Back to Journals » Therapeutics and Clinical Risk Management » Volume 16
Effect of Time Interval Between LEEP and Subsequent Hysterectomy on Postoperative Infectious Morbidity
Authors Ni T, Meng Y, Li Y, Chen Q, Huang Y, Wang L, Qian X, Wang Y
Received 3 July 2020
Accepted for publication 21 August 2020
Published 10 September 2020 Volume 2020:16 Pages 839—847
DOI https://doi.org/10.2147/TCRM.S270590
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Deyun Wang
Ting Ni,1 Yaping Meng,1 Yuhong Li,1 Qinfang Chen,1 Yong Huang,1 Lihua Wang,1 Xiaolei Qian,1 Yudong Wang1– 3
1Department of Gynecologic Oncology, International Peace Maternity and Child Health Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai 200030, People’s Republic of China; 2Shanghai Municipal Key Clinical Specialty, International Peace Maternity and Child Health Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai 200030, People’s Republic of China; 3Shanghai Key Laboratory of Embryo Original Disease, International Peace Maternity and Child Health Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai 200030, People’s Republic of China
Correspondence: Yudong Wang Email [email protected]
Objective: This study aimed to provide insight into the effect of time interval between loop electrosurgical excision procedure (LEEP) and subsequent hysterectomy on postoperative infectious morbidity in cervical neoplasia patients.
Methods: In this retrospective cohort study, a total of 1172 medical records of patients who were diagnosed with high grade cervical intraepithelial neoplasia (HSIL) or invasive cancer underwent a subsequent hysterectomy after LEEP at the International Peace Maternity and Child Health Hospital (IPMCH) in Shanghai, China from January 2008 to December 2019 were collected. The study outcome was postoperative infectious morbidity within 30 days after a hysterectomy. Overall and surgical approach specific effect of time interval on infectious morbidity was estimated using logistic regression in crude and adjusted models.
Results: There was an inverse association between time interval and postoperative infectious morbidity in HSIL or invasive cancer patients (OR=0.99, 95% CI: 0.98– 1.00, p=0.0079). When trisecting time interval into three parts, the top tertile time interval (34– 90 days) was also inversely associated with infectious morbidity compared with bottom tertile (0– 16 days), independent of stage, surgical approach, operative time and estimated blood loss (OR=0.66,95% CI: 0.43– 1.00, P=0.0487). A test for interaction between time interval and surgical approach on infectious morbidity was significant (P values for interaction= 0.0352). Longer time interval significantly reduced the risk of infectious morbidity in the laparoscopic group (OR = 0.37, 95% CI: 0.17– 0.78), while no statistically significant effects were observed in patients who underwent vaginal or open abdominal hysterectomy.
Conclusion: The time interval and surgical approach can interactively affect the risk of postoperative infectious morbidity in cervical neoplasia patients who underwent a hysterectomy after LEEP. Our data suggest that compared with vaginal or open abdominal hysterectomy, laparoscopic hysterectomy required a longer time interval (34– 90 days) to reduce the risk of infectious morbidity.
Keywords: time interval, LEEP, hysterectomy, cervical cancer
Introduction
Generally, since the 1950s, patients diagnosed with early cervical cancer by cold knife conization (CKC) subsequently undergo hysterectomy within 48 h or 6 weeks to reduce intra- and postoperative complications,1,2 although this interval is still under investigation. Loop electrosurgical excision procedure (LEEP), which is a more convenient, safer, and cheaper procedure compared to CKC, has been widely used to diagnose and treat cervical neoplasia. However, the appropriate timing of performing subsequent hysterectomy after LEEP is still not well determined.
In the previous study, Kim et al3 reported that different time intervals between LEEP and abdominal hysterectomies were not significantly associated with postoperative complications in patients with cervical neoplasia. It indicated that an open, type I hysterectomy could be conducted at any time. While the result of Sullivan et al4 showed that time interval between excision and definitive minimally invasive surgery (MIS) should be greater than 6 weeks to reduce surgical complications, however, with cervical incision procedure including both CKC and LEEP. Recently, Yin et al5 further reported that simple hysterectomy should be performed greater than 4 weeks after conization for CIN III or stage IA1 patients to reduce postoperative infection, with an emphasis on iodoform treatment. Apparently, the results did not consist with either of these studies. Both cervical excision procedure and surgical approach for hysterectomy varied. The time interval groups were all divided according to previous CKC studies or clinical experience, and the results changed along with the way of group division. None of the studies analyzed the time interval as a continuous variable. Additionally, the study outcomes also varied. Meanwhile, as not only surgical approach,6–9 but also lots of other surgical factors10–12 might significantly associate with postoperative complications, the independent effect of time interval on postoperative complication was not fully studied. Also, as small sample size studies, the low incidence of exact complications might not be enough to power the analysis to some extent.
Thus, in our study, as LEEP was emphasized, we aimed to clarify the independent effect of time interval between LEEP and final surgical treatment on postoperative infectious morbidity. Time interval was analyzed both as continuous and categorical variable.
Materials and Methods
Data of cervical neoplasia patients who underwent LEEP and subsequent hysterectomy between January 2008 and December 2019 at the Department of Gynecologic Oncology, International Peace Maternity and Child Health Hospital (IPMCH), Shanghai, China were collected in this retrospective cohort study. The project was approved by the Institutional Review Board of IPMCH: ref. no. (GKLW) 2017–125. The data are anonymous in our study, and the requirement for informed consent was therefore waived, as reported previously.13 The patient data are confidential and complied with the Declaration of Helsinki. No standardized timing of hysterectomy was established at IPMCH during the study period.
Patients who had a definitive hysterectomy after LEEP within a time interval of 90 days were included in this study. The exclusion criteria included: 1) conducted subsequent surgery in more than 90 days, 2) underwent repeat LEEP before hysterectomy, 3) combined with other cancers, 4) with hyperthyroidism or preoperative infection, 5) treated with immunosuppressive agents or steroids, 6) treated with preoperative concurrent chemoradiotherapy or neoadjuvant chemotherapy, 7) with incomplete information.
Indications for LEEP included unsatisfactory colposcopy, positive endocervical curettage, discrepancies of greater than two grades between Pap smear and colposcopic examination, and suspicion of microinvasive diseases. Indications for simple hysterectomy included cases diagnosed with high grade cervical intraepithelial neoplasia (HSIL) to International Federation of Gynecology and Obstetrics (FIGO, 2018) stage IA1 without lymphovascular space invasion (LVSI) when preservation of fertility was not desired. Patients with FIGO stage IA1 with positive LVSI, IA2, IB1-IB2, and IIA1 underwent radical (or modified radical) hysterectomy with pelvic lymphadenectomy with or without a para-aortic lymph node biopsy. The indication for hysterectomy in HSIL patients was: 1) with positive margins after LEEP and had no desire to preserve fertility, 2) combined with benign lesions such as uterine leiomyoma and adenomyosis, 3) poor follow-up condition. The simple and radical hysterectomies were accomplished using either laparoscopic, vaginal, or open abdominal approach in our study. Cases converted from laparoscopic or vaginal were included in open approach.
Our hospital is a public large tertiary care center that serves a stable patient population, and all the patients were operated by the same oncological team with extensive experience in different surgical procedures. Both patient care and criteria for postoperative outcomes evaluation were standardized. Patients’ demographic and clinical information were collected from the electronic medical records by two reviewers in our group. After the data were collected, the patients’ charts were rereviewed by another investigator to ensure consistency.
The study outcome was postoperative infectious morbidity including surgical site infection (SSI), nonsurgical site infection (NSSI), and febrile morbidity. SSI was defined by the Centers for Disease Control and Prevention criteria, comprising superficial SSI (skin and subcutaneous tissue) and deep SSI (fascial, muscle layers, and organ space.14 NSSI included the urinary tract and other infections. Febrile morbidity of unknown origin was defined as a body temperature greater than 38°C on two separate occasions at least 6 hours apart, excluding the first 24 hours after hysterectomy.15 In addition, data of intraoperative and noninfectious postoperative complications were also collected.
A second-generation cephalosporin was administered as a single dose half an hour before surgeries, while in the case of a penicillin allergy in which clindamycin was used. Patients with operative time exceeding 3 hours, or with an estimated blood loss >1500 mL received an additional dose of the same antibiotic. Postoperative evaluation was scheduled 30 days after surgery. If a patient was lost to follow up, we attempted to make a phone call and asked if she had any remaining symptoms attributed to the surgery or received any further treatment after hysterectomy.
Statistical Analysis
To achieve an even distribution in each group, time interval was divided into three tertiles (T1-bottom, T2-middle, and T3-top): T1 median (range): 11 (0–16) days, T2: 24 (17–33) days and T3: 48 (34–90) days. Time interval was analyzed both as continuous and categorical variables. The χ2 test was used for comparisons between time interval tertiles in categorical variables. We performed multivariate logistic regression analysis to determine the independent association between time interval and postoperative infectious morbidity, with an adjustment for the potential confounders including cancer stage, surgical approach, operative time and estimated blood loss. Covariates were included as potential confounders if they changed the estimates of time interval on infectious morbidity by more than 10%. The variance inflation factor (VIF) among the covariates in multivariate analysis was determined, and a VIF > 5 was considered indicating multicollinearity in our study. We then performed tests for the interaction between time interval and confounders which entered the final model. The results were presented as odds ratios (OR) with 95% confidence interval (CI). An OR > 1 indicated an increased risk of infectious morbidity, while an OR < 1.0 indicates reduced risk. A smooth curve fitting and a generalized additive model (GAM) was used to address the relationship of time interval with infectious morbidity. Analyses were performed using R project (http://www.R-project.org) and Empower (R) (www.empowerstats.com, X&Y solutions, Inc. Boston MA), and a P-value < 0.05 was considered statistically significant.
Results
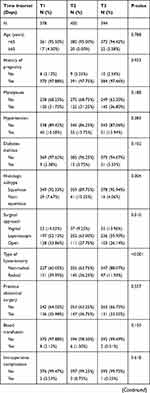
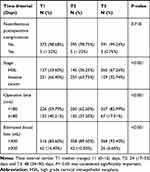
During the study period, 1172 patients with complete and valid data were eligible for this study. Among them, 210 patients were diagnosed with infectious morbidity, for an incidence of 17.92%. The median time between LEEP and subsequent hysterectomy was 25 days (range 0–90 days). The distribution of surgical time interval was displayed in Figure 1 and Figure S1. The baseline characteristics of the included participants stratified by time interval tertiles are shown in Table 1. The proportion of patients experienced intraoperative (p=0.618) and noninfectious postoperative complications (p=0.718) did not differ significantly between time interval tertiles. The details of perioperative complications were shown in Table 2.
 | |
 | |
 |
Table 1 Patient Baseline Characteristics by Time Interval Tertiles |
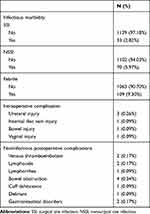 |
Table 2 Details of Perioperative Complications |
 |
Figure 1 The distribution of time interval between loop electrosurgical excision procedures (LEEP) and subsequent hysterectomy for our population by surgical approach. |
The effect of risk factors on infectious morbidity was shown in Table 3. Cancer stage, surgical approach, operative time, estimated blood loss and type of hysterectomy were significantly associated with infectious morbidity. Among them, type of hysterectomy was excluded in the final regression model and subgroup analysis because of multicollinearity with a VIF > 5.
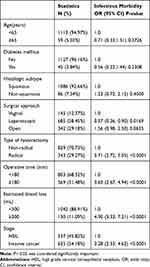 |
Table 3 Effect of Risk Factors on Infectious Morbidity |
There was a decline in postoperative infectious morbidity with increasing time interval in HSIL or invasive cancer patients (OR=0.99, 95% CI: 0.98–1.00, p=0.0079) after adjusting for stage, surgical approach, operative time and estimated blood loss (Table 4). When trisecting time interval into three parts, the top tertile (34–90 days) time interval was also inversely associated with infectious morbidity compared with bottom tertile (0–16 days), independent of the same confounders (OR=0.66,95% CI: 0.43–1.00, P=0.0487). The rate of postoperative infectious morbidity in the T1, T2, and T3 groups was 24.6%, 17.0%, and 11.7%, respectively (Figure 2).
 |
Table 4 Univariate and Multivariate Analysis Between Time Interval and Infectious Morbidity |
Figure 3 presents the association between time interval and infectious morbidity, stratified by surgical approach. The green line, which represents laparoscopic hysterectomy, shows a decline in infectious morbidity with increasing time interval after adjusting for covariables. In contrast, no such association was found in the vaginal and open hysterectomy group (Table S1).
 |
Figure 3 Smooth curves between time interval and postoperative infectious morbidity stratified by surgical approach. The model was adjusted for stage, operative time and estimated blood loss. |
A test for interaction between time interval and surgical approach on infectious morbidity was significant (P values for interaction= 0.0352). Longer time interval significantly reduced the risk of infectious morbidity in laparoscopic group (OR = 0.37, 95% CI: 0.17–0.78), while no statistically significant effects were observed in patients who underwent vaginal or open hysterectomy (Table 5).
 |
Table 5 Subgroup Analysis and Interaction Test Between Covariates and Time Interval on Infectious Morbidity |
Discussion
In our study, we found that in cervical neoplasia patients, there was a reduced risk in postoperative infectious morbidity with increasing time interval between LEEP and subsequent hysterectomy. This effect was modified by the surgical approach. In the laparoscopic group, longer time intervals significantly reduced the risk of infectious morbidity, while no statistically significant effects were observed in other surgical approaches.
In several institutions, the LEEP-to-hysterectomy time interval was determined according to the CKC-to-hysterectomy interval or clinical experience. The preferred time interval varied in different group division ways. Besides, the population size and distribution of patients does not allow for a balanced evaluation of different time interval levels.4 To achieve an even distribution in each group, the time interval was divided into three tertiles in our study, and it was analyzed both as continuous and categorical variable. In a brief review of articles of time interval and hysterectomy, our study was the first study till now in which time interval was treated as a continuous variable. The results showed that the longer the time interval, the fewer the infectious morbidity. However, as is known, the cervix takes within 90 days to heal after LEEP. Besides, in order to achieve a balance between disease progression and postoperative complication, time interval more than 90 days were not included in our study. Thus, the top tertile time interval (34–90 days) might be acceptable in decision making for surgeons, with significantly reduced infectious morbidity compared with bottom tertile (0–16 days), respectively.
In the largest series to date, Tae Kim et al3 reviewed the records of 338 patients who underwent abdominal extended hysterectomies after LEEP and reported that the postoperative complications such as fever and surgical region infection were not significantly different among various time interval groups (< 48 h, 48 h-6 weeks and >6 weeks). It was consisted with our subgroup analysis that in the open abdominal group, no statistically significant effect of time interval on infectious morbidity was observed. Other studies focusing on laparoscopic hysterectomies4,16 suggested that time interval between excision and definitive invasive surgery should be greater than 6 weeks to reduce surgical complications. However, the cervical excision procedures were combined with LEEP and CKC, with a dominant proportion of CKC (89/138, 64.49% and 37/55, 67.27%) in both of the two studies.4,16 In fact, due to fewer complications and less discomfort, LEEP is now used in preference to CKC for the diagnosis and treatment of cervical intraepithelial neoplasia. In our study, the excision procedure was LEEP alone.
In addition, except for infectious morbidity, other surgical complications such as ureter and vascular injuries were mixed in the analysis of postoperative complications in previous studies.4,16 However, these injuries might be correlated with type of hysterectomy and surgical skills other than time interval. It is reported that bladder and ureteral injuries were the most common intraoperative complication in radical hysterectomy, and they were associated with para-aortic lymphadenectomy or a larger number of resected lymph nodes.17 Furthermore, the low incidence of exact complications might not be enough to power the analysis. In our study, the intraoperative and noninfectious postoperative complications did not differ in different time interval groups. The study outcome in our cohort was focused on infectious morbidity, including SSI, NSSI and febrile morbidity. Among them, febrile morbidity of unknown origin accounts for the majority of infectious morbidity. For decades, studies have stated that the administration of prophylactic antibiotics could markedly decrease the postoperative febrile morbidity observed after performing vaginal, abdominal, and radical hysterectomy.15,18-22 In our hospital, the prophylactic antibiotic was routinely administered in a single dose half an hour before surgery. Patients with operative time exceeding 3 hours, or with an estimated blood loss >1500 mL received an additional dose of the same antibiotic.
In a similar study focused on postoperative infection, Yin et al5 reported that simple hysterectomy should be performed at least 4 weeks after conizations to reduce postoperative infection. It also demonstrated that the postoperative infection rates in the 1–2-week, 4–6-week, and 6-week groups were 60.0%, 9.1%, and 0%, respectively.5 However, the excision procedure and surgical approach were also mixed, and the risk factors including estimated blood loss and operating time which might affect the results were not thoroughly adjusted. In our study, after adjusting for stage, surgical approach, operative time and estimated blood loss, the increased time interval was still associated with reduced infectious morbidity. In further subgroup analysis, longer time interval significantly reduced the risk of infectious morbidity in the laparoscopic group, while no statistically significant effects were observed in patients underwent a vaginal or open hysterectomy. It has been recognized that laparoscopic hysterectomy required greater expertise and longer time to master than vaginal and open approach. The inflammatory reaction and hypervascularity of paracervical tissue caused by cervical operation might increase the technical difficulty of laparoscopic surgery, thus resulted in increased perioperative morbidity.9,23 We hypothesize that the delayed time interval allows the cervix to heal and thus reduces the infectious morbidity in laparoscopic hysterectomy. Furthermore, while the previous studies were all conducted in smaller sample sizes, our study, involving a total of 1172 patients, is known to have the largest sample size in determining the effect of time interval between LEEP and subsequent hysterectomy.
In the present cohort, however, we did not evaluate the association between time interval and intraoperative or noninfectious postoperative complications due to the low incidence of exact complications, which might not be enough to power the analysis. And these complications were more likely to be correlated with other risk factors other than time interval. Further larger-scale studies with a specific focus on intraoperative or noninfectious postoperative complications are required. For patients diagnosed with invasive cancer, subsequent hysterectomy was performed earlier because of fear and anxiety during the waiting period. Thus, the time distribution for our population was not normal. The smooth curve representing the relationship between time interval and infectious morbidity indicated a linear association between time and infectious morbidity in the laparoscopic group. However, no exact cut off value was concluded. The nonlinear association which might be existed in the open abdominal group was not further evaluated, either. For the other limitations, the data were retrospectively collected from one institution, which might limit the study’s generalizability. Long-term cancer-specific factors such as recurrence-free survival and overall survival were not assessed in this investigation.
In conclusion, the time interval and surgical approach can interactively affect the risk of postoperative infectious morbidity in cervical neoplasia patients who underwent hysterectomy after LEEP. Our data suggest that compared with vaginal or open hysterectomy, laparoscopic hysterectomy required a longer time interval (34–90 days) to reduce the risk of infectious morbidity.
Funding
This study was supported by the National Natural Science Foundation of China (No. 81172477 and 81402135), the Project of the Science and Technology Commission of Shanghai Municipality (No. 17441907400), Shanghai Jiao Tong University Medicine-Engineering Fund (No. YG2017MS41) and Shanghai Municipal Key Clinical Specialty (No. SHDC12014208).
Disclosure
The authors declare no conflicts of interest for this work.
References
1. Cavanagh D, Rutledge F. The cervical cone biopsy hysterectomy sequence and factors affecting the febrile morbidity. Am J Obstet Gynecol. 1960;80:53–59. doi:10.1016/S0002-9378(16)36416-X
2. DeCenzo JA, Malo T, Cavanagh D. Factors affecting cone-hysterectomy morbidity. A study of 200 patients. Am J Obstet Gynecol. 1971;110:380–384. doi:10.1016/0002-9378(71)90733-2
3. Tae Kim Y, Sung Yoon B, Hoon Kim S, et al. The influence of time intervals between loop electrosurgical excision and subsequent hysterectomy on the morbidity of patients with cervical neoplasia. Gynecol Oncol. 2005;96:500–503. doi:10.1016/j.ygyno.2004.10.032
4. Sullivan SA, Clark LH, Staley AS, et al. Association between timing of cervical excision procedure to minimally invasive hysterectomy and surgical complications. Gynecol Oncol. 2017;144:294–298. doi:10.1016/j.ygyno.2016.11.037
5. Yin X, Zhu L, Tan W, et al. Time intervals between prior cervical conization and posterior hysterectomy influence postoperative infection in patients with cervical intraepithelial neoplasia or cancer. Med Sci Monitor. 2018;24:9063. doi:10.12659/MSM.911892
6. Os Reis R, Andrade CEMC, Frumovitz M, et al. Radical hysterectomy and age: outcomes comparison based on a minimally invasive vs an open approach. J Minim Invasive Gynecol. 2018;25(7):1224–1230. doi:10.1016/j.jmig.2018.03.002
7. Uppal S, Liu JR, Reynolds RK, et al. Trends and comparative effectiveness of inpatient radical hysterectomy for cervical cancer in the United States (2012–2015). Gynecol Oncol. 2019;152(1):133–138. doi:10.1016/j.ygyno.2018.09.027
8. Salicru S, Gil-Moreno A, Montero A, et al. Laparoscopic radical hysterectomy with pelvic lymphadenectomy in early invasive cervical cancer. J Minim Invasive Gynecol. 2011;18:555–568. doi:10.1016/j.jmig.2011.05.003
9. Steed H, Rosen B, Murphy J, et al. A comparison of laparoscopic-assisted radical vaginal hysterectomy and radical abdominal hysterectomy in the treatment of cervical cancer. Gynecol Oncol. 2004;93:588–593. doi:10.1016/j.ygyno.2004.04.003
10. Barber EL, Harris B, Gehrig PA. Trainee participation and perioperative complications in benign hysterectomy: the effect of route of surgery. Am J Obstet Gynecol. 2016;215(2):
11. Park SH, Lee JY, Nam EJ, et al. Prediction of perioperative complications after robotic-assisted radical hysterectomy for cervical cancer using the modified surgical Apgar score. BMC Cancer. 2018;18(1):908. doi:10.1186/s12885-018-4809-4
12. Samlal RA, van der Velden J, Schilthuis MS, et al. Influence of diagnostic conization on surgical morbidity and survival in patients undergoing radical hysterectomy for stage IB and IIA cervical carcinoma. Eur J Gynaecol Oncol. 1997;18:478–481.
13. Filion KB, Azoulay L, Platt RW, et al. A multicenter observational study of incretin-based drugs and heart failure. N Engl J Med. 2016;374(12):1145–1154. doi:10.1056/NEJMoa1506115
14. Till SR, Morgan DM, Bazzi AA, et al. Reducing surgical site infections after hysterectomy: metronidazole plus cefazolin compared with cephalosporin alone. Am J Obstet Gynecol. 2017;217(2):
15. Ghezzi F, Cromi A, Siesto G, et al. Infectious morbidity after total laparoscopic hysterectomy: does concomitant salpingectomy make a difference? BJOG. 2009;116(4):589–593. doi:10.1111/j.1471-0528.2008.02085.x
16. Li H, Jang JY, Chen K, et al. The influence of interval between conization and laparoscopic radical hysterectomy on the morbidity of patients with cervical cancer. Eur J Gynaecol Oncol. 2012;33:601–604.
17. Machida H, Matsuo K, Furusawa A, et al. Profile of treatment-related complications in women with clinical stage IB-IIB cervical cancer: A nationwide cohort study in Japan. PLoS One. 2019;14(1):e0210125. doi:10.1371/journal.pone.0210125
18. Peipert JF, Weitzen S, Cruickshank C, et al. Risk factors for febrile morbidity after hysterectomy. Obstet Gynecol. 2004;103(1):86–91. doi:10.1097/01.AOG.0000109219.24211.30
19. Sevin BU, Ramos R, Lichtinger M, et al. Antibiotic prevention of infections complicating radical abdominal hysterectomy. Obstet Gynecol. 1984;64:539–545.
20. Micha JP, Kucera PR, Birkett JP, et al. Prophylactic mezlocillin in a radical hysterectomy. Obstet Gynecol. 1987;69:251–254.
21. Polk BF, Tager IB, Shapiro M, et al. Randomised clinical trial of perioperative cefazolin in preventing infection after hysterectomy. Lancet. 1980;1:437–440. doi:10.1016/S0140-6736(80)90994-0
22. Mittendorf R, Aronson MP, Berry RE, et al. Avoiding serious infections associated with abdominal hysterectomy: a meta-analysis of antibiotic prophylaxis. Am J Obstet Gynecol. 1993;169:1119–1124. doi:10.1016/0002-9378(93)90266-L
23. Malinak LR, Jeffrey RA, Dunn WJ. The conization-hysterectomy time interval: a clinical and pathologic study. Obstet Gynecol. 1964;23(3):317–329.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

