Back to Journals » Drug Design, Development and Therapy » Volume 16
Effect of Weight-Adjusted Phenylephrine, Norepinephrine, and Metaraminol for Elective Cesarean Delivery on Neonatal Acid–Base Status: A Randomized Controlled Trial
Authors Liu T, Cheng Z, Zou S, Xu C, Pan S, Zeng H, Shan Y, Feng Y, Zhang H
Received 5 July 2022
Accepted for publication 6 September 2022
Published 21 September 2022 Volume 2022:16 Pages 3215—3223
DOI https://doi.org/10.2147/DDDT.S381048
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Anastasios Lymperopoulos
Tianyu Liu,1,* Zhiyong Cheng,2,* Shiya Zou,3,* Chao Xu,4 Shoudong Pan,3 Huabei Zeng,2 Yidong Shan,2 Yi Feng,1 Hong Zhang1
1Department of Anesthesiology, Peking University People’s Hospital, Beijing, People’s Republic of China; 2Department of Anesthesiology, Suqian Maternity Hospital, Suqian, Jangsu, People’s Republic of China; 3Department of Anesthesiology, Capital Institute of Pediatrics Affiliated Children’s Hospital, Beijing, People’s Republic of China; 4Department of Anesthesiology, Zhongshan Hospital, Fudan University, Shanghai, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yi Feng; Hong Zhang, Department of Anesthesiology, Peking University People’s Hospital, 11 Xizhimen South Street, Xicheng District, Beijing, People’s Republic of China, Tel +86-13601083503 ; +86-13311281808, Email [email protected]; [email protected]
Purpose: Many previous trials have compared the effects of different vasoactive drugs on cesarean section patients, but their infusion rate is based on experience rather than high-quality evidence. It is difficult to judge whether the effect of vasoactive drug comes from the better choice or a more appropriate at rates of vasoactive drugs. The effect of vasoactive drugs at the rates of the 90% effective dose needs to be verified and compared.
Patients and Methods: Women undergoing elective caesarean delivery under combined spinal-epidural anaesthesia were randomized to receive phenylephrine or norepinephrine or metaraminol infusion at the rate that was assumed to be the 90% effective dose. Anesthetic management was standardized and included fluid loading with 10 mL/kg of Ringer. The primary outcome was the umbilical artery pH.
Results: 78 patients were included. The umbilical artery pH was not significantly different among the three groups (phenylephrine group: 7.33 ± 0.03 vs norepinephrine group: 7.33 ± 0.04 vs metaraminol group: 7.33 ± 0.04, P = 0.99). There were no significant differences in the incidence of hypotension, hypertension, bradycardia, and nausea and vomiting among the three groups. The SBP of the phenylephrine group was significantly higher than that of the metaraminol group (adjustive P value = 0.005).
Conclusion: Phenylephrine (0.54 μg/kg/min) or metaraminol (2 μg/kg/min) or norepinephrine (0.08 μg/kg/min) administered to healthy patients with elective cesarean section after spinal anesthesia has no significant effect on the acid-base balance of the fetus.
Keywords: cesarean section, vasoactive drugs, foetal acid-base balance, randomized controlled trial
Introduction
During cesarean delivery, hypotension after spinal anesthesia is a frequent maternal complication that has adverse effects on both the mother and fetus.1,2 The infusion of vasoactive drugs remains the most effective treatment method.3–5 However, controversies exist regarding the best choice and the appropriate rate of vasoactive drugs.
The vasoactive drug that is currently recommended by obstetric anesthesia guidelines is phenylephrine, which replaced the use of ephedrine.3,6,7 Recently, an increasing number of studies have focused on other drugs. Norepinephrine and metaraminol have recently been considered better alternatives to phenylephrine because β1 adrenergic receptor antagonism can mitigate the reflex decrease in cardiac output.8–10 Two recent network meta-analyses determined that metaraminol and norepinephrine might be better choices than phenylephrine in preventing hypotension and improving the fetal acid–base balance.11,12
In a large-sample noninferiority study of 664 patients, Ngan Kee et al found that there was no significant difference in umbilical artery pH (UA-pH) between the norepinephrine group (mean, 7.289; 95% confidence interval [CI], 7.284–7.294) and the phenylephrine group (mean, 7.287; 95% CI, 7.281–7.292) (mean difference between groups, 0.002; 95% CI, −0.005 to 0.009).13 In another noninferiority trial, when compared with the phenylephrine group, the metaraminol group had a significantly higher UA-pH and a lower incidence of hypotension with a dose ratio of 5:1 for metaraminol: phenylephrine.9 However, it is notable that the rates of the three vasoactive drugs were estimated based on clinical experience, and the authors were very cautious about the interpretation of the findings, especially as they were not convinced that the rates were equipotent. It is difficult to determine whether the effects of vasoactive drugs are a result of the better choice or a more appropriate rate of vasoactive drugs.
After several dose–response studies, the 90% effective dose (ED90) of weight-adjusted infusions of phenylephrine (0.54 μg/kg/min), norepinephrine (0.08 μg/kg/min) and metaraminol (2 μg/kg/min) for preventing hypotension during cesarean delivery was determined.14–16 However, these ED90s were estimated through the use of a probit regression instead of determining a 10% incidence of hypotension at these dosing rates; therefore, the effects of phenylephrine (0.54 μg/kg/min), metaraminol (2 μg/kg/min) and norepinephrine (0.08 μg/kg/min) on the fetus and the mother need to be verified and compared by clinical trials.
When considering the above context, we assumed that phenylephrine (0.54 μg/kg/min) and norepinephrine (0.08 μg/kg/min) have no significant difference in their effects on the fetal acid–base balance, and that metaraminol (2 μg/kg/min) can significantly improve the acid–base balance of the fetus compared with phenylephrine with a dose ratio of 4:1 for metaraminol: phenylephrine.9,13 We aimed to perform a three-group trial to compare the effects of phenylephrine, norepinephrine and metaraminol with the ED90 on the fetal acid–base balance, which may be helpful for guiding clinical practice.
Materials and Methods
Study Design and Participants
This study complies with the Declaration of Helsinki. This study was approved by the Ethics Committee of Suqian Maternity Hospital (SFY2021-HZ08-11), and written informed consent was obtained from all subjects participating in the trial. The trial was registered prior to patient enrollment at clinicaltrials.gov (NCT04991662, Principal investigator: Chao Xu, Date of registration: Aug-20-2021).
The inclusion criteria were as follows: (1) Singleton pregnancy at term scheduled to be delivered via elective cesarean delivery; (2) Height 150 cm-180 cm; (3) American Society of Anesthesiologists (ASA) II–III; and (4) body mass index (BMI) less than 35 kg/m2.
The exclusion criteria were as follows: (1) Transverse presentation, suspected fetus macrosomia; (2) uterine abnormalities (eg, large fibroids, bicornuate uterus); (3) polyhydramnios; (4) ruptured membranes, oligohydramnios; (5) intrauterine growth restriction; (6) gestational or nongestational hypertension, diabetes, or eclampsia; (7) hypertensive disorders or any condition associated with autonomic neuropathy (such as diabetes mellitus for more than 10 years), renal failure; (8) contraindications for combined spinal-epidural anesthesia; or (9) participants who declined to sign informed consent forms.
Randomization and Blinding
Subjects were randomized by use of a random number table (n = 78) provided by the randomization website http://tools.medsci.cn/rand; the allocation was hidden using an opaque envelope. The intervention drugs were made by an investigator who was not involved in the trial and labeled with groups 1, 2 and 3. Patients and doctors were blinded to the grouping.
Intraoperative Procedures
Patients fasted for 6–8 hours but were allowed clear liquids up to 2 hours prior to surgery. There was no premedication.
Upon arrival in the operating theatre, peripheral venous access was obtained in the patients’ left arm. Patients were monitored with electrocardiography (ECG), noninvasive blood pressure (NIBP) and pulse oximetry (SpO2); 5 L/minute of oxygen was supplied via a nasal cannula.17 After the woman had been in the operating room for 5 minutes, the average of the three SBP values was taken in the supine position as the baseline SBP. The interval between the three measurements was 2 minutes, until the difference between the three consecutive measurements and the mean value did not exceed 10%. The baseline SBP was used to determine whether patients had hypotension (SBP reduction >20% baseline value or SBP < 90 mmHg) during cesarean delivery.
Combined spinal-epidural anesthesia was performed by the same two anesthesiologists. Combined spinal-epidural anesthesia was performed in the left lateral position using a needle through needle technique with a 16-G epidural needle using the loss of resistance to air at the estimated L2-L3 vertebral interspace. A 25-G pencil-point spinal needle was advanced through the epidural needle, and the flow of CSF was demonstrated; 2 mL ropivacaine 0.75% and 1mL CSF was injected intrathecally at a rate of 1 mL per 10s. The spinal needle was removed, an epidural catheter was advanced 3–5 cm into the epidural space, and the epidural needle removed. The end of the spinal injection was time zero.
The sensory block level was measured by assessing loss of painful pinprick sensation using an 18 gauge epidural needle and we removed patients with a sensory level higher than T4. Patients were excluded if the block was lower than T6 by 10 minutes after injection.18
The intervention drugs were prepared by researchers who did not participate in the surgery (phenylephrine 108μg/mL, norepinephrine 16μg/mL, metaraminol 400μg/mL). Immediately after spinal injection, patients were placed in 15° left-tilt position with a wedge (50 cm long, 28 cm wide, and 7.5 cm high, consisting of dense sponges) and injected with metaraminol or phenylephrine or norepinephrine at 0.3mL/kg/h according to the group; 10 mL/kg of Ringer lactate was instilled quickly within 10–15 minutes; then, the infusion was changed to a uniform slow infusion.14–16
Immediately after the delivery of the fetus, UA blood and umbilical vein (UV) blood were taken from double-clamped segment of the umbilical cord and transported by an investigator blinded to the group assignment to a Roche Cobas b 123 POC blood gas analyzer (Roche Group, Basel, Switzerland) within 30s. Apgar scores were rated at 1 and 5 minutes after delivery; the clinicians assessing the neonates and assigning Apgar scores were blind to group allocation.
The NIBP was cycled every minute until delivery and then changed to every 2 minutes. Reactive hypertension was defined as an increase in SBP to ≥120% of the baseline values, and was managed by stopping the infusion; the infusion was restarted when SBP returned to <120% of the baseline value. Maternal hypotension (SBP reduction >20% baseline value or SBP < 90 mmHg) was treated with intervention drugs injected at double the initial rate. A dose of 0.5 mg of atropine was administered for an HR < 50 bpm without hypotension. If the patient’s baseline SBP was less than 90 mmHg, the target was to maintain an SBP at or above 90 mmHg. If the hypotension (SBP reduction >20% baseline value or SBP < 90 mmHg) was not improved for 3 consecutive minutes under this regime, the patient could be given additional phenylephrine, additional ephedrine, or additional intravenous fluids, and the position could be adjusted. If all of the above are still ineffective, patients could be given epinephrine. The dose of additional vasoactive drugs was determined by the anesthesiologist. The time of first hypotension use was recorded, as was additional vasopressor use before delivery.
Statistical Analysis
The primary outcome was the UA-pH.19 The secondary outcome indicators included other blood gas analysis results of UA blood and UV blood, incidence of hypotension. Apgar scores at 1 and 5 minutes after delivery, SBP within 12 min after the end of spinal injection (based on clinical experience, most fetuses have already been delivered at this time point), and the presence of hypertension (SBP ≥ 120% baseline value) nausea (the patient was queried at the end of the operation), vomiting (vomiting 10 mL or more of liquid or vomiting solids or the observation that the patient retched), and bradycardia (heart rate (HR) was ≤60 bpm) before delivery.13
Data analysis was performed using SPSS 21.0 (SPSS, Inc, Chicago, IL). For quantitative data, we used the Shapiro–Wilk test to evaluate for a normal distribution: normally distributed data (such as age and BMI) are expressed as the mean ± standard deviation (X ± S). The comparison of quantitative data between groups was tested by analysis of variance (ANOVA) and the least significant difference (LSD) method, and repeated measurement data were compared using a linear mixed-effects model. The group, time and group-by-time interaction were fixed effects, and the random effect was a random intercept for subjects. Nonnormally distributed data are expressed as the median (M) and interquartile range (IQR). The Kruskal–Wallis H-test was used to compare data between groups in non-normal distributions. Count data are expressed by proportions, and the comparison of different groups was performed with the Cochrane Armitage χ2 test. Kaplan–Meier curves were used to assess the time to the first incidence of hypotension after anesthesia, and the Log rank test was used to compare the first rescue time in the 3 groups. Post hoc pairwise comparison was performed using Bonferroni test. A P value of less than or equal to 0.05 was considered statistically significant.
Sample size calculation was performed using ANOVA with PASS 11.0 (NCSS, LLC, Kaysville, USA) software.20 The primary outcome of the trial is the UA pH.19 The trial carried out by Crawford et al determined that a 15° tilt could cause a difference of 0.039 in the UA pH, which led to the 15° tilt position being widely used clinically; therefore, we estimate that a UA pH difference of 0.03 has clinical significance.21 According to previous trials, we estimated that compared with the phenylephrine group, the norepinephrine group has no significant difference in UA-pH (both 7.28), while the metaraminol group can increase UA-pH to 7.31.4,9,13 We estimated (based on pilot data) within group SDs of 0.03. Considering the possible pairwise comparison, α = 0.05/3 = 0.017 and β = 0.2, a total of 63 patients was obtained. Accounting for possible dropout, each group contained 26 patients.
Results
This trial was conducted at Suqian Maternity Hospital from October to December 2021. A total of 112 women were screened during the trial, of which 78 women met the inclusion criteria and were randomized (Phenylephrine group, n = 26; Norepinephrine group, n = 26; Metaraminol group, n = 26). No patients were lost to follow-up. Data from 78 patients were included in the analysis (Figure 1).
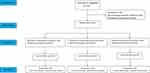 |
Figure 1 Consolidated Standards of Reporting Trials (CONSORT) flow diagram. |
Baseline Data
No clinically important differences were found in the baseline data (Table 1).
 |
Table 1 Baseline Data of Maternal |
Neonatal Outcomes
UA blood and UV blood were successfully obtained from the correct vessels. The primary outcome (neonatal UA pH) was not significantly different among the three groups (phenylephrine group: 7.33 ± 0.03 vs norepinephrine group: 7.33 ± 0.04 vs metaraminol group: 7.33 ± 0.04, P = 0.99). The difference in the means between phenylephrine group and norepinephrine group was −0.002 (98.3% CI, −0.023 to 0.02, P = 0.89); between the phenylephrine group and metaraminol group was −0.001 (98.3% CI, −0.022 to 0.021, P = 0.96); and between the norepinephrine and metaraminol group was 0.001 (98.3% CI, −0.02 to 0.022, P = 0.93).
No significant difference was found in UA base excess (BE), UV BE, or UV pH. There was no fetus with an Apgar score of less than 7 in 1 minute or 5 minutes after delivery (Table 2).
 |
Table 2 Neonatal Acid–Base Status and Apgar Score |
Maternal Hemodynamics
A linear mixed-effects model was used to examine the changes in SBP over the first 12 minutes after anesthesia. The time-by-group term interaction was not statistically significant (P = 0.27). There was significant difference in the SBP over time (P < 0.001). There was a significant difference in the SBP among three groups (F = 5.05, P = 0.007); after a pairwise comparison, we only found that patients in phenylephrine group had higher SBP than patients in metaraminol group (adjustive P value = 0.005) (Figure 2). Only at 12 min after anesthesia, we found patients in phenylephrine group had higher SBP than patients in metaraminol group (adjustive P value = 0.04).
 |
Figure 2 Maternal Mean Systolic Blood Pressure After Spinal Anesthesia. |
The incidence of hypotension (SBP reduction >20% baseline value or SBP < 90 mmHg) has no significant difference among the three groups (P = 0.08). The incidence of hypertension (P = 0.36), bradycardia (P = 0.13) and nausea and vomiting (P = 0.31) also have no significant difference among the three groups (Table 3).
 |
Table 3 Other Outcome Measures |
Two patients in each of the three groups still had hypotension 3 minutes after the double-dosed trial drug treatment, and they were all given an additional 10 mg of ephedrine.
We used Kaplan–Meier survival curves and the Log rank test to compare the time points of the first occurrence of hypotension after anesthesia (Figure 3). The results showed that there is no significant difference among groups (P = 0.06).
 |
Figure 3 Kaplan–Meier curves for time to first incidence of hypotension after anesthesia. |
Discussion
Currently, an increasing number of cesarean section patients receive prophylactic vasoactive drug infusion after spinal anesthesia, which has greater advantages for patients compared to bolus administration and can reduce the workload of anesthesiologists.22 However, the drug infusion rate of many trials is based on clinical experience rather than evidence, so it is difficult to judge whether the effect of vasoactive drugs comes from the better choice or a more appropriate rate.9,13,23,24 Some dose–response trials used probit regression analyses to estimate the ED90 of phenylephrine, norepinephrine and metaraminol for the prevention of intraoperative hypotension during cesarean section.14–16 However, probit regression is a mathematical estimation, and its confidence interval is very wide; therefore, the results need to be verified by clinical trials.
In this trial, we used 15 mg of ropivacaine for spinal anesthesia. Three groups of patients were treated with metaraminol (2 μg/kg/min), phenylephrine (0.54 μg/kg/min) or norepinephrine (0.08 μg/kg/min) at the initial rate, and the results of this study ultimately demonstrated several interesting discoveries. The incidence of hypotension in the phenylephrine group was 12%, and the incidence of hypotension in the norepinephrine group and the metaraminol group was 38% and 27%, respectively. The incidence of nausea and vomiting in the phenylephrine group was lower than that in the norepinephrine and metaraminol groups. This indicates that the previous estimates of ED90 for phenylephrine, norepinephrine and metaraminol may be underestimated. The reasons for these results may be as follows: first, compared to previous trials that estimated the ED90 of phenylephrine, norepinephrine, and metaraminol, our study used different doses and different specific gravities of drugs for spinal anesthesia; second, the confidence interval of ED90 obtained by probit regression is wide, which may lead to a deviation in the median of ED90; third, it is worth noting that this analysis is based on secondary outcome indicators and should be interpreted with caution and that the differences were not statistically significant but only numerically significant.
The UA pH of the three groups was almost the same (P = 0.99). This may be because the duration of hypotension was short; only two patients in each group had hypotension lasting more than 3 minutes and required additional vasoactive drug treatment. The occurrence of hypotension in a short period of time does not lead to significant differences in the fetal acid–base status.25 This shows that anesthesiologists need not be too indecisive in the choice of vasoactive drugs but should pay more attention to the infusion rate, which can effectively prevent hypotension.
This trial had some limitations. First, in addition to the three articles we selected, there have been other trials that determined the appropriate infusion rate of vasoactive drugs during cesarean section.26–28 We ultimately chose the results of the trials that were conducted in the same clinical center because we believe that this can effectively reduce heterogeneity.14–16 However, it is worth noting that these three trials used different spinal anesthesia drugs (10 mg of hyperbaric bupivacaine plus 5 μg of sufentanil or 15 mg of ropivacaine). According to reports, the use of 15 mg of ropivacaine in spinal anesthesia has a shorter sensory and motor block time than 10 mg of bupivacaine, and there was no significant difference in the mother’s SBP or diastolic blood pressure between the two groups.29 Therefore, 15 mg of ropivacaine was used for spinal anesthesia in our trial, and we believe that the choice of spinal anesthesia drugs will not have a significant impact on the main results of our trial. Second, oxygen supplementation can treat nausea and cerebral blood deoxygenation following spinal anesthesia, so we administered oxygen inhalation immediately after anesthesia instead of only when the patient’s blood oxygen saturation was less than 92%. However, this may influence the umbilical artery blood gas measurements. Third, this trial was a single-center clinical trial with a small sample size, and the generalizability of its results may be limited; our finding is only directly applicable to healthy women with a BMI ≤35 kg/m2 who are undergoing elective cesarean delivery of a normal weight singleton fetus.
Conclusion
Phenylephrine (0.54 μg/kg/min), metaraminol (2 μg/kg/min) or norepinephrine (0.08 μg/kg/min) administered to patients with elective cesarean section after spinal anesthesia had no significant effect on the acid–base balance of the fetus. There was no significant difference in the incidence of intraoperative complications among the three groups; however, the SBP of the phenylephrine group was significantly higher than that of the metaraminol group.
Data Sharing Statement
The individual participant’s data underlying published results reported in this study can be accessed with approval from the corresponding author (Hong Zhang. Email: [email protected]) after 6 months of publication of the main results. The study protocol, statistical analysis plan, and clinical study report will also be available.
Acknowledgment
This work was supported by the Department of Anesthesiology of Peking University People Hospital and Suqian Maternity Hospital.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Dyer RA, Reed AR, van Dyk D, et al. Hemodynamic effects of ephedrine, phenylephrine, and the coadministration of phenylephrine with oxytocin during spinal anesthesia for elective cesarean delivery. Anesthesiology. 2009;111:753–765. doi:10.1097/ALN.0b013e3181b437e0
2. Reynolds F, Seed PT. Anaesthesia for caesarean section and neonatal acid-base status: a meta-analysis. Anaesthesia. 2005;60:636–653.
3. American Society of Anesthesiologists Task Force on Obstetric Anesthesia. Practice guidelines for obstetric anesthesia: an updated report by the American Society of Anesthesiologists task force on obstetric anesthesia and the Society for Obstetric Anesthesia and Perinatology. Anesthesiology. 2016;124:270–300.
4. Lee AJ, Landau R, Mattingly JL, Meenan MM, Smiley RM. Left lateral table tilt for elective cesarean delivery under spinal anesthesia has no effect on neonatal acid-base status: a randomized controlled trial. Anesthesiology. 2017;127:241–249.
5. Liu TY, Zou SY, Guo LL, et al. Effect of different positions during surgical preparation with combined spinal-epidural anesthesia for elective cesarean delivery: a randomized controlled trial. Anesth Analg. 2021;133:1235–1243.
6. Kinsella SM, Carvalho B, Dyer RA, et al. Consensus Statement Collaborators. International consensus statement on the management of hypotension with vasopressors during caesarean section under spinal anaesthesia. Anaesthesia. 2018;73:71–92.
7. Ngan Kee WD, Lee A, Khaw KS, Floria FN, Karmakar MK, Gin T. A randomized double-blinded comparison of phenylephrine and ephedrine infusion combinations to maintain blood pressure during spinal anesthesia for cesarean delivery: the effects on fetal acid-base status and hemodynamic control. Anesth Analg. 2008;107:1295–1302.
8. Ngan Kee WD, Lee SW, Ng FF, Tan PE, Khaw KS. Randomized double-blinded comparison of norepinephrine and phenylephrine for maintenance of blood pressure during spinal anesthesia for cesarean delivery. Anesthesiology. 2015;122:736–745.
9. McDonnell NJ, Paech MJ, Muchatuta NA, Hillyard S, Nathan EA. A randomised double-blind trial of phenylephrine and metaraminol infusions for prevention of hypotension during spinal and combined spinal-epidural anaesthesia for elective caesarean section. Anaesthesia. 2017;72:609–617.
10. Theodoraki K, Hadzilia S, Valsamidis D, Stamatakis E. Prevention of hypotension during elective cesarean section with a fixed-rate norepinephrine infusion versus a fixed-rate phenylephrine infusion. Alpha double-blinded randomized controlled trial. Int J Surg. 2020;84:41–49. doi:10.1016/j.ijsu.2020.10.006
11. Fitzgerald JP, Fedoruk KA, Jadin SM, Carvalho B, Halpern SH. Prevention of hypotension after spinal anaesthesia for caesarean section: a systematic review and network meta-analysis of randomised controlled trials. Anaesthesia. 2020;75:109–121.
12. Singh PM, Singh NP, Reschke M, Ngan Kee WD, Palanisamy A, Monks DT. Vasopressor drugs for the prevention and treatment of hypotension during neuraxial anaesthesia for caesarean delivery: a Bayesian network meta-analysis of fetal and maternal outcomes. Br J Anaesth. 2020;124:95–107.
13. Ngan Kee WD, Lee SWY, Ng FF, Le A. Norepinephrine or phenylephrine during spinal anaesthesia for Caesarean delivery: a randomised double-blind pragmatic non-inferiority study of neonatal outcome. Br J Anaesth. 2020;125:588–595.
14. Fu F, Xiao F, Chen W, et al. A randomised double-blind dose-response study of weight-adjusted infusions of norepinephrine for preventing hypotension during combined spinal-epidural anaesthesia for Caesarean delivery. Br J Anaesth. 2020;124:108–114.
15. Xiao F, Shen B, Xu WP, Feng Y, Ngan WD, Chen XZ. Dose-response study of 4 weight-based phenylephrine infusion regimens for preventing hypotension during cesarean delivery under combined spinal-epidural anesthesia. Anesth Analg. 2020;130:187–193.
16. Xiao F, Xu WP, Yao HQ, Fan JM, Chen XZ. A randomized double-blinded dose-dependent study of metaraminol for preventing spinal-induced hypotension in caesarean delivery. Front Pharmacol. 2021;12:608198.
17. Hirose N, Kondo Y, Maeda T, Suzuki T, Yoshino A, Katayama Y. Oxygen supplementation is effective in attenuating maternal cerebral blood deoxygenation after spinal anesthesia for cesarean section. Adv Exp Med Biol. 2016;876:471–477.
18. Nor NM, Russell IF. Assessing blocks after spinal anaesthesia for elective caesarean section: how different questions affect findings from the same stimulus. Int J Obstet Anesth. 2013;22:294–297.
19. Johnson JW, Richards DS, Wagaman RA. The case for routine umbilical blood acid-base studies at delivery. Am J Obstet Gynecol. 1990;162:621–625.
20. Julious SA. Sample sizes for clinical trials with normal data. Stat Med. 2004;23:1921–1986.
21. Crawford JS, Burton M, Davies P. Time and lateral tilt at Caesarean section. Br J Anaesth. 1972;44:477–484.
22. Ngan Kee WD. Phenylephrine infusions for maintaining blood pressure during spinal anesthesia for cesarean delivery: finding the shoe that fits. Anesth Analg. 2014;118:496–498.
23. Aragao FF, Aragao PW, Martins CA, Salgado Filho N, Barroqueiro Ede S. Comparison of metaraminol, phenylephrine and ephedrine in prophylaxis and treatment of hypotension in cesarean section under spinal anesthesia. Rev Bras Anestesiol. 2014;64:299–306.
24. Bhardwaj N, Jain K, Arora S, Bharti N. A comparison of three vasopressors for tight control of maternal blood pressure during cesarean section under spinal anesthesia: effect on maternal and fetal outcome. J Anaesthesiol Clin Pharmacol. 2013;29:26–31.
25. Maayan MA, Schushan EI, Todris L, Etchin A, Kuint J. Maternal hypotension during elective cesarean section and short-term neonatal outcome. Am J Obstet Gynecol. 2010;56:e1–e5.
26. Chen Y, Zou L, Li Z, et al. Prophylactic norepinephrine infusion for postspinal anesthesia hypotension in patients undergoing cesarean section: a randomized, controlled, dose-finding trial. Pharmacotherapy. 2021;41:370–378.
27. Hasanin AM, Amin SM, Agiza NA, et al. Norepinephrine infusion for preventing postspinal anesthesia hypotension during cesarean delivery: a randomized dose-finding trial. Anesthesiology. 2019;130:55–62.
28. Liu TY, Gao XX, Zou SY, et al. Randomised double-blind dose-response study of weight-adjusted infusions of metaraminol for preventing hypotension with combined spinal-epidural anaesthesia for elective Caesarean delivery in the supine position. Br J Anaesth. 2022;10:20–22.
29. Olapour A, Akhondzadeh R, Rashidi M, Gousheh M, Homayoon R. Comparing the effect of bupivacaine and ropivacaine in cesarean delivery with spinal anesthesia. Anesth Pain Med. 2020;10:94155.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
