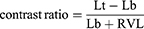Back to Journals » Clinical Ophthalmology » Volume 17
Effect of Violet Light-Filtering and Manufacturing Improvements in an Extended Depth-of-Focus Intraocular Lens on Visual Performance
Authors van der Mooren M, Alarcon A, Jenkins Sanchez MD, Chang DH
Received 10 November 2022
Accepted for publication 15 February 2023
Published 1 March 2023 Volume 2023:17 Pages 701—709
DOI https://doi.org/10.2147/OPTH.S396823
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Marrie van der Mooren,1 Aixa Alarcon,1 Mark D Jenkins Sanchez,1 Daniel H Chang2
1Johnson & Johnson Surgical Vision, Inc., Groningen, Netherlands; 2Empire Eye and Laser Center, Bakersfield, CA, USA
Correspondence: Marrie van der Mooren, Johnson & Johnson Surgical Vision, AMO Groningen BV, Van Swietenlaan 5, Groningen, 9728, NX, Netherlands, Tel +31620709324, Email [email protected]
Purpose: To assess the experimental visual performance and dysphotopsia characteristics of the new Tecnis Symfony OptiBlue extended-depth-of-focus with violet light-filtering (ZXR00V) intraocular lens (IOL) compared with the colorless Tecnis Symfony (ZXR00) IOL.
Methods: Range of vision was assessed with simulated visual acuity defocus curves, predicted by white light through focus modulation transfer function (MTF) measurements. The clinical visual acuity defocus curve of the ZXR00 IOL was used to validate the predicted range of vision. Image quality was compared by measuring white light MTF at a spatial frequency of 15 cycles per degree (c/deg) for 3 mm and 5 mm pupil diameters with optical powers of 5 D, 20 D, and 34 D using the average corneal eye (ACE) model with the average spherical and chromatic aberration of the cataract population. Effects on dysphotopsias were predicted by measurement and computer simulation of light scatter (straylight parameter) and subsequent determination of retinal veiling luminance (RVL) in vitro. Contrast enhancement under challenging light conditions was calculated based on the effects in RVL.
Results: The simulated visual acuity defocus curves and image quality outcomes were comparable between the ZXR00V and ZXR00 IOLs. The area under the straylight curve for the straylight parameter showed a 19% improvement in halo performance with ZXR00V versus ZXR00. A 12% to 17% reduction in RVL was achieved in favor of ZXR00V over ZXR00, which enhanced contrast vision by 9% to 13% under challenging light conditions
Conclusion: The violet light-filtering technology and improved manufacturing of ZXR00V delivers a comparable range of vision and tolerance to refractive error to ZXR00 while mitigating dysphotopsias and enhancing contrast vision.
Keywords: dysphotopsia profile, presbyopia-correcting IOL, retinal veiling luminance, straylight parameter, violet light-filtering IOL
Introduction
Surgical correction of presbyopia involves a balance between visual quality, range of vision and dysphotopsias. Early multifocal intraocular lenses (IOLs) provided excellent near vision but were associated with a high incidence of halos in low-light situations, limitations in intermediate vision and loss of contrast sensitivity.1–6 Extended depth-of-focus (EDF) IOLs provide a continuous range of vision, with the benefit of lowering incidence rates of halos and other dysphotopsias (eg, night glare/flare, halos, starbursts and spider webs),1,5–7 and may be a preferable presbyopia-correcting IOL option in patients who want optimal contrast sensitivity in dim lighting.8
Tecnis Symfony EDF IOLs incorporate a diffractive echelette design to increase the range of vision by creating an elongated focal point and achromat technology to increase the retinal image contrast by correcting chromatic aberration.1 Previous studies with the original colorless Tecnis Symfony (model ZXR00) IOL have reported comparable or better distance and intermediate visual acuity and relatively low incidence rates of dysphotopsias compared with multifocal IOLs.3,7 Nevertheless, some patients still report severe and persistent low-light dysphotopsias and may not be fully satisfied with their visual experience.4,7,9 Therefore, it remains important for surgeons to provide proper counseling regarding the risks of dysphotopsias prior to implantation.
The violet light filter of the Tecnis Symfony OptiBlue IOL (model ZXR00V) has a sharp cutoff at 425 nm, thus providing the optimal balance between photoreception and photo protection10 and a spectral transmission curve approaching that of a healthy crystalline lens of a young adult11 (Figure 1). The ZXR00V IOL is also manufactured with a high-resolution lathing process that yields a smoother surface, with the aim to further reduce the rate of dysphotopsias (Figure 2). This study assessed the preclinical effects of violet light-filtering and high-resolution lathing of the ZXR00V IOL on visual performance and dysphotopsias versus the ZXR00 IOL.
Materials and Methods
The ZXR00V and ZXR00 IOLs (both from Johnson & Johnson Surgical Vision, Inc., Santa Ana CA, USA) are diffractive EDF IOLs which have a diffractive echelette pattern on the posterior surface that is designed to elongate the focus and provide improved visual acuity at intermediate distances.1 The echelettes have a diffractive achromatic component to compensate for the chromatic aberration of the cornea.1 Both models share the same base IOL platform with identical mechanical properties, allowing the IOL to be in the same axial position in the capsular bag.
Preclinical Visual Performance
The visual performance of the ZXR00V and ZXR00 IOLs was predicted using an ISO standard-compliant average corneal eye (ACE) model that reproduces for 6 mm entrance pupil 0.27 μm of spherical aberration (Zernike coefficient c[4,0]) and 1 D of longitudinal chromatic aberration in the spectacle plane for the wavelength range 450 nm to 650 nm.12,13 This dedicated optical setup for the assessment of IOL image quality allowed for the taking of modulation transfer function (MTF) measurements at different apertures, spatial frequencies (through-frequency curve) and focal planes (through-focus curve). The area under the MTF curve (MTFa) was summed from a spatial frequency of 1 cycle per millimeter (c/mm) to 50 c/mm for a 20 D IOL and 3 mm pupil diameter for a defocus range from +0.5 D to −3.5 D in 0.5 D steps.14 The obtained through-focus MTFa was then used to predict the visual acuity defocus curve and to assess the range of vision.
The image visual-quality of the ZXR00V and ZXR00 IOLs was assessed by measuring white-light MTF for optical powers of 5 D, 20 D, and 34 D using pupil aperture diameters of 2 mm, 3 mm and 5 mm at a spatial frequency of 15 cycles per degree (c/deg), equivalent to 50 c/mm with a 20 D IOL.14 For the assessment of tolerance to refractive error, both the defocus curve and MTF level were assessed. For each model, 10 individual lenses were measured for each power, yielding a total of 30 measurements per model per pupil diameter. The mean and standard deviation of the individual measurements were calculated for each IOL model. The simulated defocus curves based on 3 mm pupil white-light MTFa measurements for ZXR00V and ZXR00 were then compared to clinical visual acuity defocus curve of ZXR00.1
Preclinical Assessment of Dysphotopsias
The effect of violet light-filtering and manufacturing improvements of ZXR00V versus ZXR00 on preclinical assessment of dysphotopsias was determined by in vitro measurements and computer simulation models of light scatter.
The straylight parameter15 was calculated by in vitro assessment of halos using a model eye with realistic eye dimensions with a 4-mm aperture, a cornea exhibiting the average spherical and chromatic aberration, a CCD detector and a fiber with 2 mm diameter powered by a xenon car headlight placed approximately 2.8 m on-axis in front of the model eye.16 This setup is able to discriminate performance of monofocal, multifocal and extended range of vision IOL lenses as shown by the intensity recordings in Figure 8 from Weeber et al16 using a small extended light source. Halos were assessed by measuring the radial intensity profiles for the ZXR00V and ZXR00 IOLs. A total of 32 individual images tested at different shutter times were recorded for each IOL model, resulting in high-dynamic-range halo images with more than seven decades of intensity. Three of these images were recorded for each IOL, and the mean radial intensity profiles were averaged up to 1 degree and multiplied by the square of the field angle to obtain the straylight parameter. The total intensity across the full field of 1 degree was used as normalization and the area under the curve for the straylight parameter was used to compare the outcomes for ZXR00V versus ZXR00.
A computer simulation study using MATLAB software (MathWorks Inc., USA) was also performed, and radial intensity profiles were determined using the same theoretical corneal eye model used in the in vitro assessment.17 The geometrical shape of the IOLs was modelled including the echelette profiles. Wavefront propagation calculation assured that the induced phase by the echelettes was included to calculate the radial intensity. The eye model is based on realistic eye dimensions and a uniform lighting source and the photopic sensitivity of the eye was used to calculate the straylight parameter for the ZXR00V and ZXR00 IOLs. Pupil sizes used for the study ranged from 3 to 5 mm, and angular size of the extended light source ranged from 0.7 to 2.40 arcminute. The straylight level of a healthy crystalline lens calculated from the standard glare observer15,18 was used as a threshold level. The area between the simulated straylight parameter for the ZXR00V and ZXR00 IOLs and the threshold curve of the crystalline lens was used as basis for comparison.
Effect of Reduction of Retinal Veiling Luminance on Contrast
Integration of the straylight parameter over an angular range was used to calculate the potential reduction in retinal veiling luminance (RVL)19 with ZXR00V versus ZXR00. Under challenging light conditions, such as night driving with low road luminance and an oncoming car with intense headlights, the relative contrast enhancement was calculated for the scenario of a pedestrian crossing the road, as would be predicted to be observed by a pseudophakic patient implanted with either the ZXR00V or ZXR00 IOLs. The contrast is defined as the difference of the luminance target (pedestrian, Lt) and background luminance (road, Lb) divided by sum of luminance background and RVL as per the following equation:
Pedestrian luminance of 2 cd/m2 and 5 cd/m2 and road luminance ranging between 0.2 and 1 cd.m2 were chosen as realistic values for challenging light conditions at night.20
Results
Preclinical Visual Performance
Visual performance between the ZXR00V and ZXR00 IOLs was consistent and comparable across all pupil diameters and lens powers. Optical bench testing in the ACE model resulted in the same MTF at 15 c/deg for the ZXR00V and ZXR00 IOLs (Figure 3). The simulated visual acuity defocus curves were based on MTFa data and were comparable between ZXR00V and ZXR00 (Figure 4). The defocus curves predicted by MTFa were representative of clinical performance, as evidenced by the good approximation of the predicted values to the average value of visual acuity obtained in the clinical study.1 Based on these defocus curves and identical MTF levels across pupil diameter and optical powers, it is expected that ZXR00V will deliver the same through-focus visual acuity performance as ZXR00 for all pupil sizes between 5 D and 34 D. Consequently, the same range of vision and tolerance to refractive error between the two IOLs would be expected in the clinical setting.
 |
Figure 4 Simulated visual acuity defocus curve based on 3 mm pupil white-light modulation transfer function area measurements for ZXR00V and ZXR00 and the clinical visual acuity defocus curve of the ZXR00.1 The simulated defocus curve of ZXR00 is not visualized when it overlaps with the simulated defocus curve of ZXR00V. |
Preclinical Assessment of Dysphotopsias
Analysis of the area under the curve for the straylight parameter by the experimental in vitro study showed a 19% improvement in straylight performance with ZXR00V versus ZXR00. The computer simulation study showed a straylight improvement of 7% to 11% in favor of ZXR00V over ZXR00, depending on the extent of the light source and eye pupil diameter. An increase in the extent of light source and a decrease in pupil size lowers both area under straylight parameter and retinal veiling luminance for both IOL models. Based on both the experimental and simulation results, the predicted reduction in RVL ranged from 12% to 17% in favor of ZXR00V versus ZXR00.
The night driving scene with a crossing pedestrian in front of an oncoming car is shown as observed by a pseudophakic patient (Figure 5). This traffic scenario has a high dynamic range because of the intense car headlight relative to the low road luminance and the image could not be displayed in actual levels of light intensity but as an illustration of the observed traffic situation. The enhancement in visibility of the crossing pedestrian was calculated using the contrast formula for 2 cd/m2 and 5 cd/m2 of pedestrian luminance and for 2 cd/m2 of RVL for ZXR00 and 1.7 cd/m2 for ZXR00V (representing a 15% reduction) for road luminance ranging from 0.2 to 1 cd/m2 (Figure 6). The reduction in RVL provided by violet light filtering and high-definition lathing enhances contrast vision under challenging light conditions by 9% to 13%.
Discussion
Characterizing the preclinical visual performance profiles of new EDF and other presbyopia-correcting IOLs is a useful exercise that could influence IOL selection in the clinical setting. Optical bench testing through MTF metrics offers a high correlation with clinical measurements of visual acuity defocus.14 In this study, the preclinical assessment of the ZXR00V IOL demonstrated similar through-focus visual acuity performance versus the colorless (ultraviolet-light-filtering) ZXR00 IOL. The white-light MTF level for 2 mm, 3 mm, and 5 mm pupil diameters were almost identical between ZXR00V and ZXR00, indicating that the range of vision and tolerance to refractive error are comparable between the two IOL models. These findings provide preclinical evidence to support ZXR00V and ZXR00 having equivalent visual performance.
Violet light-filtering IOLs that continue to transmit blue light have been shown to deliver equivalent color perception along with improved patient satisfaction in day and night vision versus colorless IOLs that only block ultraviolet light.21,22 Blue light is crucial to scotopic and mesopic vision, as well as playing an important role in human circadian rhythms.22,23 Blue light transmission to the eye reduces with age and may reduce the ability to walk on uneven surfaces in dimly lit environments, increasing the risk of falls in older adults.22,24–26 Violet light-filtering IOLs block the transmission of wavelengths in the range of 380 nm to 460 nm but continue to transmit blue light (which is in the range of 460 nm to 500 nm), offering better scotopic and melanopsin photoreception than blue light-filtering IOLs.10,22 Although shallow cut-off filters induce variations in the transmission characteristics across the IOL power range, the sharp cut-off of the OptiBlue violet light filter (Figure 1) provides consistent transmission characteristics across the entire IOL power range despite the variation in IOL thickness.10
Violet light-filtering IOLs may additionally reduce the light scatter that manifests clinically as dysphotopsias (ie, halos, night glare, starbursts and spiderwebs),22 one of the leading causes of patient dissatisfaction following IOL implantation.27 In this study, the area under the straylight curve for the ZXR00V IOL demonstrated improved straylight performance versus the ZXR00 IOL and consequently reduced veiling luminance irrespective of the glare source size, glare source type and eye pupil diameter. Both IOL models showed a decrease in both area under straylight parameter and retinal veiling luminance for a decreasing pupil diameter due to a lower density of echelettes in the center of the optic. The decrease for an increasing extent of the light source is due to a decreased normalized light intensity distribution at the retinal plane. Based on both the in vitro and simulation results, a reduction of RVL ranging from 12% to 17% could be achieved in favor of ZXR00V versus ZXR00, and the reduction in RVL observed with ZXR00V enhanced contrast vision by 9% to 13% under challenging light conditions. Even if the pedestrian in the night-driving scenario chose to increase their safety by increasing their luminance from 2 cd/m2 to 5 cd/m2 or more (eg, by the use of reflective safety clothing), the contrast enhancement results are still valid. Violet light filtering does not reduce white light MTF outcomes because of the low spectral sensitivity for violet light while it does reduce intraocular light scatter more than colorless IOLs because light scatter behaves reciprocal with wavelength to the fourth power. This means that violet light scatters more than red light.22 A study comparing spectacle wearers with a clear filter versus a violet light filter (cutoff at 426 nm) found preference and visual performance improvements under glare conditions in favor of the violet light filter due reduced intraocular scatter.28 The manufacturing improvement provides a smoother and higher defined surface profile which results in lower small angle and wide angle light scatter.29 It is known that light scatter influences dysphotopsia but it also manifests changes in absolute and contrast detection thresholds.30
These results suggest that the OptiBlue violet light-filtering technology and manufacturing improvements in the Tecnis Symfony ZXR00V are likely to further mitigate dysphotopsias versus ZXR00 by reducing light scatter and enhancing contrast vision under challenging light conditions.
A strength of this study is that the preclinical assessments of visual performance have previously been demonstrated to predict visual acuity and range of vision in the clinical setting;14 however, one limitation is that the straylight measurements and simulations used to predict dysphotopsias have not yet been validated. Despite the lack of validation data, we believe the preclinical data generated in this study may be useful in predicting the dysphotopsia profiles experienced by patients implanted with these IOLs. Indeed, early clinical findings with the violet light-filtering ZXR00V support the preclinical findings of this study, with a lower proportion of patients reporting dysphotopsia complaints compared to patients implanted with colorless ZXR00 lenses (manuscript in preparation). In a separate study with monofocal IOLs, a higher proportion of patients implanted with a violet light-filtering monofocal IOL (ZV9003) reported no difficulty when driving during the day31 and at night21 compared with patients implanted with a colorless monofocal IOL (ZA9003).
Conclusions
This study provided preclinical evidence that the violet light-filtering technology and improved manufacturing of the EDF Tecnis Symfony OptiBlue IOL (model ZXR00V) provided a range of vision and tolerance to refractive error that was comparable to the colorless Tecnis Symfony IOL (model ZXR00), while mitigating dysphotopsias and enhancing contrast vision. Clinical studies of ZXR00V are underway to confirm these observations.
Abbreviations
ACE, average corneal eye; EDF, extended depth-of-focus; IOL, intraocular lens; Lb, background luminance; Lt, luminance target; MTF, modulation transfer function; MTFa, area under the modulation transfer function curve; RVL, retinal veiling luminance.
Acknowledgments
Medical writing assistance was provided by Bridget Healy, MBChB, MPH (ApotheCom, Yardley, PA) and was funded by Johnson & Johnson Surgical Vision, Inc.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was funded by Johnson & Johnson Surgical Vision, Inc., which participated in the design and conduct of the study.
Disclosure
Marrie van der Mooren, Aixa Alarcon, and Mark D Jenkins Sanchez are employees of Johnson & Johnson Surgical Vision, Inc. Daniel H. Chang is an investigator and consultant for Johnson & Johnson Surgical Vision, Inc., and an investigator for AcuFocus. He reports grants, personal fees, non-financial support from Johnson & Johnson Vision and grants from Acufocus, Inc, outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. Chang DH, Janakiraman DP, Smith PJ, et al. Visual outcomes and safety of an extended depth-of-focus intraocular lens: results of a pivotal clinical trial. J Cataract Refract Surg. 2022;48(3):288–297. doi:10.1097/j.jcrs.0000000000000747
2. Calladine D, Evans JR, Shah S, Leyland M. Multifocal versus monofocal intraocular lenses after cataract extraction. Cochrane Database Syst Rev. 2012;(9):CD003169. doi:10.1002/14651858.CD003169.pub3
3. Britton JJL, El-Defrawy S, Wong BM, et al. Patient satisfaction and visual function following implantation of trifocal or extended range of vision intraocular lenses. Clin Ophthalmol. 2022;16:669–676. doi:10.2147/opth.S339385
4. Escandón-García S, Ribeiro FJ, McAlinden C, Queirós A, González-Méijome JM. Through-focus vision performance and light disturbances of 3 new intraocular lenses for presbyopia correction. J Ophthalmol. 2018;2018:6165493. doi:10.1155/2018/6165493
5. Kohnen T, Suryakumar R. Extended depth-of-focus technology in intraocular lenses. J Cataract Refract Surg. 2020;46(2):298–304. doi:10.1097/j.jcrs.0000000000000109
6. Akella SS, Juthani VV. Extended depth of focus intraocular lenses for presbyopia. Curr Opin Ophthalmol. 2018;29(4):318–322. doi:10.1097/icu.0000000000000490
7. Hamid A, Sokwala A. A more natural way of seeing: visual performance of three presbyopia correcting intraocular lenses. Open J Ophthalmol. 2016;6:176–183. doi:10.4236/ojoph.2016.63025
8. Mencucci R, Favuzza E, Caporossi O, Savastano A, Rizzo S. Comparative analysis of visual outcomes, reading skills, contrast sensitivity, and patient satisfaction with two models of trifocal diffractive intraocular lenses and an extended range of vision intraocular lens. Graefes Arch Clin Exp Ophthalmol. 2018;256(10):1913–1922. doi:10.1007/s00417-018-4052-3
9. TECNIS Symfony® Extended Range of Vision Intraocular Lenses (IOLs), Lens Model ZXR00 and Toric Lens Models ZXT150, ZXT225, ZXT300, and ZXT375 [package insert]. Santa Ana, CA: Johnson & Johnson Surgical Vision, Inc.; 2017.
10. van de Kraats J, van Norren D. Sharp cutoff filters in intraocular lenses optimize the balance between light reception and light protection. J Cataract Refract Surg. 2007;33(5):879–887. doi:10.1016/j.jcrs.2007.02.020
11. Artigas JM, Felipe A, Navea A, Fandiño A, Artigas C. Spectral transmission of the human crystalline lens in adult and elderly persons: color and total transmission of visible light. Invest Ophthalmol Vis Sci. 2012;53(7):4076–4084. doi:10.1167/iovs.12-9471
12. van der Mooren M, Weeber H, Piers P. Verification of the average cornea eye ACE model. Invest Ophthalmol Vis Sci. 2006;47(13):309.
13. Norrby S, Piers P, Campbell C, van der Mooren M. Model eyes for evaluation of intraocular lenses. Appl Opt. 2007;46(26):6595–6605. doi:10.1364/ao.46.006595
14. Alarcon A, Canovas C, Rosen R, et al. Preclinical metrics to predict through-focus visual acuity for pseudophakic patients. Biomed Opt Express. 2016;7(5):1877–1888. doi:10.1364/boe.7.001877
15. van der Mooren M, van den Berg T, Coppens J, Piers P. Combining in vitro test methods for measuring light scatter in intraocular lenses. Biomed Opt Express. 2011;2(3):505–510. doi:10.1364/boe.2.000505
16. Weeber HA, Meijer ST, Piers PA. Extending the range of vision using diffractive intraocular lens technology. J Cataract Refract Surg. 2015;41(12):2746–2754. doi:10.1016/j.jcrs.2015.07.034
17. Piers PA, Norrby NE, Mester U. Eye models for the prediction of contrast vision in patients with new intraocular lens designs. Opt Lett. 2004;29(7):733–735. doi:10.1364/ol.29.000733
18. Vos JJ, Van den Berg TJ. Report on disability glare. CIE Collection. 1999;135(1):1–9.
19. van den Berg TJ. Analysis of intraocular straylight, especially in relation to age. Optom Vis Sci. 1995;72(2):52–59. doi:10.1097/00006324-199502000-00003
20. Fotios S, Gibbons R. Road lighting research for drivers and pedestrians: the basis of luminance and illuminance recommendations. Light Res Tech. 2018;50(1):154–186. doi:10.1177/1477153517739055
21. Canovas C, Weeber H. A, Trentacost D, Janakiraman P, Tarantino N, Raheja M, Piers P. Optical and visual performance of violet blocking intraocular lenses. Investigative Ophthalmology & Visual Science. 2019;60(9):3717.
22. Chang D, Pastuck T, Rosen R, Hollmann S, Babic T, Stapars A. Violet and blue light: impact of high-energy light on vision and health. J Ophthalmic Stud. 2020;3(2). doi:10.16966/2639-152X.119
23. Park JW, Choi CY. Comparative spectrophotometer analysis of ultraviolet-light filtering, blue-light filtering, and violet-light filtering intraocular lenses. Korean J Ophthalmol. 2022;36(1):1–5. doi:10.3341/kjo.2021.0157
24. Mainster MA. Violet and blue light blocking intraocular lenses: photoprotection versus photoreception. Br J Ophthalmol. 2006;90(6):784–792. doi:10.1136/bjo.2005.086553
25. Cuthbertson FM, Peirson SN, Wulff K, Foster RG, Downes SM. Blue light-filtering intraocular lenses: review of potential benefits and side effects. J Cataract Refract Surg. 2009;35(7):1281–1297. doi:10.1016/j.jcrs.2009.04.017
26. Mainster MA, Findl O, Dick HB, et al. The blue light hazard versus blue light hype. Am J Ophthalmol. 2022;240:51–57. doi:10.1016/j.ajo.2022.02.016
27. Masket S, Fram NR. Pseudophakic dysphotopsia: review of incidence, cause, and treatment of positive and negative dysphotopsia. Ophthalmology. 2021;128(11):e195–e205. doi:10.1016/j.ophtha.2020.08.009
28. Cozza F, Compagnoni MM, Airoldi C, et al. The effects of two longpass filters on visual performance. J Optom. 2020;13(2):102–112. doi:10.1016/j.optom.2019.07.001
29. Harvey JE. Integrating optical fabrication and metrology into the optical design process. Appl Opt. 2015;54(9):2224–2233. doi:10.1364/AO.54.002224
30. Westheimer G, Liang J. Influence of ocular light scatter on the eye’s optical performance. JOSA A. 1995;12(7):1417–1424. doi:10.1364/JOSAA.12.001417
31. TECNIS Synergy™ IOL with TECNIS Simplicity® Delivery System [package insert]. Santa Ana, CA: Johnson & Johnson Surgical Vision, Inc.; 2021.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.