Back to Journals » Journal of Inflammation Research » Volume 12
Effect of thermal spring water on human dendritic cell inflammatory response
Authors Eliasse Y, Galliano MF , Redoules D, Espinosa E
Received 26 April 2019
Accepted for publication 28 June 2019
Published 22 July 2019 Volume 2019:12 Pages 181—194
DOI https://doi.org/10.2147/JIR.S213594
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Yoan Eliasse1,2, Marie-Florence Galliano,3 Daniel Redoules,4 Eric Espinosa1,2
1INSERM U1037, Centre de Recherche en Cancérologie de Toulouse (CRCT), Toulouse F-31037, France; 2Université De Toulouse, Université Paul Sabatier, Toulouse F-31062, France; 3Research and Development, Pierre Fabre Dermo-Cosmétique, Toulouse, France; 4Global Medical Direction, Laboratoire Dermatologique Avène, Pierre Fabre Dermo-Cosmétique, Toulouse, France
Background: Hydrotherapy appears as a valuable therapeutic tool in the management of patients suffering from chronic skin inflammatory diseases. Nevertheless, the underlying immune mechanisms of these beneficial effects remain poorly understood. To better understand the biological effects of thermal spring water on the immune system, we investigated the effects of Avène thermal spring water (ASW) on dendritic cells as key cells participating in the control of the immune response.
Methods: Dendritic cells (DCs) were generated from human monocytes and matured with LPS in ASW-based culture medium or in dexamethasone supplemented culture medium as an anti-inflammatory treatment. The phenotypes and abilities of these DCs to produce cytokines and induce allogeneic T cell response was next assessed.
Results: We showed that ASW modulated the differentiation of monocytes into DCs and impacted the DC maturation upon LPS priming. We observed a reduction of the CD83, CD86, CD1a and HLA-DR molecule expression and a decrease of IL-12 and IL-23 production whereas IL-10 production was increased. LPS-primed DCs generated in presence of ASW exhibited a reduced capacity to induce naive CD4+, T cell proliferation and IFN-γ and IL-17 production.
Conclusion: Our study showed that ASW is endowed with an immunomodulatory potential. ASW limits the DC stimulatory capacity of Th1 and Th17 cell responses by impairing their maturation, IL-12 and IL-23 production and accessory cell function.
Keywords: dendritic cells, hydrotherapy, inflammation, naive CD4+, T cells, dexamethasone
Introduction
Skin constitutes the first barrier and line of defense against pathogens and noxious substances. It is an immunologically active tissue with several resident immune cells and an important pool of recirculating lymphocytes. Due to its strategic location, skin is continuously exposed to a multitude of pathogens but also physical and chemical aggressions, which can lead to different types of inflammatory diseases. Spa therapy is widely used to treat inflammatory skin diseases, such as atopic dermatitis, psoriasis, rosacea, seborrheic dermatitis and others.1 The mechanisms underlying spa therapy are ill-understood and might incorporate chemical, thermal and immunomodulatory effects.2–4 Several studies showed that thermal spring waters decrease the pro-inflammatory abilities of keratinocytes.5–7 Avène thermal spring water (ASW) is commonly used in France to treat atopic dermatitis and psoriasis. Previous studies reported that ASW is endowed with an anti-inflammatory potential.7–9 Because dendritic cells (DCs) are key players in the control of the immune response, we investigated in this study the effects of ASW on human DCs.
DCs are highly specialized antigen presenting cells (APCs), which are crucial for the induction and the control of immune responses.10,11 In steady state, DCs are sentinel cells equipped with a huge panel of pattern recognition receptors (PRRs) allowing them to detect virtually all pathogens or tissue damages. They are in an immature state, prone to antigen capture and with tolerance-inducing functionality. Upon PRR stimulation, DCs differentiate in a fully mature state, allowing them to migrate in secondary lymphoid organs and initiate adaptive responses. DCs activate antigen-specific CD4+ T cells by providing three main signals.12 During the maturation process, DCs upregulate cell surface expression of antigen presenting molecules such as MHC-class II and CD1a molecules (signal 1) as well as T cell costimulatory molecules such as CD80 and CD86 (signal 2).13 Depending on the environmental cues detected and the PRR engaged, DCs drive T helper cell differentiation by producing cytokines (signal 3).14
Several groups of DCs reside in the skin in steady state;15,16 they control skin homeostasis and start immune responses.17 Moreover, upon inflammation, skin is infiltrated by myeloid DCs that are often referred to as inflammatory DCs.15,16,18 In chronic pathologies such as auto-immune diseases or allergy, these cells sustain a continuous activation of the adaptive immune system at sites of inflammation.19,20 Depending on the tissue microenvironment, Inflammatory DCs termed TipDCs (TNF-α/iNOS-producing DC) have been described in psoriasis21,22 or after bacterial infection.23 Inflammatory dendritic epidermal cells (IDECs) were described in atopic dermatitis.24 Inflammatory DCs are assumed to be monocyte derived, but their development is poorly known.15
DC maturation represents a key event in the immune response and is tightly controlled. Immature DCs are weak stimulators of T cell responses and are viewed as tolerogenic to ensure the maintenance of immune homeostasis. Tolerogenic DCs express low levels of costimulatory molecules and produce notably the immunomodulatory cytokine IL-10.25 Several clinical attempts were made to harness DC maturation in order to dampen the immune response in autoimmunity, graft rejection or chronic inflammatory diseases.17 A wide array of mediators such as IL-10, dexamethasone, vitamin D3 or rapamycin were used to induce a tolerogenic phenotype in DCs.26,27 In this study, we investigated the effects of ASW on DC maturation and functions. We used the classical model of DC derived from human monocytes in ASW-supplemented medium or in distilled water with or without dexamethasone-supplemented media as controls.
Materials and methods
Spring water collection and processing
ASW was collected in 2018 from the Avène thermal center (Laboratoire de l’Eau, Avène, France) using an aseptic procedure. Briefly, a single operator wearing sterile nitrile surgical gloves collected 2,000 mL water with a sterile 50 mL syringe. The water was filtered through 0.20-µm pore cellulose nitrate membranes and stored at 4°C. All experiments were performed within 3 months of water filtration and storage. The hydrochemical analysis of ASW is provided in Table S1.
Media
RPMI-1640 powder (R6504 Sigma) supplemented with 2.0 g sodium bicarbonate NaHCO3 was reconstituted with Avène spring water or UltraPure™ DNase/RNase-Free Distilled Water (Invitrogen). Basal media were next supplemented with 10% FCS, 1% penicillin (10,000 U/mL) and streptomycin (10 mg/mL), Sodium pyruvate (1 mmol/L), HEPES (N-2-hydroxyethylpiperazine-N-2-ethane sulfonic acid, 10 mmol/L) all from GIBCO, the pH of the media was adjusted to 7.0 and the media were next sterilized immediately by filtration using a 0.22µm pore size unit. ASW-based complete RPMI medium was termed R10ASW, Ultrapure distilled water-based complete RPMI medium was termed R10CTW and R10CTW supplemented with 10–7 mol/L dexamethasone (Sigma Aldrich) was termed R10DEX.
Cell purification
Peripheral blood mononuclear cells (PBMCs) were isolated from buffy coats (Etablissement Français du Sang) by Ficoll-Hypaque gradient centrifugation (specific gravity, 1.077 g/mL; Amersham Biosciences, USA) density.CD14+ cells were positively separated by anti-CD14 magnetic beads according to the Manufacturer’s instructions (EasySep™ Human CD14 Positive Selection Kit II, Stemcell technologies). Isolated cells routinely contained more than 95% of CD14+ cells. Naive CD4+ T cells were isolated from PBMCs using EasySep™ Human Naïve CD4+ T Cell Isolation Kit II (Stemcell technologies). The cell population obtained, contained >95% of CD3+ CD4+ CD45RA+ CD45RO− cells, as assessed by flow cytometry.
Generation and culture of MoDCs
Purified CD14+ monocytes were seeded at 5×105 cells per well of a 12-well plate in 2 mL of R10ASW or R10CTW or R10DEX supplemented with GM-CSF (500 IU/mL) and IL-4 (10 ng/mL) (both from PeproTech, Rocky Hill, NJ) and cultured for 5 days at 37°C, 5% CO2. Half of the medium was replaced on Day 3. Non-adherent cells (immature DC) were collected at day 5 and analyzed by flow cytometry to assess CD11c expression; only cultures with more than 90% CD11c+ cells were used for experiments. Cells (1x105 per well in a U-bottom 96 plate) were next matured with 100 ng/mL LPS (Ultrapure LPS from E. coli 055:B5, Invivogen, France) for 2 additional days, in their respective media: R10ASW,R10CTW or R10DEX. In another set of experiments, iDC were matured with 2µg/mL of peptidoglycan (PGN) and 10µg/mL of lipoteichoic acid (LTA, both from Invivogen, France).
Phenotype analysis of MoDC by FACS
Anti-human leukocyte antigen class II-APC-vio770 (MHC-II) (clone REA805), anti-CD1a PE-Vio770® (clone REA736), anti-CD14 Vioblue® (clone REA599), anti-CD86 APC (clone: FM95), anti-CD83 Viobright® (clone REA714) and anti-CD11c PECy5 (clone: B-ly6) mAbs (All from Miltenyi Biotec except the CD11c from Becton Dickinson) were used for direct immunofluorescence staining to characterize the phenotype of DC generated in the indicated conditions. Briefly, collected cells were washed in phosphate-buffered saline (PBS) containing 10% human serum and were stained with indicated fluorescent labeled antibodies, plus a viability dye (Miltenyi Biotec) for 30 min at 4°C. Cells were stained with isotype-matched control antibodies to evaluate non-specific binding in parallel experiments. The cells were then washed and acquired using a MACSQuant® Analyzer 10 instrument (Miltenyi Biotec). Analysis was performed on gated population that excluded doublets and dead cells. Data were analyzed using FlowJo software version X 10.0.7.r2 (Tree Star).
Cytokine quantitation
1x105 iDCs were matured with LPS (100 ng/mL) for 48 hrs in their respective media: R10ASW, R10CTRL or R10DEX. Supernatants were collected and stored at −80°C. IL-10, IL-6, IL-12p70, IL-23 and TNF amounts were measured by a bead-based multiplex assay (LEGENDplex™, Biolegend) following the Manufacturer’s recommendations. Flow cytometric analysis were performed with a MACSQuant® Analyzer 10 instrument (Miltenyi Biotec). Data were analyzed using FlowJo software version X 10.0.7.r2 (Tree Star).
Allogeneic naive CD4+ T cell stimulation by MoDC
Mixed lymphocyte reactions (MLRs) were performed in 96 wells round-bottom microtiter plates (Falcon 353 077) by adding 105 allogeneic naive CD4+ T cells to varying numbers of DCs in 200µL complete RPMI (RPMI 1640 Medium, supplemented with 10% FCS, 1% penicillin (10,000 U/mL) and streptomycin (10 mg/mL), Sodium pyruvate (1 mmol/L), HEPES (N-2-hydroxyethylpiperazine-N-2-ethane sulfonic acid, 10 mmol/L) all from GIBCO and GlutaMAX™ (Invitrogen). In some experiments, T cells were previously stained with CTV (Thermofisher Scientific), according to the manufacturer’s recommendations, in order to measure the proliferation by flow cytometry. Cultures were maintained for 5 days at 37°C in a 5% CO2 humidified atmosphere. The proliferative response was measured after 5 days of culture by CTV dilution analysis by flow cytometry. Briefly, cells were harvested, washed with PBS-EDTA and stained with anti-CD4-PE (clone RPA-T4) and viability dye eFluor™ 780 (eBioscience) for 30 min at 4°C. Cells were washed with PBS-EDTA and analyzed by flow cytometry using a MACSQuant® Analyzer 10 instrument (Miltenyi Biotec). In parallel experiments performed with unstained T cells at DC:T cell ratio 1:10, secreted cytokines (IL-4, IL-17, IL-22, TNF and IFN-γ) were quantified using a bead-based multiplex immunoassay (MACSPlex, Miltenyi Biotec) following the manufacturer’s instructions. In parallel experiments performed with unstained T cells at DC:T cell ratio 1:10, cells were stimulated for 4 h with phorbol myristate acetate (PMA) (50 ng/ml; Sigma) and ionomycin (500 ng/ml; Sigma) in presence of GolgiPlug (BD Biosciences). Cells were next stained with anti-CD4-vioBlue antibody and viobility 405/520 fixable viability dye (Miltenyi Biotec) for 30 min at 4°C. Cells were next fixed with 4% PFA, permeabilized with 0,1% Saponine (Sigma Aldrich) and then were incubated with anti-IFN-γ-APC, anti-IL-17-APCvio770, anti-IL-22-FITC and anti-IL-4-PEvio770 antibodies (Miltenyi Biotec) for 45 min at 4°C. Cells were washed, and cytokine profiles were analyzed using a MACSQuant® Analyzer 10 instrument (Miltenyi Biotec); the data were analyzed using FlowJo software version X 10.0.7.r2 (Tree Star). The percentage of cytokine+ cells was analyzed among viable CD4+ cells.
Statistical analysis
GraphPad Prism software (version 6; GraphPad Software, La Jolla, CA) was used. Means were compared between groups using One-way ANOVA (Dunnett’s Multiple Comparison Test) or Friedman Test with Dunn’s post hoc test.
Results
ASW impairs MoDC differentiation and maturation
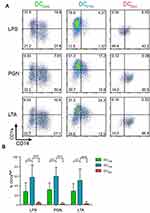
To analyze the impact of ASW on DC functions, we generated monocyte-derived dendritic cells in ASW-based RPMI basal culture medium (Figure 1A). We first analyzed the effects of ASW on DC differentiation and maturation. CD14+ cells were differentiated into DCs with IL-4 and GM-CSF for five days in ASW-based or control water (CTW)-based medium or CTW-based medium plus dexamethasone (inhibits DC differentiation and maturation). Loosely adherent cells were next harvested and DC differentiation was assessed by analyzing the expression of CD11c by flow cytometry. More than 95% of the cells expressed CD11c in the three culture conditions tested (Figure 1A). We next evaluated whether ASW impacted the yield of iDC generated and we observed that the number of iDCs collected was similar in the ASW and CTW conditions while dexamethasone significantly decreased the number of iDCs obtained (Figure 1B). ASW is poorly mineralized and does not contains minerals expected to have an impact on cell viability (Table S1). Likewise, ASW-based medium did not present any cytotoxicity for DCs as measured with the viability dye by flow cytometry (Figure 1B). iDC were next treated with LPS for 48h and tested for the expression of CD83, CD86, CD1a, HLA-DR and CD14. During the differentiation into DCs, monocytes are expected to lose CD14 and gain CD1a expression. CD1a expression was significantly lower in DC matured with ASW (DCASW) as compared to DC matured with CTW (DCCTW) (Figure 1B). As a control, DC matured with CTW supplemented with dexamethasone (DCDEX) did not express CD1a and remained CD14+. The joint analysis of CD86 and CD83 expression revealed that the proportion of fully mature DCs (CD86highCD83high) was significantly reduced among DCASW as compared to DCCTW (Figure 1C). Accordingly, the expression of CD86 (gMFI fold increase over isotype control) was lower in DCASW compared to DCCTW (Figure 1D). The three sets of DCs expressed HLA-DR on their surface and we observed a slight reduction of HLA-DR expression in DCASW compared to DCCTW (Figure 1E). Because several Gram-positive bacteria are known to invade the skin, we also analyzed whether ASW impairs DC maturation induced by Gram-positive PAMPs such as peptidoglycan (PGN) and lipoteichoic acid (LTA). Following maturation with LTA or PGN, DCASW exhibited a reduced expression of CD1a compared to DCCTW (Figure 2).
Taken together, these results show that ASW reduces the expression of differentiation and maturation markers on the DC surface upon priming with LPS, PGN or LTA.
DCs generated in ASW-based medium harbor a diminished ability to prime naive CD4+ T cells
Upon maturation with LPS, DCs are expected to acquire the ability to efficiently prime naive allogeneic-CD4+ T cells. We therefore examined the capacity of DCASW to prime and activate T cells. We co-cultured DCASW or control DCs (DCCTW and DCDEX) with sort-purified and CTV-labelled naive CD4+ T cells from allogeneic healthy donors at different ratios. After 5 days of co-culture, CD4+ T cell proliferation was measured by flow cytometry analysis of CTV dilution. We observed that DCASW induced a smaller fraction of naive CD4+ T cells to proliferate compared to DCCTW, regardless of the T cell:DC ratio. As expected, control DCDEX barely induced CD4+ T cell proliferation (Figure 3).
ASW decreases the amount of IL-12 and IL-23 and augments the amount of IL-10 produced by DCs upon LPS-induced maturation
Upon maturation, DCs produce a panel of cytokines that participate to inflammation and orient T helper cell responses.12 We first analyzed the impact of ASW on cytokine production by DCs upon maturation induced by LPS. DCs cultured either with ASW-Based or CTW-based culture media were tested for IL-12p70, IL-23, IL-6, IL-10 and TNF production by the collection of cell-free culture supernatant 48 hrs after LPS stimulation. As shown in Figure 4, DCASW produced smaller amounts of IL-12p70 and IL-23 but higher amount of IL-10 as compared to DCCTW. By contrast, TNF production was not affected and IL-6 production was barely impacted by ASW.
CD4+ T cells differentiated with DCASW show a reduced production of IFN-γ and IL-17
We next investigated the abilities of DCASW, DCCTW and DCDEX to drive CD4+ T cells differentiation by measuring the IL-4, IL-17, TNF, IL-22 and IFN-γ production by CD4+ T cells in the co-culture supernatants. IL-4, IL-17 and IL-22 were not detected for almost all the donors tested (data not shown). The amount of IFN-γ produced was lower with DCASW as compared to DCCTW (Figure 5A). By contrast, TNF production was not statistically different between cocultures with DCASW and DCCTW, only cocultures with DCDEX exhibited a decreased TNF production (Figure 5B).
We also used flow cytometry to analyze intracellular cytokine expression in CD4+ T cells restimulated by PMA and ionomycine after 5 days of MLR. This readout allowed us to detect some CD4+ T cells producing IL-17 (Figure 5C). ASW reduced slightly, but significantly the ability of DCASW to induce IL-17 production by CD4+ T cells. CD4+ T cells producing IL-4 or IL-22 were observed only in a few donors, making statistical analysis impossible (data not shown). This analysis clearly showed that the percentage of CD4+ T cells producing IFN-γ decreased when the T cells response was generated with DCASW compared to DCCTW (Figure 5D).
Discussion
In this study, we analyzed the effects of ASW on DC differentiation, maturation and functions. We show that ASW dampens DC differentiation and maturation and decreases their ability to elicit inflammatory T helper cell responses in MLR.
ASW is poorly mineralized (266 mg/L) and characterized by a significant amount of silicates, a low sodium concentration, a calcium/magnesium ratio of 1:2, and a high diversity of trace elements (Table S1).28 Moreover, ASW contains organic compounds derived from microorganisms such as Aquaphilus dolomiae.28–30 Prolonged exposure to spring water during spa is expected to allow these compounds to permeate into the skin. We sought to analyze the impact of ASW on the immune response. Because DCs are crucial for the induction and the control of immune responses, we investigated the effect of thermal spring water on DC differentiation, maturation and functions.
To assess the impact of ASW on DCs, we generated monocyte-derived DCs, the gold standard in vitro model for DCs. Monocytes cultured for 5 days in presence of GM-CSF and IL-431 resemble immature myeloid dendritic cells.32 These iDC matured with LPS or inflammatory cytokines are close to in vivo inflammatory DCs.15,33–35 Since monocytes that infiltrate inflamed tissues are expected to differentiate and maturate in a continuous process, we analyzed the effects of ASW on both steps of differentiation and LPS-induced maturation (Figure 1A), by including this water in the composition of the culture medium. Ultra-pure commercial distilled water, with or without dexamethasone, were used as experimental controls.
When monocytes are differentiated into iDCs, they are expected to upregulate MHC class II molecules and CD1a at the same time, while losing CD14 molecules expression.32 Upon maturation with LPS, iDCs upregulate costimulation molecules such as CD80 and CD86.32 We observed that ASW induced significant changes in the phenotype of LPS-matured DCs. CD1a expression was highly variable among individuals (Figure 1B) as previously reported by Cernadas et al.36 Nevertheless, by testing DCs generated from a large number of donors (n=27), we clearly showed that CD1a expression was strongly reduced by ASW. The main role of CD1a is to present lipid or glycolipid antigens to T cells, but it also appears as a marker of monocyte to DC differentiation because it is principally induced during the differentiation step.37 Different studies have compared the CD1a+ and CD1a− populations of MoDC and showed that CD1a+ are more prone to produce IL-12 and polarize CD4+ T cells toward a Th1 phenotype.36,37 Moreover CD1a+ MoDC exhibited a more differentiated phenotype than their CD1a− counterparts.38 In agreement with these reports, our results show that a reduced expression of CD1a in DCASW is associated with a lower production of IL-12 and a reduced ability to generate Th1 cells. In light of these results, ASW dampens DC differentiation and limits their capacity to induce inflammatory T cell responses. In addition to LPS, we also tested PAMPs from Gram positive bacteria, to mature DCs in parallel experiments. We observed that ASW decreased the CD1a expression in DCs matured in presence of LTA or PGN. These results indicate that ASW seems to decrease DC maturation regardless of the priming stimulus.
Besides CD1a, ASW reduced the expression of the DC maturation markers CD86, CD83 and HLA-DR upon LPS stimulation. CD83 is mainly known as a marker of mature DC39 while CD86 and HLA-DR are key molecules for CD4+ T cell activation. Again, we observed a large variability of responses among the donors and a significant reduction of the maturation of DCASW compared to the negative control DCCTW. Nevertheless, ASW inhibitory effect on DC maturation was not as effective as the dexamethasone-treatment excepted for HLA-DR expression. We observed that dexamethasone did not impact HLA-DR expression in agreement with previous reports.40,41 In order to determine whether ASW was able to impair the main function of DCs (i.e to induce naive T cell activation) we tested the stimulatory capacity of DCASW in MLR. We observed that ASW reduced the ability of the DCs to induce allogeneic naive CD4+ T cell proliferation. Naive CD4+ T cells are indeed tightly dependent on the costimulatory signals to be activated and we can speculate that the semi immature phenotype of DCs induced by ASW is responsible for their reduced ability to drive naive CD4+ T cell proliferation.
During cognate interaction with CD4+ T cells, DCs produce cytokines such as IL-12, IL-23, IL-10 or IL-6 that drive T helper cell differentiation.12 Hence, we evaluated the effects of ASW on cytokine production by DCs after the maturation step. We observed that DCs generated from some donors were not able to produce IL-12 nor IL-23 in response to LPS. Concerning DCs that are able to produce IL-12 or IL-23 in response to LPS, we observed a strong reduction of IL-12 production and a slight decrease of IL-23 production in DCASW. Because IL-12 and IL-23 drive Th1 and Th17 cell differentiation respectively, we analyzed the production of IFN-γ and IL-17 by CD4+ T cell generated in the MLRs. We observed a significant reduction of IFN-γ production and IFN-γ+ CD4+ T cells, indicating that DCASW have a reduced ability to drive Th1 responses. We were not able to detect IL-17 production by bead-based multiplexed assay in all the conditions tested, but we observed a decrease in the % IL-17+ CD4+ T cells generated with DCASW as compared to DCCTW. This low percentage of CD4+ T cells producing IL-17, is consistent with reports in the literature42 and can be explained by the fact that we only used LPS as a stimulus for DC maturation. LPS is known to promote IL-12 production by DCs and thereby to drive mainly Th1 response, whereas LPS alone is a poor inductor of IL-2343 and therefore of Th17 response. It is noteworthy that TNF production in the supernatant of the MLR was similar with DCASW and DCCTW. TNF can be produced by DCs and by a large part of activated CD4+ T cells, without being limited to a defined Th subset.
Moreover, we showed that ASW did not impact TNF production and slightly reduced IL-6 production by DCs upon LPS-induced maturation. Interestingly, DCASW exhibited an increased production of IL-10 as compared to DCCTW. As expected, the IL-10 production was the highest with dexamethasone treatment.40 IL-10 is an inhibitory cytokine, that can be produced by virtually all the leukocytes. The main role of IL-10 is to limit the extent of the immune response, in order to protect the host against excessive immune reaction associated with infection, autoimmunity, and allergy.
Conclusion
Collectively, these results indicate that ASW fine tunes DC maturation by decreasing their ability to produce some cytokines such as IL-12, IL-23 and to a lesser extent IL-6, by fostering IL-10 production without impacting TNF production. This ASW-induced DC phenotype and functions, indicate that ASW favors the generation of immunomodulatory DCs, which might explain the beneficial effects of ASW-based hydrotherapy in the treatment of inflammatory skin diseases. It is tempting to speculate that ASW properties might rely on microbial molecules from endemic bacteria found in this thermal water, such as Aquaphilus dolomiae that was shown to promote IL-10 production and reduce CD4+ T cell activation by DCs.30
Ethics approval and informed consent
Buffy coats from healthy donors were obtained through the Etablissement Français du Sang (EFS Midi-Pyrénées, Purpan University Hospital, Toulouse, France) and processed following standard ethical procedures (Helsinki protocol), after obtaining written informed consent from each donor and approval by the local ethics committee (Comité de Protection des Personnes Sud-Ouest et Outremer II).
Abbreviation list
ASW, Avène thermal spring water; CTV, Celltrace violet; DC, dendritic cell; DEX, dexamethasone; iDC, immature dendritic cell; MLR, mixed lymphocyte reaction; MoDC, monocyte-derived dendritic cell; PAMP, pathogen-associated molecular pattern, PBMC, peripheral blood mononuclear cell.
Acknowledgments
This research was supported by INSERM (Institut national de la santé et de la recherche médicale) and by Pierre Fabre Dermo-Cosmétique (PFDC). The funding body had no role in the design and conduct of the study, data collection, data management, data analysis and interpretation, writing the manuscript, or decision to submit results.
Author contributions
All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
D.R and M-F.G are employees of Pierre Fabre Dermo-Cosmétique. Y.E. reports grants from Laboratoires Pierre Fabre, during the conduct of the study. E.E. reports grants from Laboratoires Pierre Fabre, during the conduct of the study. He also received grants and personal fees from Laboratoires Pierre Fabre, outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. Matz H, Orion E, Wolf R. Balneotherapy in dermatology. Dermatol Ther. 2003;16(2):132–140.
2. Valitutti S, Castellino F, Musiani P. Effect of sulfurous (thermal) water on T lymphocyte proliferative response. Ann Allergy. 1990;65(6):463–468.
3. Celerier P, Richard A, Litoux P, Dreno B. Modulatory effects of selenium and strontium salts on keratinocyte-derived inflammatory cytokines. Arch Dermatol Res. 1995;287(7):680–682.
4. Bajgai J, Fadriquela A, Ara J, et al. Balneotherapeutic effects of high mineral spring water on the atopic dermatitis-like inflammation in hairless mice via immunomodulation and redox balance. BMC Complement Altern Med. 2017;17(1):481.
5. Hahn HJ, Kim JS, Kim YH, Lee YB, Yu DS, Kim J-W. Investigation of immune-regulatory effects of mageumsan hot spring via protein microarray in vitro. Ann Dermatol. 2018;30(3):322–330.
6. Lee H-P, Choi Y-J, Cho K-A, et al. Effect of spa spring water on cytokine expression in human keratinocyte hacat cells and on differentiation of CD4+ T cells. Ann Dermatol. 2012;24(3):324–336.
7. Zoller N, Valesky E, Hofmann M, et al. Impact of different spa waters on inflammation parameters in human keratinocyte HaCaT cells. Ann Dermatol. 2015;27(6):709–714.
8. Portales P, Aries MF, Licu D, et al. Immunomodulation induced by avene spring water on Th1- and Th2-dependent cytokine production in healthy subjects and atopic dermatitis patients. Skin Pharmacol Appl Skin Physiol. 2001;14(4):234–242.
9. Joly F, Charveron M, Aries MF, et al. Effect of Avene spring water on the activation of rat mast cell by substance P or antigen. Skin Pharmacol Appl Skin Physiol. 1998;11(2):111–116.
10. Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392(6673):245–252. doi:10.1038/32588
11. Collin M, McGovern N, Haniffa M. Human dendritic cell subsets. Immunology. 2013;140(1):22–30. doi:10.1111/imm.12117
12. Kapsenberg ML. Dendritic-cell control of pathogen-driven T-cell polarization. Nat Rev Immunol. 2003;3(12):984–993. doi:10.1038/nri1246
13. Banchereau J, Briere F, Caux C, et al. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. doi:10.1146/annurev.immunol.18.1.767
14. Walsh KP, Mills KHG. Dendritic cells and other innate determinants of T helper cell polarisation. Trends Immunol. 2013;34(11):521–530. doi:10.1016/j.it.2013.07.006
15. Kashem SW, Haniffa M, Kaplan DH. Antigen-Presenting Cells in the Skin. Annu Rev Immunol. 2017;35(1):469–499. doi:10.1146/annurev-immunol-051116-052215
16. Clausen BE, Stoitzner P. Functional specialization of skin dendritic cell subsets in regulating T cell responses. Front Immunol. 2015;6:534. doi:10.3389/fimmu.2015.00534
17. Chu -C-C, Di Meglio P, Nestle FO. Harnessing dendritic cells in inflammatory skin diseases. Semin Immunol. 2011;23(1):28–41. doi:10.1016/j.smim.2011.01.006
18. Zaba LC, Krueger JG, Lowes MA. Resident and “inflammatory” dendritic cells in human skin. J Invest Dermatol. 2009;129(2):302–308. doi:10.1038/jid.2008.225
19. Coquerelle C, Moser M. DC subsets in positive and negative regulation of immunity. Immunol Rev. 2010;234(1):317–334. doi:10.1111/j.0105-2896.2009.00887.x
20. Wakim LM, Waithman J, van Rooijen N, Heath WR, Carbone FR. Dendritic cell-induced memory T cell activation in nonlymphoid tissues. Science. 2008;319(5860):198–202. doi:10.1126/science.1151869
21. Lowes MA, Chamian F, Abello MV, et al. Increase in TNF-α and inducible nitric oxide synthase-expressing dendritic cells in psoriasis and reduction with efalizumab (anti-CD11a). Proc Natl Acad Sci U S A. 2005;102(52):19057–19062. doi:10.1073/pnas.0509736102
22. Hansel A, Gunther C, Ingwersen J, et al. Human slan (6-sulfo LacNAc) dendritic cells are inflammatory dermal dendritic cells in psoriasis and drive strong T(H)17/T(H)1 T-cell responses. J Allergy Clin Immunol. 2011;127(3):787–U456. doi:10.1016/j.jaci.2010.12.009
23. Serbina NV, Salazar-Mather TP, Biron CA, Kuziel WA, Pamer EG. TN/iNOS-producing dendritic cells mediate innate immune defense against bacterial infection. Immunity. 2003;19(1):59–70.
24. Wollenberg A, Kraft S, Hanau D, Bieber T. Immunomorphological and ultrastructural characterization of Langerhans cells and a novel, inflammatory dendritic epidermal cell (IDEC) population in lesional skin of atopic eczema. J Invest Dermatol. 1996;106(3):446–453.
25. Raker VK, Domogalla MP, Steinbrink K. Tolerogenic dendritic cells for regulatory T cell induction in man. Front Immunol. 2015;6:569. doi:10.3389/fimmu.2015.00569
26. Naranjo-Gómez M, Raïch-Regué D, Oñate C, et al. Comparative study of clinical grade human tolerogenic dendritic cells. J Transl Med. 2011;9(1):89. doi:10.1186/1479-5876-9-89
27. Navarro-Barriuso J, Mansilla MJ, Martínez-Cáceres EM. Searching for the transcriptomic signature of immune tolerance induction—biomarkers of safety and functionality for tolerogenic dendritic cells and regulatory macrophages. Front Immunol. 2018;9:2062.
28. Guerrero D, Garrigue E. Annales de dermatologie et de venereologie. Eau thermale d’Avène et dermatite atopique: Avène’s thermal water and atopic dermatitis. 2017;144(Suppl 1):S27–s34. doi:10.1016/S0151-9638(17)31040-2
29. Aries MF, Hernandez-Pigeon H, Vaissiere C, et al. Anti-inflammatory and immunomodulatory effects of Aquaphilus dolomiae extract on in vitro models. Clin Cosmet Investig Dermatol. 2016;9:421–434. doi:10.2147/CCID.S113180
30. Martin H, Laborel-Preneron E, Fraysse F, et al. Aquaphilus dolomiae extract counteracts the effects of cutaneous S. aureus secretome isolated from atopic children on CD4(+) T cell activation. Pharm Biol. 2016;54(11):2782–2785. doi:10.3109/13880209.2016.1173069
31. Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J Exp Med. 1994;179(4):1109–1118. doi:10.1084/jem.179.4.1109
32. Palucka KA, Taquet N, Sanchez-Chapuis F, Gluckman JC. Dendritic cells as the terminal stage of monocyte differentiation. J Immunol. 1998;160(9):4587–4595.
33. Grassi F, Dezutter-Dambuyant C, McIlroy D, et al. Monocyte-derived dendritic cells have a phenotype comparable to that of dermal dendritic cells and display ultrastructural granules distinct from Birbeck granules. J Leukoc Biol. 1998;64(4):484–493. doi:10.1002/jlb.64.4.484
34. Cheong C, Matos I, Choi J-H, et al. Microbial stimulation fully differentiates monocytes to DC-SIGN/CD209(+) dendritic cells for immune T cell areas. Cell. 2010;143(3):416–429. doi:10.1016/j.cell.2010.09.039
35. Sander J, Schmidt SV, Cirovic B, et al. Cellular differentiation of human monocytes is regulated by time-dependent interleukin-4 signaling and the transcriptional regulator NCOR2. Immunity. 2017;47(6):1051–1066.e1012.
36. Cernadas M, Lu J, Watts G, Brenner MB. CD1a expression defines an interleukin-12 producing population of human dendritic cells. Clin Exp Immunol. 2009;155(3):523–533.
37. Faivre V, Lukaszewicz AC, Alves A, Charron D, Payen D, Haziot A. Human monocytes differentiate into dendritic cells subsets that induce anergic and regulatory T cells in sepsis. PLoS One. 2012;7(10):e47209.
38. Gogolak P, Rethi B, Szatmari I, et al. Differentiation of CD1a− and CD1a+ monocyte-derived dendritic cells is biased by lipid environment and PPARγ. Blood. 2007;109(2):643–652.
39. Zhou LJ, Tedder TF. Human blood dendritic cells selectively express CD83, a member of the immunoglobulin superfamily. J Immunol. 1995;154(8):3821–3835.
40. Xia C-Q, Peng R, Beato F, Clare-Salzler MJ. Dexamethasone induces IL-10-producing monocyte-derived dendritic cells with durable immaturity. Scand J Immunol. 2005;62(1):45–54.
41. Boks MA, Kager-Groenland JR, Haasjes MSP, Zwaginga JJ, van Ham SM, Ten Brinke A. IL-10-generated tolerogenic dendritic cells are optimal for functional regulatory T cell induction — a comparative study of human clinical-applicable DC. Clin Immunol. 2012;142(3):332–342.
42. Bellanger AP, Pallandre JR, Borg C, Loeffert S, Gbaguidi-Haore H, Millon L. Human monocyte-derived dendritic cells exposed to microorganisms involved in hypersensitivity pneumonitis induce a Th1-polarized immune response. Clin Vaccine Immunol. 2013;20(8):1133–1142.
43. Napolitani G, Rinaldi A, Bertoni F, Sallusto F, Lanzavecchia A. Selected Toll-like receptor agonist combinations synergistically trigger a T helper type 1–polarizing program in dendritic cells. Nat Immunol. 2005;6:769.
Supplementary materials
 |
Table S1 Mineral composition of Avène spring water (cf ref.28) |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.