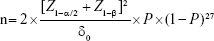Back to Journals » Therapeutics and Clinical Risk Management » Volume 13
Effect of treatment with single total-dose intravenous iron versus daily oral iron(III)-hydroxide polymaltose on moderate puerperal iron-deficiency anemia
Authors Iyoke CA , Emegoakor FC, Ezugwu EC, Lawani LO, Ajah LO , Madu JA, Ezegwui HU, Ezugwu FO
Received 6 May 2016
Accepted for publication 10 November 2016
Published 17 May 2017 Volume 2017:13 Pages 647—653
DOI https://doi.org/10.2147/TCRM.S112227
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Garry Walsh
Chukwuemeka Anthony Iyoke,1 Fausta Chioma Emegoakor,1 Euzebus Chinonye Ezugwu,1 Lucky Osaheni Lawani,2 Leonard Ogbonna Ajah,1 Jude Anazoeze Madu,3 Hyginus Uzo Ezegwui,1 Frank Okechukwu Ezugwu4
1Department of Obstetrics and Gynaecology, University of Nigeria, Enugu Campus, 2Department of Obstetrics and Gynaecology, Federal Teaching Hospital, Abakaliki, 3Department of Haematology, University of Nigeria, Nsukka, 4Department of Obstetrics and Gynaecology, College of Medicine, Enugu State University, Enugu, Nigeria
Background: Iron-deficiency anemia is the most common nutritional cause of anemia in pregnancy and is often responsible for puerperal anemia. Puerperal anemia can impair postpartum maternal and neonatal well-being.
Objective: To determine the effect of treatment of moderate puerperal iron-deficiency anemia using a single intravenous total-dose iron dextran versus daily single dose oral iron(III)-hydroxide polymaltose.
Methodology: A randomized controlled study in which postpartum women with moderate iron-deficiency anemia were randomized into treatment with either a single total-dose intravenous iron dextran or with daily single doses of oral iron(III)-hydroxide polymaltose tablets for 6 weeks. Effects on hemoglobin concentration using either method were compared at 6 weeks postpartum. Analysis was per protocol using SPSS version 17 for windows. P-values ≤0.05 were considered significant.
Results: Two hundred eighty-four women were recruited for the study: 142 women received single total dose intravenous infusion of iron dextran while 142 received daily oral iron(III)-hydroxide polymaltose tablets. Approximately 84.0% (237/282) completed the study and were analyzed including 81% (115/142) of those randomized to injectable iron therapy compared to 85.9% (122/142) of those randomized to oral treatment. The proportions of women who had attained hemoglobin concentration of at least 10 g/dL by the 6 weeks postpartum visit did not differ significantly between cases and controls (95.7% vs 94.3%; P=0.73). Similarly, the mean increases in hemoglobin following either therapeutic route were comparable (1.03±0.56 g/dL for intravenous iron and 0.97±0.46 g/dL for the oral group; P=0.42).
Conclusion: Single total-dose intravenous iron for treatment of puerperal iron-deficiency anemia was as effective as daily single doses of ferric iron tablets. For puerperal patients with iron-deficiency anemia in whom compliance with and tolerability of oral iron are not certain, a single total-dose intravenous iron can be safely offered.
Keywords: effect, intravenous, iron, puerperal, anemia
Introduction
Postpartum anemia is a major contributor to puerperal complications with the risk being greatest among those women who were anemic in pregnancy.1,2 Anemia (occurring in pregnancy or postpartum) has been implicated as a significant cause of direct and indirect maternal and perinatal morbidity and mortality.3–8 The associations between anemia and reduced work performance/productivity,9,10 impaired cognition8 and depression11,12 have been documented and postpartum anemia could predispose parturients to maternal infections, tiredness and other morbidities.13 These could cause women to experience difficulty caring for their babies and impair the emotional bonding between the mother and her baby.14 Treatment of puerperal anemia is, therefore, important for improving maternal and newborn health during breastfeeding and for the restoration of the maternal iron and hemoglobin statuses before subsequent conception.
Prevalences of puerperal anemia have been documented as 27.2% among low income women in USA,15 70% in India2 and 25.2% in Croatia.1 In Enugu, Nigeria, a prevalence of 47.5% for puerperal anemia was obtained in a recent study on low risk women.16 The overall prevalence of postpartum anemia is estimated to be in the range of 40%–80% in the developing countries.17
The leading cause of puerperal anemia is prepartum iron-deficiency anemia;3 other causes include excessive blood loss at delivery1,18 and infection in women with cesarean section,1 malaria, human immunodeficiency virus (HIV), hemoglobinopathies, etc. Preexisting maternal anemia is implicated in lowering hematologic reserves and as such worsens blood loss during delivery.21–23 Other risk factors for postpartum anemia include multiple births, prepregnancy obesity and nonpractice of exclusive breastfeeding.1,22
Postpartum anemia and the consequent maternal complications seem to be a neglected area of obstetric practice in sub-Saharan Africa. For instance, there are hardly any protocols for screening parturients for postpartum anemia the way it is done for antenatal cases. Besides, there are few studies on this significant cause of maternal morbidity in the subregion. However, evidence suggests that postpartum anemia is common and that iron-deficiency anemia may be responsible for up to 15% of puerperal anemia.1,2,15–17
A recent review by Breyman et al concluded that in cases of postpartum anemia where the hemoglobin was <9.5 g/dL, intravenous iron was the ideal, as it tended to lead to more rapid recovery in hemoglobin level.23 Drawbacks to oral iron therapy include noncompliance to treatment by patients, malabsorption of ingested iron and poor tolerance by patients.24 Where any of these factors obtains, recourse to parenteral iron can also be undertaken in order to correct iron-deficiency anemia. Although parenteral therapy is more expensive than oral iron preparations, the benefits of parenteral iron far outweigh its higher costs.25 In many cases, use of intravenous iron has lower risks of adverse events than blood transfusion and as long as the patient is stable is a better alternative.26
Anecdotal evidence suggests that women with moderate puerperal iron-deficiency anemia in our setting may receive inadequate treatment due to the fact that parturients either find it inconvenient to comply with oral iron therapy or see no reason to do so once normal delivery had been accomplished. A one-off treatment with parenteral total-dose iron therapy could overcome this problem of noncompliance if it gives comparable increase in hemoglobin concentration to oral therapy. The aim of this study was to compare the treatment of moderate iron-deficiency anemia among postpartum women with a single total-dose intravenous iron dextran compared to daily oral iron(III) hydroxide polymaltose tablets.
Methods
The study was carried out in the two tertiary health care institutions in Enugu namely University of Nigeria Teaching Hospital, Ituku Ozalla and Enugu State University Teaching Hospital, Parklane, Enugu. Both centers offer both primary maternity care and tertiary health services. The annual delivery rate in each of these two centers ranges between 1,500 and 2,000.
The study population included all women who had singleton vaginal deliveries in the study centers during the study period from April 2013 to October 2015. This was a randomized controlled study. Cases were defined as women who had postpartum hemoglobin concentration of 6–7.9 g/dL associated with red blood cell features of iron deficiency at 48 hours or later following vaginal delivery of a singleton pregnancy and who were treated with single total-dose intravenous infusion of iron dextran (intervention). Controls were defined as women who had hemoglobin concentration of 6–7.9 g/dL associated with red blood cell features of iron deficiency at 48 hours or later following vaginal delivery of a singleton pregnancy and who were treated with single daily oral doses of iron(III)-hydroxide polymaltose tablet (standard treatment).
The minimum sample size (n) for this study was derived by the formula for sample size calculation for two independent samples in an equivalence trial with a categorical variable as main outcome measure:
|
where n is the size per group; p is the response rate of standard treatment group; p0 is the response rate of new drug treatment group; zx is the standard normal deviate for a one- or two-sided x; d is the real difference between two treatment effect; and δ0 is a clinically acceptable effect size.
Assuming P=50% since we had no previous study on the use of single dose oral therapy for treatment; Z1–α/2=1.96, Zβ=0.845, δ0=18% and substituting in the equation; n=121; assuming a loss to follow-up of 18%, and adding to this, n=142.8 For convenience we chose 142 as sample size for each arm of the study.
All the women who had vaginal delivery during the period of the study were counseled and screened for postpartum anemia by determining hemoglobin concentration and also examining blood film for red cell morphology. For the purpose of the study, hemoglobin level <10 g/dL was considered as anemia in line with the definition of anemia in Nigerian hospitals.28 Postpartum period was defined as the period between delivery of the baby and 6 weeks after. Moderate anemia was defined as hemoglobin concentration of 6–7.9 g/dL. Iron-deficiency anemia was defined as the presence of hemoglobin level <10 g/dL associated with blood film features of red cell hypochromia and microcytosis with or without anisocytosis and poikilocytosis.
The inclusion criteria for the study included vaginal delivery of singletons occurring within 48 hours, moderate anemia with features of iron deficiency, hemodynamic stability and written consent to participate in the study. The exclusion criteria included severe anemia, that is, Hb<6 g/dL; mild anemia, that is, Hb 8–9.9 g/dL; secondary postpartum hemorrhage; vaginal delivery of multiple pregnancy; cesarean delivery, genotype SS; HIV infection; comorbidities, such as hypertensive disorders, renal pathology and refusal of consent.
Consenting women were then recruited into the study and were randomized into receiving intravenous iron (cases) and receiving oral iron (controls). Based on sample size of 284, numbers 1 to 284 were randomly split into two equal groups using computer generated random numbers. The numbers in each group were written on identical square cards with the letter “i” for cases and “o” for controls. The cards were then placed each in a small opaque envelop and sealed. The sealed envelopes containing all the numbers were put in a nontransparent bag. Each study subject to be recruited was asked to dip her hand in the bag and pick one of the envelopes. She was assigned the number which appeared on the card enclosed in the envelop and this number was used to allocate her to either case or control according to the earlier randomization. The ratio of cases to controls was 1:1. Cases and controls were matched by age (within 2 years), parity (nulliparity, multiparity and grandmultiparity) and marital status.
The protocol for the administration of intravenous iron was as follows: A vial of iron dextran (Imferon MD®, Shreya Life Sciences Pvt Ltd, Mumbai, India) contained 5 mL of parenteral iron equivalent to 250 mg elemental iron. The dose of intravenous iron administered was determined from the deficit in hemoglobin concentration calculated as the difference between the expected hemoglobin (of 10 g/dL) and the patient’s hemoglobin concentration at 48 hours postpartum. It was assumed that 250 mg of parenteral iron would increase hemoglobin concentration by 1 g/dL per week.29 Therefore, the required dose in milligram was obtained by multiplying each participant’s deficit in hemoglobin concentration by 250 to get the amount of iron dextran in milligrams to be administered. Fifty percent of this value was also added to replenish the iron stores. The cases received, in addition, 0.4 mg of folic acid tablets daily. The calculated amount was added into and mixed with an infusion of 0.9% Normal saline to a maximum of six vials of iron dextran (ie, 1,500 mg) in 1 L of saline. Then, intravenous access was secured and a premedication consisting of chlorpheniramine 10 mg was administered intramuscularly. Thirty minutes after, a test dose was first administered by giving the iron dextran infusion at a slow rate of 10 drops per minute >30 minutes while the patient was monitored for hypersensitivity reaction. Subsequently, if there was no reaction, the infusion was allowed to run at 30 drops per minute till the end of the infusion. If any degree of adverse/allergic reaction was noticed either during the test dose or afterwards while the infusion was running, the patient was given some rescue medications that include intravenous hydrocortisone 200 mg statim and promethazine 50 mg statim and the iron infusion was discontinued.
For oral iron, each tablet of Fegem® (Torrent Pharmaceuticals Ltd, Gujarat, India) contained iron(III)-hydroxide polymaltose complex equivalent to 100 mg elemental iron and also 350 μg of folic acid. The drug came in chewable form to encourage. To enhance compliance with oral therapy among the control group, an individualized drug administration card containing every day of the 6 weeks postpartum was issued to patients where they ticked each day’s dose taken. Besides, participants were sent weekly SMS reminders to facilitate compliance. Participants were instructed to bring the drug administration card along while coming for the 6 weeks follow-up appointment. Blood samples were taken for hemoglobin estimation at the 6 weeks visit.
The data collected were analyzed using the statistical package for social sciences (SPSS) computer software version 17.0 for windows (SPSS Inc., Chicago, IL, USA). Data analysis were done per protocol (patients who did not complete the study were excluded). The target hemoglobin concentration was 10 g/dL for both cases and controls for the reason that this was the threshold level for definition of anemia in Nigeria.28 The main outcome measure was the proportion of women who had attained a hemoglobin concentration of at least 10 g/dL by the 6 weeks postpartum visit among cases and controls. Categorical variables for the groups were compared using chi-square. Comparison of means of hemoglobin levels was done with Student’s t-test. P-value ≤0.05 was considered significant.
Ethical clearance for the study was obtained from the Research Ethics Committee of the University of Nigeria Teaching Hospital, Enugu. All the hematinics were given to participants free of charge and any extra day stayed in the hospital for the purpose of the study was not surcharged.
Results
Two hundred eighty-four consenting women who met the eligibility criteria were enrolled and randomized into two equal groups of 142 women each. Only 84.6% (237/280) completed the study and were used for analysis while 15.4% (43/280) were lost to follow-up. Approximately 82% (115/140) of those randomized to injectable iron therapy completed the study compared to 87% (122/140) of those randomized to oral treatment. Figure 1 is a flowchart of the study.
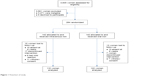  | Figure 1 Flowchart of study. |
Table 1 shows the sociodemographic characteristics of the study participants. The study groups were comparable in all sociodemographic parameters except on educational status. The average age of participants was 29±5.38.
  | Table 1 Sociodemographic characteristics for the two groups |
Table 2 shows the proportions of women who attained the target hemoglobin of 10 g/dL anemia at 6 weeks postpartum. The proportion of women who attained the target hemoglobin by 6 weeks postpartum was 95.7% (110/115) among women treated with single total-dose intravenous iron and 94.3% (115/122) among those treated with oral iron tablets. The difference was not statistically significant (P=0.51).
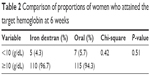  | Table 2 Comparison of proportions of women who attained the target hemoglobin at 6 weeks |
Table 3 shows the mean hemoglobin levels of the two study groups at 48 hours postpartum and 6 weeks postpartum periods. The changes in mean hemoglobin between the time of recruitment and at 6 weeks following either therapeutic route were comparable (1.03±0.56 g/dL for intravenous iron and 0.97±0.46 g/dL for the oral group; P=0.42). The average rate of rise of hemoglobin was also similar in the two groups (0.17/week vs 0.16/week), respectively for iron dextran and oral groups.
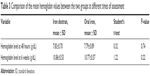  | Table 3 Comparison of the mean hemoglobin values between the two groups at different times of assessment |
Table 4 for shows the pattern of adverse reactions observed among cases and controls. No case of severe anaphylactic reaction was observed or reported in any of the groups.
  | Table 4 Adverse effects of treatment of postpartum anemia for each group |
Discussion
Two recent studies from our center had shown that the level of anemia at 6 weeks postpartum was high among low risk parturients and that iron-deficiency anemia contributed significantly to this finding.16,30 From anecdotal evidence, it would appear that considerable uncertainty about patient’s compliance with daily oral iron postdelivery existed and that noncompliance with oral therapy could be contributing to this high level of puerperal anemia. In order to address the problem of compliance with oral iron following delivery, we conducted this study to determine if a single total dose of intravenous iron given to parturients 48 hours after delivery would be as effective as the current practice of daily oral doses of ferric iron given for 6 weeks.
Our study showed that the use of single total-dose intravenous iron dextran was as effective as daily oral iron given for 6 weeks in the treatment of postpartum iron-deficiency anemia. Although the oral treatment group had a slightly higher proportion of women with persistent anemia at 6 weeks, the proportions of women who still had anemia after treatment in the two groups were not significantly different. Given that both groups had comparable mean hemoglobin at the commencement of treatment, this result suggests that a single total dose intravenous iron may be useful in addressing the problems of noncompliance or intolerance of oral iron among postpartum women.
Our findings were similar to that of a multicenter randomized controlled study by Breyman et al.31 Their study compared the effectiveness of treatment of 349 women with postpartum anemia defined as 10.5 g/dL, using intravenous iron carboxymaltose and oral ferrous sulfate. The study found comparable increase in the hemoglobin levels of the two groups throughout the 12-week study period and concluded that intravenous iron carboxymaltose was as effective oral iron. Westad et al32 also in their study of 128 anemic postpartum women using intravenous iron sucrose versus oral iron sulfate observed that both groups of women had similar hemoglobin values at 4 weeks postpartum. A similar study to ours was also done by Bhandal and Russell33 comparing oral versus intravenous iron sucrose in the treatment of postpartum anemia. They found that women treated with intravenous sucrose had higher hemoglobin within the 1 week of the study but the difference became insignificant by 6 weeks postpartum.
In contrast, some studies found significant differences in the effectiveness of iron therapy for the treatment of postpartum anemia based on the routes of administration. Aggarwal et al34 in India, comparing oral and intravenous iron in treatment of postpartum anemia, noted a significant difference in increase in mean hemoglobin level in the two groups, 5.54 versus 6.08 g/dL (P<0.004), respectively. They noted a faster increase in hematocrit value in the intravenous group from first to fourth postpartum week with 80% of the intravenous group and 40% of the oral group attaining their target hemoglobin of 12 g/dL. Similarly, Seid et al,35 Kharde et al36 and Van Wyck et al37 observed that the use of intravenous ferric carboxymaltose was associated with statistically significant rise in hemoglobin than in the oral group during treatment of postpartum anemia.
We observe that differences in study designs especially specific study populations and clinical characteristics of study participants may explain the differences in the findings of different studies comparing routes of iron administration for the treatment of postpartum anemia. Besides, differences in dietary cultures may affect absorption of iron and hence, response to oral iron intake.
Our study also found no clinically significant adverse drug reaction or anaphylaxis in any of the groups. Although iron dextran is not now the parenteral iron of first choice38 in the presence of newer products with better side effect profiles, its use in this study was based on its availability relative to newer parenteral formulations. It is noteworthy that with the drug administration protocol used in this study, iron dextran was not associated with serious adverse events. However, the significance of our study lies in the fact that it has provided evidence for advocating for the use of single total-dose intravenous iron for addressing noncompliance and intolerability of oral iron in postpartum iron-deficiency anemia. Newer parenteral iron formulations with better side effect profiles than iron dextran can only improve the advantages of this approach.
Limitations
The limitations of this study included the fact we could not exclude cases of Thalassemia from those who had iron-deficiency anemia due to nonfunctional facilities for hemoglobin electrophoresis. Besides the nonuse of the popular Ganzoni formula for determining intravenous iron requirements may have underestimated the iron requirements of participants. Weekly measurements of levels of hemoglobin during the study period were not done thereby making it impossible to determine the weekly changes in hemoglobin concentration. Besides, the women on oral iron were not directly observed as they took the drugs so that absolute compliance with daily oral iron was not guaranteed.
Conclusion
We conclude that single total-dose intravenous iron for treatment of puerperal iron-deficiency anemia could be as effective as daily single doses of ferric iron tablets taken for 6 weeks. For puerperal patients with iron deficiency anemia in whom compliance with and tolerability of oral iron are not certain, a single total-dose intravenous iron can be safely offered.
Disclosure
The authors report no conflicts of interest in this work.
References
Milasinovic L, Kapamadziga A, Dobric L, Petrovic D. Postpartum anemia–incidence and etiology. Med Pregl. 2000;53(7–8):394–399. Croatian. | ||
Somdatta P, Reddaiah VP, Singh B. Prevalence of anemia in the postpartum period: a study of a North Indian village. Trop Doct. 2009;39(4):211–215. | ||
Ekanem AD, Etuk SJ, Samson-Akpan U. The influence of cultural practice on puerperal anaemia. Int J Gynaecol Obstet. 1996;55(2):169–170. | ||
Harrison KA. Maternal mortality in developing countries. Br J Obstet Gynaecol. 1989;96(1):1–3. | ||
Brabin BJ, Hakimi M, Pelletier D. An analysis of anemia and pregnancy-related maternal mortality. J Nutr. 2001;131(2S):604S–614S; discussion 614S–615S. | ||
Shu EN, Ogbodo SO. Role of ascorbic acid in the prevention of iron-deficiency anaemia in pregnancy. Biomed Res. 2005;16(1): 40–44. | ||
Iyengar K. Early Postpartum maternal morbidity among rural women of Rajasthan, India: a community-based study. J Health Popul Nutr. 2012;30(2):213–225. | ||
Kalaivani K. Prevalence and consequence of anaemia in pregnancy. Indian J Med Res. 2009;130(5):627–633. | ||
Scholz BD, Gross R, Schultink W, Sastroamidjojo S. Anemia is associated with reduced productivity of women workers even in less-physically-strenuous tasks. Br J Nutr. 1997;77(1):47–57. | ||
Husani DK, Gunadi H. Evaluation of nutritional anemia intervention among anaemic female workers on a tea plantation. In: Iron Deficiency and Work Performance. Washington, DC: The Nutrition Foundation; 1983:73–78. | ||
Corwin EJ, Murray-Kolb LE, Beard JL. Low hemoglobin level is a risk factor for postpartum depression. J Nutr. 2003;133(12):4139–4142. | ||
Beard JL, Hendricks MK, Perez EM, et al. Maternal iron deficiency anemia affects postpartum emotions and cognition. J Nutr. 2005;135(2):267–272. | ||
Gibbs RS. Clinical risk factors for puerperal infection. Obstet Gynaecol. 1980;55(Suppl 5):178S–184S. | ||
Ballin A, Berar M, Rubinstein U, Kleter Y, Hershkovitz A, Meytes D. Iron state in female adolescents. Am J Dis Child. 1992;146(7):803–805. | ||
Bodnar LM, Scanlon KS, Freedman DS, Siega-Riz AM, Cogswell ME. High prevalence of postpartum anemia among low-income women in the United States. Am J Obset Gynaecol. 2001;185(2):438–443. | ||
Emegoakor CF, Iyoke CA, Ezegwui HU, Umeora OU, Lawani LO, Madu AJ. Rates and determinants of peripartum and puerperal anemia in Enugu, Nigeria. Niger J Clin Pract. 2016;19(6):709–714. | ||
Milman N. Postpartum anemia 1: definition, prevalence, causes and consequences. Ann Hematol. 2011;90(11):1247–1253. | ||
Bergmann RL, Richter R, Bergman KE, Dudenhausen JW. Prevalence and risk factors for early postpartum anemia. Eur J Obstet Gynecol Reprod Biol. 2010;150(2):126–131. | ||
Anorlu RI, Oluwole AA, Abudu OO. Sociodemographic factors in anemia in pregnancy at booking in Lagos, Nigeria. J Obstet Gynecol. 2006;26(8):773–776. | ||
Buseri FI, Uko EK, Jeremiah ZA, Usanga EA. Prevalence and risk factors of anaemia among pregnant women in Nigeria. Open Hematol J. 2008;2(1):14–19. | ||
Kavle JA, Stoltfuz RJ, Witter F, Tielsch JM, Khalfan SS, Caulfield LE. Association between anaemia during pregnancy and blood loss at and after delivery among women with vaginal births in Pemba Island, Zanzibar, Tanzania. J Health Popul Nutr. 2008;26(2):232–240. | ||
Bodnar LM, Siega-Ritz AM, Miller WC, Cogswell ME, Mc Donald T. Who should be screened for postpartum anemia? An evaluation of current recommendations. Am J Epidemiol. 2002;156(10):903–912. | ||
Breymann C, Honegger C, Holzgreve W, Surbek D. Diagnosis and treatment of iron-deficiency anaemia during pregnancy and postpartum. Arch Gynaecol Obstet. 2010;282(5):577–580. | ||
Kumpf VJ. Parenteral iron supplementation. Nutr Clin Pract. 1996;11(4):139–146. | ||
Moore RA, Gaskell H, Rose P, Allan J. Meta-analysis of efficacy and safety of intravenous ferric carboxymaltose (Ferinject) from clinical trial reports and published trial data. BMC Blood Disord. 2011;11:4. | ||
Chertow GM, Mason PD, Vaage-Nilsen O, Ahlmén J. On the relative safety of parenteral iron formulations. Nephrol Dial Transplant. 2004;19(6):1571–1575. | ||
Zhong B. How to calculate sample size in randomized controlled trial? J Thorac Dis. 2009;1(1):51–54. | ||
Ogunbode O. Anaemia in pregnancy. In: Okonofua F, Odunsi K, editors. Contemporary Obstetrics and Gynaecology for Developing Countries. 1st ed. Benin City: Women’s Health and Action Research Centre; 2003:515–529. | ||
Casanueva E, Pfeffer F, Drijanski A, Fernandez-Gaxiola AC, Gutierrez-Valenzuela V, Rothenberg SJ. Iron and folate status before pregnancy and anemia during pregnancy. Ann Nutr Metab. 2003;47(2):60–63. | ||
Emegoakor FC, Iyoke CA, Ezegwui HU, Ezugwu FO, Umeora OU, Ibeagha IO. Rate and predictors of low serum ferritin levels among healthy parturient women in Enugu, Nigeria. J Blood Med. 2015;6:261–267. | ||
Breyman C, Gliga F, Bejenariu C, Strizhova N. Comparative efficacy and safety of intravenous ferric carboxymaltose in the treatment of postpartum iron deficiency anemia. Int J Gynecol Obstet. 2008;101(1):67–73. | ||
Westad S, Backe B, Salvesen KA, et al. A 12-week randomised study comparing intravenous iron sucrose versus oral ferrous sulphate for treatment of postpartum anaemia. Acta Obstet Gynaecol Scand. 2008;87(9):916–923. | ||
Bhandal N, Russell R. Intravenous versus oral therapy for postpartum anaemia. BJOG. 2006;113(11):1248–1252. | ||
Aggarwal RS, Mishra VV, Panchal NA, Patel NH, Deshchougule VV, Jasani AF. Comparison of oral iron and iron sucrose for treatment of anemia in postpartum Indian women. Natl J Comm Med. 2012;3(1):48–54. | ||
Seid MH, Derman RJ, Baker JB, Banach W, Goldberg C, Rogers R. Ferric carboxymaltose injection in the treatment of postpartum iron deficiency anemia: a randomized controlled clinical trial. Am J Obstet Gynecol. 2008;199(4):435.e1–e7. | ||
Kharde PS, Bangal VB, Panicker KK. Comparative study of intravenous iron sucrose versus oral iron therapy in iron deficiency anemia during postpartum period. Int J Biomed Adv Res. 2012;3(4):238–243. | ||
Van Wyck DB, Martens MG, Seid MH, Baker JB, Mangione A. Intravenous ferric carboxymaltose compound with oral Iron in treatment of postpartum anemia: a RCT. Obstet Gynecol. 2007;110(2 Pt 1):267–278. | ||
American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No 95: anemia in pregnancy. Obstet Gynecol. 2008;112(1):201–207. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.