Jinqiao Biological Technology Co., Ltd., China.
Yak beef is adult yak fillet, purchased from Datong Feng Aggregation Agriculture and animal Husbandry Technology Co., LTD.
Experimental Design
First, 115 male SD rats weighing 300 ± 50 g were adaptively reared for one week. To establish a scalding model with second-degree burns, after anesthesia, the back was shaved and routine disinfection was conducted, and then a constant temperature water bath box at 80°C± 2°C was given for 15s, and the deep second degree scald was completed with the scalding area of 20%. Ninety rats with successful modeling were selected and disinfected with iodophor and randomly divided into three equal groups (n = 30). Six healthy rats served as the blank controls and were fed the basic diet. Adopting the two-factor single-level factorial experimental design, the rats in the control group were fed with basic feed, the rats in the yak meat feed group were fed with the basic feed and yak meat at a 6:4 ratio, and the rats in the yellow beef feed group were fed with the basic feed and yellow beef at a 6:4 ratio. Six experimental rats in each group were executed on days 7, 14, 30, and 45. Their samples were obtained, and the indexes were measured. The experimental period was 45 days. The groups of experimental animals included: on day 7 after scalding, yak meat feed group (M-7), yellow beef feed group (H-7), basic feed group (J-7), and blank group (K-7); on day 14 after scalding, yak meat feed group (M-14), yellow beef feed group (H-14), and basic feed group (J-14); on day 30 after scalding, yak meat feed group (M-30), yellow beef feed group (H-30), and basic feed group (J-30); and on day 45 after scalding, yak meat feed group (M-45), yellow beef feed group (H-45), and basic feed group (J-45).
Feed Preparation
The yak tenderloin, the yellow cattle tenderloin, and the basic feed were ground separately. They were prepared into a pure basic feed, a mixture of basic feed with yak meat at a 6:4 ratio, and a mixture of basic feed with yellow beef at a 6:4 ratio. They were converted into pellet feed using a pellet feed machine, dried at 65°C, and finally stored at −20°C for later usage.
Feed Nutrient Composition Table
GB/T 6435–2014 was used to determine moisture in the feeds; GB/T 6438–2007 was used to determine crude ash in the feeds; GB/T 6432–2018 was used to determine CP in the feeds by the Kjeldahl method; GB/T 6433–1994 was used to determine the ether extract in the feeds; GB/T 20806–2006 was used to determine the neutral detergent fiber in the feeds; GB/T 20805–2006 was used to determine the acid detergent fiber in the feeds; GB/T 6436–2002 was used to determine the total Ca in the feeds; and GB/T 6437 2002 was used to determine the total P in the feeds by a spectrophotometric method. An oxygen-bomb calorimeter method was used to detect energy (Table 1).12
 |
Table 1 Feed Composition and Nutrient Level |
Feeding Management
Each group contained 30 rats that were kept in a cage. The feeding site had natural ventilation. The padding was replaced regularly to keep the cage dry, and iodophor was applied to the rat’s wounds to prevent wound inflammation. The ambient temperature and humidity were kept in an appropriate range. All the rats had free access to food and water.
Measurement Index and Method
Sampling
Six rats in each group were randomly selected for sampling on days 7, 14, 30, and 45. The rats were fasted for food and water 12 hours before sampling. The rats were anesthetized with 10% chloral hydrate, and their body weights and lengths were measured and recorded. First, the rats were sacrificed, and then about 2 cm of scalded wound skins were taken, fixed in 10% formalin, and stored at 4°C for standby.
Food Intake Amount and Daily Weight Gain Were the Same as in Section 2.6.1
Lee’s Index13
On days 0, 7, 14, 30, and 45, the body weight and length of the rats were measured, and the Lee’s indexes were calculated:
Lee’s index = (body weight × 1000)^(1/3) / body length.
The Wounds of the Scalded Rats Were Observed, and Photos Were Taken on the Days 1, 7, 14, 21, and 30 After Scalding
H&E Staining14
- Dewaxing of paraffined sections: The sections were put into xylene I for 20 minutes, xylene II for 20 minutes, absolute ethanol I for 5 minutes, absolute ethanol II for 5 minutes, and 75% ethanol for 5 minutes, after which they were rinsed with tap water.
- Hematoxylin staining: The sections were stained with hematoxylin for 3–5 minutes, rinsed with tap water, differentiated with a differentiation solution, rinsed with tap water, and then treated with a hydrochloric acid solution, after which they were rinsed with running water.
- Eosin staining: The sections were dehydrated with 85% and 95% gradient alcohol for 5 minutes and stained with eosin for 5 minutes.
- Dehydration and sealing: The sections were put into anhydrous ethanol I for 5 minutes, anhydrous ethanol II for 5 minutes, anhydrous ethanol III for 5 minutes, xylene I for 5 minutes, and xylene II for 5 minutes to make the sections transparent, and then the sections were sealed with neutral gum.
- Microscopic examination, image acquisition, and analysis were conducted.
PCNA Level15
The sections were put into xylene I for 8 minutes, xylene II for 15 minutes, absolute ethanol I for 6 minutes, absolute ethanol II for 6 minutes, 95% ethanol for 6 minutes, 85% ethanol for 6 minutes, 75% ethanol for 6 minutes, and double distilled water for 2 minutes. Tissue sections were placed in a high-pressure pot filled with citric acid (pH 6.0) antigen repair solution for antigen repair, which lasted for 2.5 minutes starting from air injection. After natural cooling, the slide was placed in phosphate-buffered saline (PBS) (pH 7.4) and washed three times on a decolorizing shaker for 5 minutes each time. The sections were incubated with 3% methanol hydrogen peroxide solution at room temperature (25°C) for 20 minutes in the dark. The slide was placed in PBS (pH 7.4) and washed three times on a decolorizing shaker for 5 minutes each time. After the sections were slightly dried by shaking, a circle was drawn around the tissue with a Pap Pen, and 5% Bovine serum albumin (BSA) was added dropwise in the circle and sealed at room temperature for 1 hour. The blocking buffer was gently shaken off, the primary antibody (prepared in a certain proportion) was added dropwise to the sections, after which the sections were placed in a wet box and incubated overnight at 4°C. The slide was placed in PBS (pH 7.4) and washed three times on a decolorizing shaker for 8 minutes each time. After the sections were slightly dried by shaking, a biotin-conjugated secondary antibody was added dropwise in the circle to cover the tissue and incubated at room temperature for 50 minutes. The slide was placed in PBS (pH 7.4) and washed three times on a decolorizing shaker for 8 minutes each time. After the sections were slightly dried by shaking, the horseradish peroxidase-labeled Streptavidin third antibody was added to the circle to cover the tissue and incubated at room temperature for 50 minutes. The slide was placed in PBS (pH 7.4) and washed three times on a decolorizing shaker for 8 minutes each time. The slide was placed in PBS (pH 7.4) and washed three times on a decolorizing shaker for 5 minutes each time. After the sections were slightly dried by shaking, the freshly prepared DAB color developing solution was added dropwise in the circle, the color development time was controlled under the microscope, and the positive color was brownish yellow. Finally, the sections were rinsed with running water to terminate coloration. Harris–hematoxylin counterstaining was performed for approximately 3 minutes. The sections were rinsed with tap water, differentiated with 1% hydrochloric acid-alcohol for several seconds, rinsed with tap water, and flushed with running water until the nuclei became blue. The sections were put into 75% alcohol for 6 minutes, 85% alcohol for 6 minutes, absolute ethanol I for 6 minutes, absolute ethanol II for 6 minutes, xylene I for 7 minutes, and xylene II for 7 minutes to be dehydrated until they became transparent. The sections were taken out of the xylene and slightly dried by airing, after which the sections were sealed with neutral gum. Microscopic examination, image acquisition, and analysis were conducted. The nuclei stained by hematoxylin were blue, and the positive expression developed by DAB was brownish yellow.
Apoptosis
A fluorescein isothiocyanate (FITC) labeling kit was used for apoptosis detection. The sections were put into xylene I for 10 minutes, xylene II for 10 minutes, xylene III for 10 minutes, absolute ethanol I for 5 minutes, absolute ethanol II for 5 minutes, absolute ethanol III for 5 minutes, and finally washed in distilled water. After the sections were slightly dried by shaking, a circle was drawn around the tissue with a Pap Pen, the tissue was added dropwise and covered with a proteinase K working solution, and the sections were incubated in a constant temperature box at 37°C for 22 minutes. The slide was placed in PBS (pH 7.4) and washed three times on a decolorizing shaker for 5 minutes each time. (Preparation method of proteinase K working solution, stock solution: PBS = 1:9). After the sections were slightly dried by shaking, the tissue was covered with the membrane permeabilization working fluid in the circle and incubated at room temperature for 20 minutes. The slide was placed in PBS (pH 7.4) and washed three times on a decolorizing shaker for 5 minutes each time. (The permeabilization buffer was 0.1% Triton. Preparation method: Triton stock solution: PBS = 1:1000) After the sections were slightly dried by shaking, a buffer was added dropwise in the circle to cover the tissue and incubated in buffer at room temperature for 10 minutes. The proper amounts of terminal deoxynucleotidyl transferase enzyme, 2´-deoxyuridine, 5´-triphosphate, and buffer were taken from the Tunel kit according to the number of sections and tissue size and mixed in a ratio of 1:5:50. The mixture was added to the circle to cover the tissue, the sections were laid flat in a wet box, incubated in an incubator at 37°C for 2 hours, and a small amount of water was added into the wet box to keep the humidity. The sections were washed three times with PBS (pH 7.4) for 5 minutes each time. After the PBS was removed, the4’,6-diamidino-2-phenylindole (DAPI) was added dropwise in the circle and incubated at room temperature in the dark for 10 minutes. The slide was placed in PBS (pH 7.4) and washed three times on a decolorizing shaker for 5 minutes each time. The sections were slightly dried by shaking and sealed with an anti-fluorescence quenching sealing agent. The sections were observed under a fluorescence microscope, and images were acquired. The DAPI-stained nuclei were blue under ultraviolet (UV) excitation, and the positive apoptotic nuclei were green.16 Tunel result calculation:
Positive cell ratio = number of positive cells / total number of cells.17
Positive cell density = number of positive cells / area of tested tissue.18
Average optical density value = cumulative optical density value / positive pixel area.19,20
Positive area ratio = positive area / tissue area.21
Positive area density = cumulative optical density value / tissue pixel area.22
Statistical Analysis of Data
The original data were sorted using Excel 2010 software, and a notability analysis was conducted using statistical software SPSS 20.0. A comparison of multiple data was conducted using the least significant difference method, and P < 0.05 was considered statistically significant. The experimental results were presented as line charts in Origin2019.
Results and Analysis
Effects of Different Daily Ration Supplements on the Feed Intake and Body Weight of Scalded Rats
As is shown in Figure 1, the feed intake of the three groups of rats increased with the increase of the feeding period; the feed intake was the least in the yak meat feed group. On day 7, the intake in the basic feed group was higher than that in the yellow beef feed group, and the feed intake in the yellow beef feed group was higher than that in the basic feed group from day 14 to day 45. The feed intake of the three groups of rats had been increasing—the increase was the fastest in the first week—and the increase in the basic feed group slowed down after one week. The increase in food intake in the yellow beef and the yak meat feed groups was rapid, and their increase rates were almost parallel; however, the feed intake in the yak meat feed group was always lower than that in the yellow beef feed group.
 |
Figure 1 Comparison of feed intake of rats. |
As is shown in Figure 2, the initial body weight of the three groups of rats was approximately 300 g. The body weights of the three groups of rats decreased after one week, and the range of body weight descent in the basic feed group was less than for the other two groups of rats. After two weeks, the body weights in the three groups of rats all increased to a certain extent. The ranges of body weight increases of the rats in the yellow beef and the yak meat feed groups were more than in the basic feed group. After 30 days, the body weight of the three groups of rats all increased rapidly. The yak meat feed group had the greatest range increase, followed by the basic feed group and then the yellow beef feed group. After 45 days, the increase in body weights of the three groups of rats all slowed down. The body weight of the rats in the yak meat feed group was the highest, and the body weight in the yellow beef feed group was the lowest.
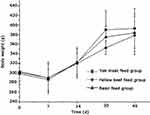 |
Figure 2 Comparison of body weight of rats. |
Effects of Different Daily Ration Supplements on Lee’s Index of Scalded Rats
As is shown in Table 2, the Lee’s indexes on days 7 and 30 after scalding in the yak meat feed group were significantly higher than those on day 45, but there was no significant difference among the remaining time points for this group. The Lee’s index before scalding and on day 14 after scalding in the yellow beef feed group was significantly higher than that on day 45, but there was no significant difference among the remaining time points for this group; the Lee’s index in the basic feed group at 45 days after scalding was significantly lower than for other time points, and there was no significant difference among the remaining time points for this group.
 |
Table 2 Lee’s Index Added in Different Diets for Scald Rats |
As is shown in Figure 3, the initial obesity index (Lee’s index) of the three groups of rats was between 300 and 310. After 7 days, the obesity index decreased the fastest in the yellow beef feed group, decreased slowly in the basic feed group, and increased in the yak meat feed group. After 14 days, the obesity index increased in the yellow beef feed group, increased slowly in the basic feed group, and decreased rapidly in the yak meat feed group. After 30 days, the obesity index decreased rapidly in the yellow beef feed group, decreased slowly in the basic feed group, and increased rapidly in the yak meat feed group. After 45 days, the obesity index of the three groups of rats all decreased, with the obesity index of the rats in the yak meat feed group being the highest and the basic feed group being the lowest. Overall, the obesity index of the three groups of rats all decreased to a certain extent after scalding. Furthermore, the obesity index in the yak beef feed group on day 45 after scalding was the closest of all groups to before scalding.
 |
Figure 3 Comparison of obesity index in rats. |
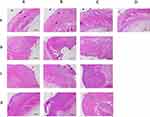
Effect of Different Daily Ration Supplements on the Scalded Wounds
One rat in each group was randomly selected, and the wounds of the rats represented for each group were wounds of the same rat on days 1, 7, 14, 21, and 30 after scalding. The wounds of the rats in all groups healed gradually after scalding, and the wounds of the rats in all groups were clearly scabbed on day 14 after scalding. On day 21, the necks of the rats in the basic feed group were ulcerated and crusted, and the rats in the yak meat feed group had the best wound healing effect. On day 30, the wounds of the three groups of rats had all recovered (Figure 4).
Effect of Different Daily Ration Supplements on the H&E Staining Condition of the Scalded Wounds
Hematoxylin and eosin-stained sections of rats in all groups were represented by the H&E staining of a rat’s wound tissue sections randomly selected from each group on days 7, 14, 30, and 45 after scalding.
As is shown in Figure 5a, the skin tissue of the rats in the yak meat feed, yellow beef, and basic feed groups demonstrate epidermal necrosis and defect and fibrinoid necrosis of the collagen fibers in the dermis. Parts of the collagen fibers were arranged irregularly, swollen, and fractured, and the damage to the muscle layer was deep. There were different degrees of necrosis in the skin appendages, such as the hair follicles and sweat glands, and the number was reduced (black arrows). The inflammatory reaction in the yak meat feed group was severe, and infiltration of many neutrophils and monocytes in the dermis and muscle layer could be observed (blue arrows).
In the skin tissue of the rats in the blank group, the repaired epidermis was intact with a thin spinous layer with mild keratinization (red arrow). The collagen fibers in the dermis were swollen and arranged irregularly (green arrow), the inflammatory reaction was mild, and a scattered distribution of a small number of lymphocytes could be observed (blue arrow).
As is shown in Figure 5b, the skin tissue of the rats in the yak meat feed group showed that the repaired epidermis was intact, with a thin spinous layer with moderate keratinization (red arrow). The collagen fibers in the dermis were swollen and arranged irregularly (green arrow), the inflammatory reaction was mild, and a scattered distribution of a small number of lymphocytes could be observed (blue arrow).
In the skin tissue of the rats in the yellow beef feed group, partially dried necrotic tissue was shed off (black arrows). The epidermis was mildly healed, and there was a new epidermis with uneven thickness and mild keratinization (red arrow). The connective tissue in the dermis was proliferated and repaired. Many fibroblasts and collagen fibers could be observed, and the numbers of hair follicles and sweat glands in the repaired wound were reduced (green arrows). The inflammatory reaction was moderate, and a scattered distribution of neutrophils and lymphocytes could be observed (blue arrow).
In the skin tissue of the rats in the basic feed group, the epidermis was moderately healed, with thick spinous layers of the new epidermis, mild keratinization, and a few elongated cutaneous processes (red arrows). Crusts were found occasionally. The dermal connective tissue proliferated and was repaired. Many fibroblasts and collagen fibers could be observed, and the numbers of hair follicles and sweat glands in the repaired wound were reduced (green arrows). The inflammatory reaction was moderate, a scattered distribution of neutrophils and lymphocytes could be observed (blue arrow), and a small number of new capillaries formed (yellow arrows).
As is shown in Figure 5c, the skin tissue of the rats in the yak meat feed group demonstrated that the epidermis of the wound was basically healed, the bridging gap was small, and there was mild keratinization (red arrow). The connective tissue in the dermis was proliferated and repaired. Many fibroblasts and collagen fibers could be observed, and the numbers of hair follicles and sweat glands in the repaired wound were reduced (green arrows). The inflammatory reaction was mild, a small number of lymphocytes could be observed (blue arrow), and a medium number of new capillaries formed (yellow arrows).
In the skin tissue of the rats in the yellow beef feed group, local dried necrotic tissue remained, forming a crust (black arrow). The epidermis repaired was intact, with thicker spinous layers of the new epidermis and a few elongated cutaneous processes (red arrows). The connective tissue in the dermis proliferated and was repaired. Many fibroblasts and collagen fibers could be observed, and the numbers of hair follicles and sweat glands in the repaired wound were reduced (green arrows). The inflammatory reaction was moderate, a scattered distribution of neutrophils and lymphocytes could be observed (blue arrow), and a small number of new capillaries formed (yellow arrows).
In the skin tissue of the rats in the basic feed group, the repaired epidermis was intact, with a thicker local spinous layer of the new epidermis with mild keratinization (red arrow). The connective tissue in the dermis proliferated and was repaired. Many fibroblasts and collagen fibers could be observed, and the numbers of hair follicles and sweat glands in the repaired wound were reduced (green arrows). The inflammatory reaction of the local dermal layer was moderate, many neutrophils and lymphocytes could be observed (blue arrow), and a small number of new capillaries formed (yellow arrows).
As is shown in Figure 5d, the skin tissue of the rats in the yak meat feed group showed that the epidermis of the wound was moderately healed, the bridging gap was larger, and the spinous layer of the new epidermis was thickened, with a small amount of extended cutaneous process with mild keratinization (red arrow). The connective tissue in the dermis proliferated and was repaired. Many fibroblasts and collagen fibers could be observed, and the numbers of hair follicles and sweat glands in the repaired wound were reduced (green arrows). The inflammatory reaction was mild, a small number of lymphocytes could be observed (blue arrow), and a small number of new capillaries formed (yellow arrows).
In the skin tissue of the rats in the yellow beef feed group, local dried necrotic tissue remained, forming a crust (black arrow). The epidermis was moderately healed, with thicker spinous layers of the new epidermis and a few elongated cutaneous processes (red arrows). The connective tissue in the dermis proliferated and was repaired. Many fibroblasts and collagen fibers could be observed, and the numbers of hair follicles and sweat glands in the repaired wound were reduced (green arrows). The inflammatory reaction was moderate, a scattered distribution of neutrophils and lymphocytes could be observed (blue arrow), and a small number of new capillaries formed (yellow arrows).
In the skin tissue of the rats in the basic feed group, the repaired epidermis was intact, with a thicker local spinous layer of new epidermis with mild keratinization (red arrow). The connective tissue in the dermis proliferated and was repaired. Many fibroblasts and collagen fibers could be observed, and the numbers of hair follicles and sweat glands in the repaired wound were reduced (green arrows). The inflammatory reaction of the local dermal layer was moderate, a scattered distribution of neutrophils and lymphocytes could be observed (blue arrow), and a small number of new capillaries formed (yellow arrows).
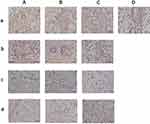
Comparison of Effects of Different Daily Ration Supplements on the PCNA Levels in the Wounds of the Scalded Rats
The tissue sections stained with PCNA in all groups were the same as those in the H&E-stained tissue sections (3.4). Hematoxylin-stained nuclei were blue, and DAB-positive nuclei were brown. Figure 6 demonstrates that PCNA-positive expression gradually decreased. The positive expression of PCNA was the highest in all groups on the day 7 after scalding; the positive expression in the basic feed group was the highest. The expression of PCNA in all groups decreased on day 14 after scalding. The positive expression of PCNA in the yak meat feed group was the lowest on day 30 after scalding, followed by the yellow beef feed group, and the positive expression of PCNA in the basic feed group was the highest. The expression of positive PCNA was the lowest in all groups on day 45 after scalding; the expression of positive PCNA was the lowest in the yak meat feed group and the highest in the basic feed group, suggesting that the recovery of the rats in the yak meat feed group was better than that in the other groups.
As is shown in Table 3, the PCNA-positive cell rate in the three groups of scalded rats decreased with the increase in feeding time. In the basic feed group, the PCNA-positive cell rate of rats increased on days 14 and 30 and decreased on day 45. Among the rats in all groups, the PCNA-positive cell rate in the yak meat feed group was the highest on day 7 and the lowest on day 45. Regarding the PCNA-positive cell rate, the rats in the yak meat feed group recovered better than those in the other two groups.
 |
Table 3 PCNA Positive Cell Rate (%) |
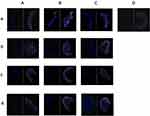
Effects of Different Daily Ration Supplements on Cell Apoptosis
The apoptosis-stained tissue sections in all groups were the same as those in the H&E-stained tissue (3.4). The nuclei stained with DAPI were blue under UV excitation using the FITC labeling kit used, and the positive apoptotic nuclei were green. In this experiment (as is shown in Figure 7), on day 7, the apoptosis of the cells in the yellow beef and basic feed groups was clear. On day 14, the apoptosis of the cells in the yellow beef and the basic feed groups was reduced. On day 30, the apoptosis of the cells was not displayed in all groups. Finally, on day 45, the apoptosis of the cells in the yellow beef and the basic feed groups was mild.
As is shown in Table 4, the positive cell rate and positive area ratio of the scalded rats in the different feed groups changed with time after scalding. In the yak meat feed group, the positive cell rate was the highest on day 7, gradually decreased on days 14 and 30, and fluctuated upward slightly on day 45. The positive area ratio for the yak meat feed group was the highest on day 7, decreased on days 7 and 14, and slightly increased on day 45. In the yellow beef feed group, the positive cell rate decreased sequentially from days 7 to 45, and the ratio of the positive area also decreased sequentially during this time. In the basic feed group, the positive cell rate and positive area ratio both decreased sequentially with time and increased to a certain extent on day 45. The positive cell rate of the yellow beef feed group was lower than in the blank group on day 45, and the positive cell rates of the yak meat and basic feed groups were lower than in the blank group on day 30. The positive area ratio of the yak meat feed group was lower than in the blank group on days 30 and 45. The positive area ratio of the yellow beef feed group was lower than in the blank group on day 45. The positive area ratio of the basic feed group was lower than in the blank group on day 30.
 |
Table 4 Positive Cell Ratio and Positive Area Ratio of Scald Rats in Different Groups |
As is shown in Table 5, the positive cell density, average optical density, and positive area density of the scalded rats in the feed groups were different. On day 7 after scalding, the positive cell density was highest in the yellow beef feed group, then the basic feed group, then the yak meat feed group, and finally, the blank group. On day 14, the positive cell density was highest in the yellow beef feed group, followed by equal amounts of yak meat and basic feed groups. On day 30, the positive cell density was highest in the yellow beef feed group, then the yak meat feed group, and then the basic feed group. On day 45, the positive cell density was highest in the basic feed group, then the yak meat feed group, and then the yellow beef feed group. The density of positive cells in the yak meat feed group decreased to a certain extent from day 7 to day 30 after scalding and was lower than that in the yellow beef and basic feed groups. The density of positive cells in the yak meat feed group increased on day 45 and was higher than in the yellow beef feed group and lower than in the basic feed group. The density of positive cells in the yellow beef feed group decreased continuously from day 7 to day 45 after scalding and was lower than that in the yak meat and basic feed groups on day 45. The density of positive cells in the basic feed group decreased from day 7 to day 30, while it increased to a certain extent on day 45 and was higher than that in the yellow beef and yak meat feed groups.
 |
Table 5 Comparison of Positive Cell Density, Average Optical Density and Positive Surface Density of Scald Rats in Different Feed Groups |
On day 7 after scalding, the average optical density was highest in the blank group, then the yak meat feed group, then the basic feed group, and finally, the yellow beef feed group. On day 14, the average optical density was highest in the yak meat feed group, then the basic feed group, and then the yellow beef feed group. On day 30, the average optical density was highest in the basic feed group, then the yak meat feed group, and then the yellow beef feed group. On day 45, the average optical density was highest in the yak meat feed group, then the yellow beef feed group, and then the basic feed group. The average optical density of the rats in the yak meat feed group decreased to a certain extent from day 7 to day 14 after scalding and also from day 30 to day 45. The average optical density in the yellow beef feed group continuously increased from day 7 to day 45. The average optical density in the basic feed group increased from day 7 to day 30, decreased on day 45, and was lower than in the yak meat and yellow beef feed groups.
On day 7 after scalding, the positive area density was highest in the yak meat feed group, then the yellow beef feed group, then the basic feed group, and finally, the blank group. On day 14, the positive area density was highest in the yellow beef feed group, then the basic feed group, and then the yak meat feed group. On day 30, the positive area density was highest in the yellow beef feed group, then the yak meat feed group, and then the basic feed group. On day 45, the positive area density was highest in the basic feed group, then the yak meat feed group, and then the yellow beef feed group. The positive area density of the rats in the yak meat feed group decreased rapidly from day 7 to day 14, while it increased to a certain extent from day 30 to day 45. The positive area density of the rats in the yellow beef feed group decreased to a certain extent from day 7 to day 45. The positive area density of the rats in the basic feed group decreased to a certain extent from day 7 to day 30 while increasing on day 45. The positive area density of the three groups of rats on day 45 after scalding overlapped with each other.
Discussion
Effects of Different Daily Ration Supplements on the Growth Performance of the Scalded Rats
After scalding, the food intake of the rats in the different feed groups was different. On day 7 after scalding, the rats’ food intake significantly reduced, which was consistent with the results of a study conducted by Chen et al.23 They found that in the first 3 days after scalding, the food intake of rats decreased significantly but returned to the normal rat level on day 4 after scalding. Overall, the food intake of rats in the yellow beef feed group was the highest, and it was the lowest in the yak meat feed group. However, after scalding, the body weight of the three groups of rats first decreased and then increased, and the body weight of the rats in the yak meat feed group was the highest; it was the lowest in the yellow beef feed group. The decrease of the obesity index in the rats after scalding may be due to the stimulation of rats, which was similar to the results of the study conducted by Dong et al,24 who reported that acupuncture was beneficial for weight loss in male and female rats with simple obesity. It might also be because the body energy consumed a lot after being scalded, and the great demand for nutrients caused the rats to lose weight. This idea was verified by the fact that the obesity index of the rats in the yak meat feed group was higher than in the other two groups. Furthermore, the obesity index of the yak beef feed group on day 45 after scalding was the closest to that of the rats before scalding.
Effect of Different Daily Ration Supplements on the Scalded Rats
Scalded rats need to consume many nutrients to maintain the body’s daily metabolism and repair requirements. Table 1 reveals that yak meat feed was richer in nutrients than yellow beef and was thus more conducive to the recovery of the scalded rats. From a macro perspective, the rats in the yak meat feed group had the best wound recovery on day 30, while the rats in the basic feed group had neck ulcerations on day 21 during the single-cage feeding process. This proved the beneficial effects of nutrients for the rats’ recovery. Chen et al25 revealed that the appropriate addition of arginine was beneficial to healing burns. Chen et al26 revealed that feeding rats with pigeon meat led to the wound healing rate of scalded rats being higher than in the control group. The results of the H&E staining also showed that the rats fed with 30% pigeon meat had a better recovery effect than those fed with 20% pigeon meat. In this study, the results of the H&E staining revealed that the recovery effect of scalded rats in the yak meat feed group was better than in the other two groups.Liu et al27 revealed that oxymatrine could increase the expression of PCNA in skin wound healing. In terms of PCNA, it can be concluded that the positive cell rate of the yak meat feed group was lower than in the other groups.
Apoptosis could cause a decline in immune function. Early enteral feeding could reduce apoptosis, thereby improving the body’s immune function.28 Removing the apoptotic cells during apoptosis was more conducive to wound healing.29 Zhou et al30 revealed that, with the passing of feeding time, the apoptosis of fibroblasts in the wounds of burned rats decreased to a certain extent under the action of alkaline calponin. Ling et al31 revealed that allicin could inhibit cell apoptosis and heal burns in rats. In this study, the decrease in the apoptotic ratio and area indicated that the wound surface of the scalded rats recovered, and the wounds of the scalded rats in the yak meat feed group recovered faster. The apoptotic ratio and area on day 30 were smaller in the yak meat feed group than in the blank control group. These indexes in the yellow beef feed group tended to be stable The apoptotic ratio and area of the basic feed group increased more significantly than the other two groups on day 45. The positive cell density decreased gradually in the yellow beef feed group while increasing on day 45 in the yak meat feed group. The average optical density of the yak meat group was higher than in the other groups at each time point, except for the blank group. The positive area density of the yak meat feed group on day 14 was lower than in the blank control group. The positive area density of the yellow beef and basic feed groups was lower than in the blank control group on day 30.
Conclusion
- For growth performance, the food intake, body weight, and Lee’s index of the yak meat feed group were all better scoring than those of the other two groups, and the postures of the rats in the yak meat feed group were close to that of healthy rats.
- From a macroscopic state perspective, the recovery of the skin wounds of the scalded rats in the yak meat feed group was better than for the rats in the other groups. The necks of the rats in the basic feed group were not scalded but ulcerated, which fully illustrated the importance of nutrients for burn recovery.
- From the pathological tissue perspective, the H&E staining and PCNA detection both showed that the skin tissue condition of the rats in the yak meat feed group was better than in the other groups, and the positive cell number was lower than in the other groups. From the apoptosis results, it could be concluded that the yak meat group recovered faster than the other groups.
- In conclusion, the rats in the yak meat feed group recovered better than those in the other groups, and the yak beef feed had the best effect on the wound healing of the scalded rats.
Data Sharing Statement
All data generated or analysed during this study are included in this article. Further enquiries can be directed to the corresponding author.
Ethics Approval and Consent to Participate
This study was conducted with approval from the Ethics Committee of Affiliated Hospital of Qinghai University (P-SL-2020089) and complied with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Acknowledgments
We would like to acknowledge the hard and dedicated work of all the staff that implemented the intervention and evaluation components of the study.
Funding
This research was funded by Qinghai Province “Kunlun Talents High-end Innovation and Entrepreneurial Talents” project, Natural Science Foundation of China (NSFC, 32060766), Qinghai Province Key Laboratory of Animal Nutrition and Feed Science for Plateau Grazing Livestock (2022-ZJ-Y17), Nature Fund of Qinghai province (2022-ZJ-926).
Disclosure
The authors declare that they have no competing interests for this work.
References
1. Geletu US, Usmael MA, Mummed YY, Ibrahim AM. Quality of cattle meat and its compositional constituents. Vet Med Int. 2021;2021:7340495. doi:10.1155/2021/7340495
2. Lancaster PA, Davis ME, Tedeschi LO, Rutledge JJ, Cundiff LV. A mathematical nutrition model adequately predicts beef and dairy cow intake and biological efficiency. Transl Anim Sci. 2021;6(1):txab230. doi:10.1093/tas/txab230
3. Xhao H, Mao J. Meat nutrition and quality analysis of Changtai Yak Beef. Hubei Agric Sci. 2017;56(16):3117–3120.
4. Li W, Wang J, Zhang C, et al. Using an integrated feature-based molecular network and lipidomics approach to reveal the differential lipids in yak shanks and flanks. Food Chem. 2022;403:134352. doi:10.1016/j.foodchem.2022.134352
5. Li WH, Gao YL, Yang XL, Xi B, Xiong L. Analysis on amino acid of different yak meat. Hubei Agric Sci. 2018;57(12):89–90.
6. Ya B, Xiong W, Li G, et al. Nutritional Composition of Yak Beef in Western Sichuan. Farm Prod Process. 2019;20:64–66.
7. Zhang Q, Hao L, Liu S, et al. Comparative research on nutritional content for adult yaks meat in different regions. Sci Technol Food Indust. 2018;39(1):302–307.
8. Guo HF, Mohd Ali R, Abd Hamid R, et al. Epidermal growth factor and tocotrienol-rich fraction cream formulation accelerates burn healing process based on its gene expression pattern in deep partial-thickness burn wound model. Int J Low Extrem Wounds. 2022;21(4):544–554. PMID: 33241700. doi:10.1177/1534734620971066
9. Mertin V, Most P, Busch M, et al. Current understanding of thermo(dys)regulation in severe burn injury and the pathophysiological influence of hypermetabolism, adrenergic stress and hypothalamic regulation-a systematic review. Burns Trauma. 2022;10:tkac031. doi:10.1093/burnst/tkac031.
10. Kurmis R, Nicholls C, Singer Y, et al. An investigation of early enteral nutrition provision in major burn patients in Australia and New Zealand. Nutr Diet. 2022;79(5):582–589. doi:10.1111/1747-0080.12746.
11. Siritientong T, Thet D, Buangbon M, et al. Nutritional support with omega-3 fatty acids in burn patients: a systematic review with meta-analysis of randomized controlled trials. Nutrients. 2022;14(14):2874. doi:10.3390/nu14142874.
12. Zhang N, Song X, Dong W, Liu L, Cui Z, Ma Y. Nutritional evaluation of fish protein hydrolysate and its application in piglet production. J Anim Sci. 2022;100(3):skab369. doi:10.1093/jas/skab369
13. Chen X, Chen S, Ren Q, et al. A metabonomics-based renoprotective mechanism analysis of empagliflozin in obese mice. Biochem Biophys Res Commun. 2022;621:122–129. PMID: 35820282. doi:10.1016/j.bbrc.2022.06.091
14. Boroujeni MM, Barzi SM, Zafari M, et al. Electrosprayed cefazolin-loaded niosomes onto electrospun chitosan nanofibrous membrane for wound healing applications. J Biomed Mater Res B Appl Biomater. 2022;110(8):1814–1826. PMID: 35195946. doi:10.1002/jbm.b.35039
15. Ding J, Lei L, Liu S, et al. Macrophages are necessary for skin regeneration during tissue expansion. J Transl Med. 2019;17(1):36. PMID: 30665437; PMCID: PMC6341552. doi:10.1186/s12967-019-1780-z
16. Nosrati H, Hamzepoor M, Sohrabi M, et al. The potential renal toxicity of silver nanoparticles after repeated oral exposure and its underlying mechanisms. BMC Nephrol. 2021;22(1):228. PMID: 34144690; PMCID: PMC8212496. doi:10.1186/s12882-021-02428-5
17. Li L, Sun R, Miao Y, et al. PD-1/PD-L1 expression and interaction by automated quantitative immunofluorescent analysis show adverse prognostic impact in patients with diffuse large B-cell lymphoma having T-cell infiltration: a study from the International DLBCL Consortium Program. Mod Pathol. 2019;32(6):741–754. doi:10.1038/s41379-018-0193-5
18. Xu-Monette ZY, Xiao M, Au Q, et al. Immune profiling and quantitative analysis decipher the clinical role of immune-checkpoint expression in the tumor immune microenvironment of DLBCL. Cancer Immunol Res. 2019;7(4):644–657. doi:10.1158/2326-6066.CIR-18-0439
19. Liu S, Jin Z, Zhang Y, et al. The glucagon-like peptide-1 analogue liraglutide reduces seizures susceptibility, cognition dysfunction and neuronal apoptosis in a mouse model of dravet syndrome. Front Pharmacol. 2020;11:136. doi:10.3389/fphar.2020.00136
20. Dogan S, Vasudevaraja V, Xu B, et al. DNA methylation-based classification of sinonasal undifferentiated carcinoma. Mod Pathol. 2019;2019:988.
21. Luo W, Ai L, Wang BF, Zhou Y. High glucose inhibits myogenesis and induces insulin resistance by down-regulating AKT signaling. Biomed Pharmacother. 2019;120:109498. doi:10.1016/j.biopha.2019.109498
22. Jiao X, Fei X, Li S, Lin D, Ma H, Zhang B. Design mechanism and property of the novel fluorescent probes for the identification of microthrix parvicella in situ. Materials. 2017;10(7):804. doi:10.3390/ma10070804
23. Chen B, Niu R. Changes of conventional nutrient metabolism in burned rats. Sichuan J Zool. 2001;2:100–102.
24. Dong N, Zhang S, Liang Y, et al. Effect of lose weight on the different gender rats with simple obesity by acupuncture. J Chin Med. 2015;30(6):846–848. doi:10.16368/j.issn.1674-8999.2015.06.292
25. Chen X, Lu S, Xu W, Chen Z, Shi J. Effect of dietary arginine supplementation on wound healing and the relationship between dose and response in burn rats. Chin J Surg. 1999;5:17–20.
26. Chen Y, Yang H, Wang Z, et al. Effect of diets with pigeon meat on wound healing in rats. J Dom Anim Ecol. 2019;40(8):42–47.
27. Liu J, Liu Y, Li J, Dai G, Zheng P. Effects of oxymatrine on PCNA, a-SMA and collagen I in the process of skin wound healing of mice. Ningxia Med J. 2022;44(1):10–13. doi:10.13621/j.1001-5949.2022.01.0010
28. Ren H, Zhao F, Zhang Q, Huang X, Wang Z. Autophagy and skin wound healing. Burns Trauma. 2022;10:tkac003. doi:10.1093/burnst/tkac003
29. Markiewicz-Gospodarek A, Kozioł M, Tobiasz M, Baj J, Radzikowska-Büchner E, Przekora A. Burn wound healing: clinical complications, medical care, treatment, and dressing types: the current state of knowledge for clinical practice. Int J Environ Res Public Health. 2022;19(3):1338. doi:10.3390/ijerph19031338.
30. Zhou B, Zhang J, Wang L, et al. Effect of calponin on fibroblast proliferation and apoptosis in burn wound of diabetic rats. Chin J Injury Repair Wound Healing. 2019;14(3):195–201.
31. Ling S, Yang L, Tao J, Wang X, Su X, Wang J. Inhibition of allicin on apoptosis of deep second degree burns wound rats and its mechanism. Med J West China. 2020;32(12):1739–1743.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.