Back to Journals » International Journal of Nanomedicine » Volume 14
Effect of superparamagnetic nanoparticles coated with various electric charges on α-synuclein and β-amyloid proteins fibrillation process
Authors Javdani N , Rahpeyma SS , Ghasemi Y, Raheb J
Received 10 October 2018
Accepted for publication 13 December 2018
Published 23 January 2019 Volume 2019:14 Pages 799—808
DOI https://doi.org/10.2147/IJN.S190354
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Anderson Oliveira Lobo
Negin Javdani,1 Sayyed Shahryar Rahpeyma,1 Younes Ghasemi,2 Jamshid Raheb1
1National Institute of Genetic Engineering and Biotechnology, Tehran, Iran; 2Pharmaceutical Sciences Research Center, Shiraz University of Medical Sciences, Shiraz, Iran
Background: Most of nanoparticles are nontoxic and have high absorption capability. Therefore, nanoparticles binding can effectively restrain fibrillation of β-amyloid and α-synuclein proteins and eventually prevent the toxicity of pathogenesis peptide of Alzheimer. Super paramagnetic iron oxide nanoparticles (SPIONs) contain iron oxide core which can be connected to a special part through magnetic coating.
Materials and methods: In this study, the effect of SPIONs with different charges was simultaneously examined on the fibrillation of both β-amyloid and α-synuclein proteins by applying Thioflavin-T assay.
Results: According to the results of the investigation on amyloid-fibrillation mechanism in both β-amyloids and α-synucleins, it was revealed that negatively-charged nanoparticles encoded to -COOH by dextran-coating were able to have a considerable absorption decrease from 17,000–12,000 after 320 minutes delay to lag phase and decrease in binding level of thioflavin-T particles to β-sheets.
Conclusion: The different concentrations of these nanoparticles and special coating of each particle had an effect on the kinetics of β-amyloid and α-synuclein fibrillations.
Keywords: SPION, α-synuclein, β-amyloid, fibrillation, Alzheimer’s disease, Parkinson’s disease
Corrigendum for this paper has been published.
Introduction
In neurodegenerative diseases, abnormal proteins are accumulated inside and outside of nerve cells, interfere with neural-network associations, and finally destroy particular neurons. Alzheimer’s disease (AD) is known by the accumulation of two proteins, namely, Aβ-protein and tau protein which are aggregated outside and inside of the neurons. Larger aggregation of Aβ which are called amyloid plaques (A-plaques), are very toxic and interfere with neuron function and association. Abnormally high levels of tau protein exist in the neurons in AD which are known as neurofibrillary tangles.1
Aβ is a peptide with a weight of 4 kD and in normal condition, only some of these segments exist in the cells and they are rapidly dialyzed; however, if the proteome of neuronal cells of this condition is disrupted and their levels increase, then spherical protein structures are formed and therefore AD is developed.2
The formation of intracellular pathogenesis inclusions in which α-synuclein protein is the main member, is the reason for the inclusion of such diseases in this group. These inclusions are recognized as Lewy bodies and Lewy neurites in case they are localized in cell body of the neurons and neural cell walls of axons. The latter case can be observed in multiple system atrophy.3
α-Synuclein is the most frequent brain-protein containing 140 amino acids and the gene of which contains seven exons. This protein lacks cysteine and tryptophan amino acids and is found in presynaptic terminals with high concentration in the form of a solution and membrane-bound. It is estimated that α-synuclein forms 1% of the total proteins in soluble cytosolic region of the brain. This protein was first described by Maroteaux et al,27 as the special nerve cell protein which is localized at presynaptic terminals and the nucleus and was named “synuclein”. It was found that there is a relationship between a mutation in α-synuclein gene and Parkinson’s disease (PD) and that the aggregations of this protein form the main composition of Lewy bodies which is the hallmark of PD.4
Some researchers believe that the pharmaceutical factories and companies and their research sections, using these studies and hypotheses, seriously begin to seek out medicines to prohibit the Aβs binding to their receptors in individuals who are susceptible of affliction with AD. They also expect that the results obtained from these studies pave the way for the onset of a revolution in AD treatment.4
Some factors are important in fibrillation such as structural factors including hydrophilic and hydrophobic model, the localization of charged region propensity for β-sheet generation, the presence of aromatic amino acids, membranous structure and environmental factors including pH, temperature, high protein concentration, oxidative stress, and mutation in peptide chain.5
The normal synthetic form of amyloid formation includes three phases. The first phase is related to nucleation which is sensitive and an inhibition phase in the amyloid process and is thermodynamically improper. The next phase is elongation and an increase in fibril size.6 This phase is the fastest one in which fibril structures are rapidly formed and the level of fluorescence or absorption of indicators like thioflavin T (ThT) and Congo red are severely changed. The final phase is the balance phase through which the level of fluorescence or absorption of amyloid-special indicators does not usually have a severe change and the system apparently has a steady state. However, researchers have demonstrated that fibrils are also active in this state and monomers are exchanged in them. In addition, lateral interactions are formed among fibrils and thus mature and thick fibrils are generated.7
The application of nanoparticles is described in the drug, protein and peptide transfer and cancer treatment. They transfer small molecules to the desired location. Usually, iron, gold and silver nanoparticles are used to study protein reactions. One of the best features of nanoparticles is that they can easily pass through the brain and blood–brain barrier. Most of them are nontoxic and also have a high free Gibbs energy in their surface which brings them a great absorption capability. In this case, the nanoparticles may influence the β-amyloid fibrillation process.8
Paramagnetic iron oxide nanoparticles have an iron oxide core, which can be attached to a specific part through the magnetic coating. They have features such as paramagnetic superpower, high saturation field, nontoxicity, biodegradability, and variable loops.9,10
Studies conducted on the effect of magnetically charged nanoparticles showed that the application of these nanoparticles as a carrier can be appropriate in treatment of many diseases. In addition, these nanoparticles, in fibrillation process, can have a crucial role in decreasing or delaying the plaque formation mechanism in A-proteins.11,12
The purpose of this study was to investigate the effect of paramagnetic iron oxide nanoparticles with different charges on the α-synuclein and β-amyloid protein fibrillation process. The main focus of this study is on the effect of the physicochemical properties of the nanoparticles used, along with their charges, concentrations and the coating, on the fibrillation of β-amyloid and α-synuclein.
Material and methods
Super paramagnetic iron oxide nanoparticles
For this study, nanoparticles were purchased from Micromod Particle Technology GmbH., Rostock, Germany. These particles contained bonding between dextran coatings with three different molecules including carboxyl, amino, and neutral groups that had negative, positive, and plain charges, respectively. In addition, such nanoparticles contained different concentrations which were applied for the investigation of their inhibition effect in Aβ and α-synuclein fibrillation. Each of the nanoparticles had concentrations of 25, 50, and 100 mg/mL. The charges of all nanoparticles were measured using zeta potential (ZP) set and the diameter of all nanoparticles was 20 nm.
Properties of trivalent and charged iron oxide nanoparticles (NanoFe3O4)
- Purity of magnetite iron nano-oxide: 98%
- Average size of iron oxide nanoparticles: 20 nm
- Surface area of iron oxide nanomaterials: 40–60 m5mg
- Trivalent nano-iron dye: black
- Shape of iron nano-oxide particles: spherical
- Density of iron oxide nanoparticles: 0.84 g/cm3
- Real density of magnetite nano-iron oxide: 1–4.8 g/cm.
Considering that SPIONs were commercially purchased for this study, their diameters as well as chemical properties are presented according to the data provided by the production company.
Magnetic charges diagrams (ZP) of iron nanoparticles are shown in Figure 1.
  | Figure 1 Magnetic charge of iron nanoparticles. |
Aβ-protein or Aβ-peptide (Aβ 1-42)
The Aβ and α-synuclein proteins as well as ThT fluorescence assay were purchased from Sigma (Sigma-Aldrich Co., St Louis, MO, USA). The ThT is a type of dye which binds to α-synuclein through fluorescence at wavelengths in the range of 380–460 nm.
Cell survival with MTT assay
MTT assay was used to evaluate the cellular effect of nanoparticles and to assess the cell survival ability. In the MTT test, the SY5Y neurons used were purchased from the Pasteur Institute of Iran (Tehran, Iran). Tetrazolium salt was used for measurement. To prepare a solution of MTT, 5 mg of this compound was dissolved in 1 mL of PBS. The cells were cultured in 96 wells microtiter plate with 10,000 cells per well and incubated at 37°C for 24 hours. Then different charged nanoparticles with 2.5, 5 and 10 mg/mL were added to the culture medium, and the control well with a culture medium containing no effect of nanoparticles was considered. After incubation for 24, 48 and 72 hours in 5% CO2, 100 μL MTT was added and incubated for 2 hours and then the culture medium was discarded. The reaction was stopped by adding ZAAZDMSO. As this solution dissolves the purple colored formazan sediment in live cells, the absorption rate was measured by the ELISA device at 570 nm. In this study, to analyze the data, SPSS software (IBM Corporation, Armonk, NY, USA) was applied and the results were analyzed by ANOVA test and the significance level was measured on P<0.05.
Investigating the structure modification level of Aβ-protein at the presence of SPIONs
To investigate the effect of various concentrations of iron oxide nanoparticles on the secondary structure of Aβ and α-synuclein proteins, nanoparticles’ different concentrations of 2.5, 5, and 10 μg/L were first incubated with Aβ-protein at 37°C for 1 hour and then circular dichroism (CD) set was used to analyze the second structure of this protein using an Aviv Model 202 CD spectrometer (Aviv biomedical, Inc., Lakewood, NJ, USA). CD spectra were recorded at room temperature; the wavelength was 1 nm, three scans were taken per sample in the range from 190 to 300 nm, and the average time was 1 second at each wavelength. It is notable that the SPIONs, themselves, induce scattering in CD which must be considered in the interpretation of the CD data.
ThT fluorescence assay
A common method for investigation of protein fibrillation process is the fluorescence technique through ThT. For this test, 0.6 mg of ThT was dissolved in 2 mL of deionized water so as to reach the final concentration of 1 mM. This solution was used as the stock.
To perform ThT fluorescence assay, 10 μL of ThT stock solution in addition to 20 μL of Aβ-protein using PBS with concentrations of 2.5, 5, and 10 μL of various charged nanoparticles were blended together (all the steps carried out in an ice cold medium) and were measured through fluorimeter set along with three negative and three positive controls at each run. The wavelength of the set was in the range of 380–460 nm.
It is worth mentioning that ThT fluorescence assay was repeated 3 times for each nanoparticle with a certain concentration and charge. Therefore, the effect of each nanoparticle on Aβ fibril formation was investigated and recorded by fluorimeter set, nine times and every 10 minutes for 600 minutes at 37°C. The curve related to fibril formation was depicted according to positive and negative controls and certain points and times in which the inhibition effect occurred were identified.
Results
Magnetic power investigation and physicochemical properties of nanoparticle
Considering that SPIONs were commercially purchased for this study, diameters as well as chemical properties of these nanoparticles were presented according to the data provided by the production company. Physicochemical data for each of the three nanoparticles are attached to this article.
The dextran coated nanoparticle samples commercially provided contained plain, positive, and negative magnetic charges and the diameter of all was 20 nm. Positive as well as negative nanoparticles were provided by NH2 and COOH ions, respectively and neutral sample was plain.
Magnetic charges calculated using ZP set include −4.15, 22.8, and −13.4 C for plain, positive, and negative charges, respectively.
Investigation of the structure modification level of Aβ-protein at the presence of SPIONs
The results obtained from the investigation of the secondary structure of Aβ indicated that an increase in nanoparticle concentration led to a decrease in level of α-helix structure showing that the interaction of Aβ-protein with iron oxide nanoparticles resulted in a severe change in the secondary structure of the proteins. Furthermore, the results also showed that increasing iron oxide concentration would slightly decrease the level of α-helix structure but this structure change was not very noticeable. Therefore, it can be concluded that the secondary structure of Aβ-proteins did not have a considerable change through interaction with iron oxide (Figure 2).
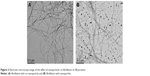  | Figure 2 Electronic microscope image of the effect of nanoparticles on fibrillation of Aβ-proteins. |
Investigation of Aβ-protein fibrillation process using the modifications in ThT fluorescence emission
To perform this test, each of these different charged nanoparticles with concentrations of 2.5, 5, and 10 μL were added to Aβ-protein and together with ThT (Table 1). The fluorescence emission was separately estimated through fluorimeter set, pH=7.4 at 37°C. Fluorescence microplates were read every 10 minutes for 10 hours (Figures 3–5).
  | Table 1 The ThT nanoparticles’ concentrations and proteins applied |
  | Figure 3 Diagram of SPIONs effect with a concentration of 2.5 μL and different charges on Aβ-protein fibrillation. |
  | Figure 4 Diagram of SPIONs effect with a concentration of 5 μL and different charges on Aβ-protein fibrillation. |
  | Figure 5 Diagram of SPIONs effect with a concentration of 10 μL and different charges on Aβ-protein fibrillation. |
Investigation of α-synuclein protein fibrillation using ThT fluorescence emission modifications
To perform this test, variously charged nanoparticles with concentrations of 2.5 (Figure 6), 5 (Figure 7), and 10 (Figure 8) μL were added to α-synuclein protein and their fluorescence emissions were estimated separately by ThT at 37°C (pH=7.4) using fluorimeter set (Table 1).
  | Figure 6 Diagram of SPIONs effect with concentration of 2.5 μL and various charges on α-synuclein protein fibrillation. |
  | Figure 7 Diagram of SPIONs effect with concentration of 5 μL and various charges on α-synuclein protein fibrillation. |
  | Figure 8 Diagram of SPIONs effect with concentration of 10 μL and various charges on α-synuclein protein fibrillation. |
According to the diagram of α-synuclein protein fibrillation mechanism, the required time period for fibril diagram formation of α-synuclein protein was 10 hours which was obtained according to fibrillation investigation of Aβ-protein at each 10-minute run of ThT fluorescence. Microplate fluorescence was read every 10 minutes for 10 hours.
Discussion
Recent studies using different methods demonstrated that multiple-shaped α-synuclein fibrils show a certain structure. These structural differences were probably due to a difference in β-sheet folding, molecular aggregation among joint levels of the sheets or the interaction of side-chains in the medium.7 In fact, slight difference in buffer condition such as pH, temperature, ion concentration, and external variables like stirring with toxics and medicines can severely affect α-synuclein aggregation and folding processes.13,14
Various studies have been carried out regarding the mechanism of the onset and ending of fibril formation and their behavioral model, fibril formation and growth diagram have been demonstrated. Fibril formation is started by a slow phase named lag phase and is followed by an increase in fibril formation severity in nucleation phase. Based on studies conducted on AD and PD, factors like salt concentration, temperature, and pH of the medium can inhibit or excite fibril formation level.11,28,29
In a study, it was revealed that normal concentration of salt in vitro would inhibit fibril formation far more than high salt concentration and this may be the reason for the interaction between amino group of proteins and salt of the medium which inhibits or delays the formation of β-sheet structure.12 A suitable temperature for the formation of protein structures according to previous studies is 37°C and the most suitable temperature was found to range between 34°C and 37°C. Of course, temperatures above 40°C disrupt the structure of the proteins.15
In our study, in order to consider the proper conditions for the application of iron oxide nanoparticles, the temperature for performing the test was considered as 37°C, that is according to body temperature, and a plain pH was also observed for the investigation of protein fibril formation.
Nowadays, the gold, silica, silver and iron nanoparticles are widely used in pharmaceutical industry across the world. Medical consumption of these nanoparticles has always been focused on their different structures as well as the ratio of level to volume and influx power into live tissues which are regarded as the main properties of nanoparticles.16 Another important factor is the detection ability of different magnetic nanoparticles, for instance, gold or trivalent iron oxide nanoparticles. The reason of why we used SPIONs in our study was the traceability of the nanoparticles.17 As it is known, SPIONs have the highest magnetic property.8
Another issue is the nontoxicity property of these nanoparticles. As it is reported, in various studies utilization of nanoparticles has led to the cytotoxicity in live cells.18 As a result, the application of toxicity evaluation tests and techniques has been recommended for medicinal and medical consumption of nanoparticles. The iron, gold, and silica nanoparticles have the least levels of toxicity for the live cells.19 Trivalent iron oxide nanoparticles used in our study were confirmed by the US FDA (Food and Drug Administration).
In a study carried out on different nanoparticles (considering that the nanoparticles had charge), Mahmoodi et al found that negatively charged nanoparticles were the most effective ones in inhibition of A-fibril formation.6
In the current study, the least concentration of the nanoparticles applied that had negative charge encoded with −COOH ion was 2.5 μL and it was investigated at 600 10-minute runs and the obtained diagram was considerably longer in lag phase, in other words, it was delayed or inhibited and the nucleation phase was shortened. What does shortening or decreasing of nucleation phase mean in the fibrillation process? It implies that the number of β-sheets formed by the process of mature fibril formation of the protein has been decreased so that lower level of ThT has been connected to these sheets compared to positive control and other charges. Therefore, it shows less fluorescence absorption compared to other diagrams. The inhibition of fibril formation was investigated at all three concentrations.20,21
The diameter of nanoparticles used in our study was 20 nm which showed a proper influx power for medicinal consumption. Finally, based on the results obtained from the investigation of A-fibril formation mechanism in two Aβ and α-synuclein proteins, it was revealed that negatively charged dextran-coated nanoparticles encoded to −COOH could have a considerable decrease from 17,000 to 12,000 after 320 minutes of lag phase delaying and decreasing the level of ThT binding to β-sheets. In the study conducted on nanoparticles and their inhibition effect in A-fibril formation, the ratio of surface to volume and their high influence power on interaction of fibril formation have been reported as the reason for inhibition effect.
Medicinal consumption as a binding factor or carrier has been recommended in various scientific articles. The reason for this was reported as the proper coating of nanoparticles on interaction with adjacent materials and of course more and better binding with these materials.22–24 Trivalent iron oxide nanoparticles are spherical, thus they can have the best surface contact with their environment compared to nanoparticles which are not spherical.25,26
In this research, following the investigation of toxicity property of nanoparticles and toxicity level of nanoparticles with respect to various concentrations and charges applied, it can be stated that the best outcome of the application and selection of trivalent iron oxide super paramagnetic nanoparticle is that it can be used as a medicinal carrier, which according to the confirmation of FDA, this certainty was obtained for the purchased nanoparticles in the present study.
According to Figure 4 which shows the difference of fibril formation mechanism with positive control in which only α-synuclein protein is used, it can be concluded that the best alternative for inhibition of A-fibril formation in α-synuclein proteins is the application of negatively, positively, and neutrally charged nanoparticles, respectively. The properties of commercially purchased super paramagnetic iron oxide nanoparticles are provided according to the physicochemical nature, charge, and ZP in the appendix.
Acknowledgements
This work was supported by the Presidency of Islamic Republic of Iran for Science and Technology, Iran National Science Foundation (grant number 91059044).
Disclosure
The authors report no conflicts of interest in this work.
References
Fink AL. Protein aggregation: folding aggregates, inclusion bodies and amyloid. Fold Des. 1998;3(1):R9–R23. | ||
Manno M, Mauro M, Craparo EF, Podest A, et al. Kinetics of different processes in human insulin amyloid formation. J Mol Biol. 2007;366(1):258–274. | ||
Sherer TB, Kim JH, Betarbet R, Greenamyre JT. Subcutaneous rotenone exposure causes highly selective dopaminergic degeneration and α-synuclein aggregation. Experimental Neurology. 2003;179(1):9–16. | ||
Cleary JP, Walsh DM, Hofmeister JJ, et al. Natural oligomers of the amyloid-beta protein specifically disrupt cognitive function. Nat Neurosci. 2005;8(1):79–84. | ||
Walsh DM, Hartley DM, Kusumoto Y, et al. Amyloid beta-protein fibrillogenesis. Structure and biological activity of protofibrillar intermediates. J Biol Chem. 1999;274(36):25945–25952. | ||
Mahmoudi M, Milani AS, Stroeve P. Synthesis, surface architecture and biological response of superparamagnetic iron oxide nanoparticles for application in drug delivery: a review. Int J Biomed Nanosci Nanotechnol. 2010;1(2/3/4):164–201. | ||
Mahmoudi M, Shokrgozar MA. Multifunctional stable fluorescent magnetic nanoparticles. Chem Commun. 2012;48(33):3957–3959. | ||
Liao YH, Chang YJ, Yoshiike Y, Chang YC, Chen YR. Negatively charged gold nanoparticles inhibit Alzheimer’s amyloid-β fibrillization, induce fibril dissociation, and mitigate neurotoxicity. Small. 2012;8(23):3631–3639. | ||
Glat M, Skaat H, Menkes-Caspi N, Margel S, Stern EA. Age-dependent effects of microglial inhibition in vivo on Alzheimer’s disease neuropathology using bioactive-conjugated iron oxide nanoparticles. J Nanobiotechnology. 2013;11(1):32. | ||
Mahmoudi M, Sant S, Wang B, Laurent S, Sen T. Superparamagnetic iron oxide nanoparticles (SPIONs): development, surface modification and applications in chemotherapy. Adv Drug Deliv Rev. 2011;63(1–2):24–46. | ||
Tauran Y, Brioude A, Coleman AW, Rhimi M, Kim B. Molecular recognition by gold, silver and copper nanoparticles. World J Biol Chem. 2013;4(3):35–63. | ||
Breydo L, Wu JW, Uversky VN. α-Synuclein misfolding and Parkinson’s disease. Biochim Biophys Acta. 2012;1822(2):261–285. | ||
Kuntworbe N, Al-Kassas R. Design and in vitro haemolytic evaluation of cryptolepine hydrochloride-loaded gelatine nanoparticles as a novel approach for the treatment of malaria. AAPS PharmSciTech. 2012;13(2):568–581. | ||
Mahmoudi M, Simchi A, Imani M. Recent advances in surface engineering of superparamagnetic iron oxide nanoparticles for biomedical applications. J Iran Chem Soc. 2010;7(S2):S1–S27. | ||
Shang W, Nuffer JH, Dordick JS, Siegel RW. Unfolding of ribonuclease A on silica nanoparticle surfaces. Nano Letters. 2007;7(7):1991–1995. | ||
Khlebtsov N, Dykman L. Biodistribution and toxicity of engineered gold nanoparticles: a review of in vitro and in vivo studies. Chem Soc Rev. 2011;40(3):1647–1671. | ||
Ai Tran HN, Sousa F, Moda F, et al. A novel class of potential prion drugs: preliminary in vitro and in vivo data for multilayer coated gold nanoparticles. Nanoscale. 2010;2(12):2724–2732. | ||
Cimini A, D’Angelo B, Das S, et al. Antibody-conjugated PEGylated cerium oxide nanoparticles for specific targeting of Aβ aggregates modulate neuronal survival pathways. Acta Biomater. 2012;8(6):2056–2067. | ||
Yang CC, Yang SY, Chieh JJ, et al. Biofunctionalized magnetic nanoparticles for specifically detecting biomarkers of Alzheimer’s disease in vitro. ACS Chem Neurosci. 2011;2(9):500–505. | ||
Sillerud LO, Solberg NO, Chamberlain R, et al. SPION-enhanced magnetic resonance imaging of Alzheimer’s disease plaques in AβPP/PS-1 transgenic mouse brain. J Alzheimers Dis. 2013;34(2):349–365. | ||
Sigalit G, Shlomo M, inventors; Bar-Ilan University, assignee. Nucleation and growth of magnetic metaloxide nanoparticles and its use. Israel patent WO 1999062079A1.1999 Dec 02. | ||
Liu G, Men P, Perry G, Smith MA. Nanoparticle and iron chelators as a potential novel Alzheimer therapy. In: Free radicals and antioxidant protocols. New York, NY: Humana Press; 2010:123–144. | ||
Cabaleiro-Lago C, Quinlan-Pluck F, Lynch I, et al. Inhibition of amyloid beta protein fibrillation by polymeric nanoparticles. J Am Chem Soc. 2008;130(46):15437–15443. | ||
Sahni JK, Doggui S, Ali J, Baboota S, Dao L, Ramassamy C. Neurotherapeutic applications of nanoparticles in Alzheimer’s disease. J Control Release. 2011;152(2):208–231. | ||
Skaat H, Shafir G, Margel S. Acceleration and inhibition of amyloid-β fibril formation by peptide-conjugated fluorescent-maghemite nanoparticles. J Nanopart Res. 2011;13(8):3521–3534. | ||
Yoo SI, Yang M, Brender JR, et al. Inhibition of amyloid peptide fibrillation by inorganic nanoparticles: functional similarities with proteins. Angew Chem Int Ed Engl. 2011;50(22):5110–5115. | ||
Maroteaux L, Campanelli JT, Scheller RH. Synuclein: a neuron-specific protein localized to the nucleus and presynaptic nerve terminal. J Neurosci. 1988;8(8):2804–2815. | ||
Harper JD, Lieber CM, Lansbury PT Jr. Atomic force microscopic imaging of seeded fibril formation and fibril branching by the Alzheimer’s disease amyloid-β protein. Chemistry & biology. 1997;4(12):951–959. | ||
Koo EH, Lansbury PT, Kelly JW. Amyloid diseases: abnormal protein aggregation in neurodegeneration. Proceedings of the National Academy of Sciences. 1999;96(18):9989–9990. |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
