Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 16
Effect of SGRQ-Defined Chronic Bronchitis at Baseline on Treatment Outcomes in Patients with COPD Receiving Nebulized Glycopyrrolate
Authors Tashkin DP, Ozol-Godfrey A, Sharma S , Sanjar S
Received 28 January 2021
Accepted for publication 21 March 2021
Published 12 April 2021 Volume 2021:16 Pages 945—955
DOI https://doi.org/10.2147/COPD.S304182
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Richard Russell
Donald P Tashkin,1 Ayca Ozol-Godfrey,2 Sanjay Sharma,2 Shahin Sanjar2
1Division of Pulmonary and Critical Care Medicine, David Geffen School of Medicine at UCLA Health Sciences, Los Angeles, CA, USA; 2Sunovion Pharmaceuticals Inc., Marlborough, MA, USA
Correspondence: Donald P Tashkin
Division of Pulmonary and Critical Care Medicine, David Geffen School of Medicine at UCLA Health Sciences, Los Angeles, CA, 90095, USA
Tel +1 310-825-3163
Email [email protected]
Background: Chronic bronchitis (CB) is one of the conditions that contribute to chronic obstructive pulmonary disease (COPD). Despite its widespread prevalence among patients with COPD and overall negative impact on treatment outcomes, the effect of CB on the efficacy of bronchodilator therapy has not been evaluated. The objective of this post hoc analysis is to assess the effect of nebulized glycopyrrolate (GLY) on lung function and health-related quality of life outcomes in patients with St George’s Respiratory Questionnaire (SGRQ)-defined CB at baseline.
Methods: Pooled data from the replicate, 12-week GOLDEN 3 and 4 studies (N=861) were grouped by CB status at baseline. The endpoints reported are changes from baseline in trough forced expiratory volume in 1 second (FEV1), SGRQ and EXAcerbations of Chronic Pulmonary Disease Tool-Respiratory Symptoms (EXACT-RS) scores. Safety of GLY was evaluated by monitoring the incidence of adverse events (AEs).
Results: Following 12 weeks of treatment, GLY 25 μg twice-daily (BID) resulted in placebo-adjusted improvements from baseline in FEV1 of 77.1 mL and 124.4 mL in the CB and non-CB groups, respectively (p< 0.0001 vs placebo in both groups). Significant improvements in SGRQ total scores were observed with GLY 25 μg BID compared with placebo, regardless of baseline CB status. Although EXACT-RS improvements were noted in both CB and non-CB groups, significant improvements were observed only in the CB group. GLY 25 μg BID was generally well tolerated through 12 weeks of treatment, with a low incidence of AEs.
Conclusion: Treatment with nebulized GLY 25 μg BID for 12 weeks resulted in significant improvements in lung function and SGRQ total scores, compared with placebo. Significant improvements in EXACT-RS total scores were observed only in the CB group. Together, these results support the use of GLY 25 μg BID in patients with COPD, regardless of their CB status.
Keywords: chronic bronchitis, COPD, LAMA, nebulized glycopyrrolate
Background
Progressive, incompletely reversible airflow limitation and persistent respiratory symptoms are the primary characteristics of chronic obstructive pulmonary disease (COPD).1 Emphysema and chronic bronchitis (CB) are the most important conditions that comprise COPD, with both conditions often coexisting in patients with COPD.2 The term emphysema refers to the destruction of alveolar walls and pathologic enlargement of the alveolar spaces.2 CB occurs due to chronic inflammation in the bronchi and can accelerate lung function decline, increase exacerbation frequency and risk of respiratory tract infections, reduce health-related quality of life (HRQoL), and raise the risk of mortality.3 At the pathophysiological level, CB is a result of excessive mucus production in response to inflammatory signals.3 CB is associated with an increase in the number of mucus-secreting goblet cells in the central and distal airways, and submucosal gland hypertrophy.4,5 This results in the overproduction and hypersecretion of mucus which, combined with poor ciliary function due to replacement of the surface ciliary epithelium by reserve cells, goblet cells and squamous metaplasia, results in decreased elimination of mucus and increased airway obstruction.6–8
CB is common in the general population (3.4–22.0% of the adults),3 while the reported prevalence of CB among patients with COPD varies widely and ranges from 7.4% to 74%.6,9–12 The wide variability in prevalence of CB in the COPD patient population and COPD clinical trials is primarily due to differences in study design and the definitions of CB used. The classic definition of CB refers to chronic cough and sputum production due to inflammation of the bronchi on most days for ≥3 months per year and for ≥2 consecutive years.9 The St George’s Respiratory Questionnaire (SGRQ) has been used in clinical trials as a surrogate for the classic definition.13 Patients who report cough and sputum production “most days of the week” or “several days of the week” in the SGRQ Symptoms domain are considered to have CB.9 The SGRQ-derived definition of CB was shown to be more sensitive than the classic definition in identifying patients with chronic cough and sputum production.13
Few clinical trials have addressed the impact of concurrent CB on lung function outcomes with bronchodilator therapy in patients with COPD. The Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) study in 2163 patients with COPD showed that patients with concurrent CB had a mean forced expiratory volume in 1 second (FEV1) that was lower by 43±20 mL per year than that of patients without CB; but CB was not associated with an accelerated rate of decline in FEV1.14 Among 1061 patients enrolled in the COPDGene® study, CB in patients with COPD was associated with worse respiratory symptoms and a greater risk for exacerbations,6 highlighting the roles played by CB in COPD disease progression. The Roflumilast and Exacerbations in patients receiving Appropriate Combination Therapy (REACT) study included patients with severe COPD and CB who are at risk of frequent exacerbations15 and showed improvements in lung function with roflumilast, an oral phosphodiesterase-4 inhibitor.
Glycopyrrolate inhalation solution (GLY; Lonhala® [Sunovion Pharmaceuticals, Inc., Marlborough, MA, USA]) 25 μg twice daily (BID) delivered via the eFlow® Closed System (CS) Nebulizer (Magnair® [PARI Pharma GmbH, Starnberg, Germany]) was approved by the US Food and Drug Administration (FDA) for the long-term maintenance treatment of airflow obstruction in patients with moderate-to-very-severe COPD.16 This was based, in part, on the results from two Phase 3 studies (Glycopyrrolate for Obstructive Lung Disease via Electronic Nebulizer [GOLDEN 3; NCT02347761] and GOLDEN 4 [NCT02347774]; Figure S1).17,18 The objective of this post hoc analysis is to assess the effect of nebulized GLY 25 μg BID on lung function, HRQoL outcomes, and safety in patients with SGRQ-defined CB at baseline using pooled data from the GOLDEN 3 and 4 studies. We utilize the SGRQ-derived definition of CB to characterize the impact of baseline CB on the efficacy and safety of nebulized bronchodilator therapy in patients with COPD.
Methods
Study Design
This is a post hoc analysis of pooled data from the GOLDEN 3 and 4 studies that were previously described (Figure S1).17 Briefly, in the multi-center, placebo-controlled, double-blind studies, patients (N=1293) with moderate-to-very-severe COPD were randomized 1:1:1 to receive either placebo or GLY (25 or 50 µg BID), via the eFlow® CS nebulizer. Patients with prior history of LAMA use were not excluded from the trials. Randomization in each study was stratified by background long-acting β2-agonist (LABA) use (yes/no) with or without inhaled corticosteroids (ICS) and by cardiovascular (CV) risk (high/low). Supplemental (ipratropium bromide) and rescue (albuterol [salbutamol]) medication use were permitted.
Patients
Detailed inclusion and exclusion criteria for the GOLDEN 3 and 4 studies have been described.17 Briefly, patients were at least 40 years of age, current or ex-smokers with ≥10 pack-year smoking history, with a clinical diagnosis of moderate-to-very-severe COPD (as defined by GOLD 2014 criteria),1 and qualifying post-bronchodilator (ipratropium 68 μg) spirometry (FEV1 <80% of predicted normal, FEV1 >0.7 L and FEV1/forced vital capacity ratio [FVC] <0.70).
Statistical Analysis
In this post hoc analysis, pooled patient data from the GOLDEN 3 and 4 studies were categorized based on the SGRQ definition of CB into those with (CB group) or without CB (non-CB group) at baseline. The SGRQ-based definition of CB is derived from the Symptoms domain of SGRQ. Patients answering “most days of the week” or “several days of the week” to both the following questions are considered to have CB: “How often do you complain of cough during the week?” and “How often do you complain of sputum production during the week?”
This analysis compared GLY and placebo treatment in patients grouped by CB status on the following endpoints: change from baseline in trough FEV1, changes from baseline in SGRQ (total and domain scores), and EXAcerbations of Chronic Pulmonary Disease Tool-Respiratory Symptoms (EXACT-RS; total and domain scores) at Week 12. Safety data were analyzed using descriptive statistics; adverse events (AEs) and serious adverse events (SAEs) were coded according to MedDRA v15.1 and summarized by treatment, system organ class, and preferred term.
Efficacy analyses were performed using the intent-to-treat (ITT) population, while the safety analyses used the safety population; both populations consisted of all randomized patients who received ≥1 dose of study drug while on the study. Only on-treatment data were used for this analysis. No multiplicity adjustments were made for the post hoc multiple comparisons. A mixed-model for repeated measures was used to analyze changes from baseline in trough FEV1 and EXACT-RS total scores at Week 12; analysis of covariance was used to measure changes from baseline in SGRQ scores. SGRQ responders, defined as patients with reduction in SGRQ total score ≥4 units (defined as minimum clinically important differences),19 were analyzed using a logistic regression model. EXACT-RS responders, defined as patients with reduction in EXACT-RS total score ≥2 units,20 were analyzed using a longitudinal logistic regression analysis. All models included covariates for baseline level of the appropriate outcome measure, CV risk (high/low), and background LABA ± ICS use (yes/no); however, data analysis did not split the groups by LABA or ICS use.
All p-value interpretations were made at the 5% significance level and all statistical procedures were performed using SAS® v9.2 and v9.4 (SAS Institute Inc., Cary, NC).
Results
Patient Demographics and Baseline Characteristics
Pooled data from patients (N=1293) from the GOLDEN 3 and 4 trials were grouped based on baseline CB (Table 1). A high prevalence of CB at baseline (65.1%) was observed in the overall patient population.
 |
Table 1 Patient Distribution by CB Status at Baseline |
Patients were generally well matched in baseline and disease characteristics between the CB and non-CB groups (Table 2). The CB group were younger than the non-CB group and the proportion of current smokers was much greater in the CB group compared with the non-CB group; however, pack-years were similar. In addition, the exacerbation history in the past 12 months shows greater incidence of exacerbations in the CB group compared with the non-CB group. Baseline lung function (FEV1) was similar between the CB and non-CB groups. Baseline SGRQ and EXACT-RS total and domain scores were higher among patients in the CB group, compared with those in the non-CB group.
 |
Table 2 Baseline Demographics and Disease Characteristics, by CB at Baseline |
Lung Function: Change from Baseline in Trough FEV1
At Week 12, GLY 25 μg BID resulted in significant improvements in change from baseline in trough FEV1 compared with placebo, regardless of CB status at baseline (p<0.0001; Figure 1A); similar results were observed with GLY 50 μg BID (Table S1). The placebo-adjusted change from baseline FEV1 with GLY 25 μg BID was greater in the non-CB group compared to the CB group (124.4 mL vs 77.1 mL, respectively; Figure 1A); similarly, the placebo-adjusted change from baseline FEV1 with GLY 50 μg BID was greater in the non-CB group compared to the CB group (148.7 mL vs 81.1 mL, respectively; Table S1). In both CB and non-CB groups, improvements from baseline in FEV1 were consistently greater with GLY 25 μg BID treatment compared with placebo at all timepoints tested during the 12-week studies (Figure 1B and C). In the CB group, the highest improvements in FEV1 with GLY 25 μg BID were noted at Week 2 (GLY: 97.6 mL vs placebo: –1.4 mL; Figure 1B); of note there was a modest decrease in the magnitude of the FEV1 improvements with GLY 25 μg BID from Week 8 to Week 12. In the non-CB group, FEV1 improvements with GLY 25 μg BID were highest at Week 8 (GLY: 108.2 mL vs placebo: 5.6 mL; Figure 1C).
Patient-Reported Outcomes
SGRQ (Total and Domain) Scores and Responder Rates
At 12 weeks, GLY 25 μg BID resulted in significant improvements from baseline in SGRQ total scores compared with placebo in both CB and non-CB groups (Figure 2A); similar results were observed with GLY 50 μg BID (Table S1). In the CB group, significant improvements in placebo-adjusted least squares (LS) mean change from baseline with GLY 25 μg BID were observed in all SGRQ domains (activity, symptoms, and impacts; Figure S2); the greatest improvements with GLY were in the SGRQ symptoms domain (Figure S2C). In the non-CB group, while numerical improvements were observed in all three SGRQ domain scores with GLY 25 μg BID compared with placebo, significant improvements from baseline with GLY 25 μg BID were observed only in the impacts domain (Figure S2B).
The odds of being an SGRQ responder (defined as ≥4 unit reduction in total score) were significantly greater with GLY compared with placebo in the CB group (odds ratio [95% confidence interval], OR [95% CI]: 1.89 [1.3, 2.73], p<0.001; Figure 2B), but not in the non-CB group (OR [95% CI]: 1.32 [0.76, 2.29], p=0.3206; Figure 2B). The odds of being an SGRQ responder were not different between patients receiving GLY 50 μg BID versus placebo in either CB group (Table S1).
EXACT-RS (Total and Domain) Scores and Responder Rates
At 12 weeks, the improvements in change from baseline in EXACT-RS total score with GLY 25 μg BID treatment at 12 weeks were significant compared with placebo only in the CB group (p<0.05; Figure 3). Although numerical improvements with GLY 25 μg BID compared with placebo were observed in each of the three EXACT-RS domain scores in both CB and non-CB groups, significant improvements with GLY 25 μg BID relative to placebo were observed only in the Breathlessness domain in the CB group. The improvements in change from baseline in EXACT-RS total score with GLY 50 μg BID were numerically, but not significantly, different from those observed with placebo (Table S1).
We analyzed the changes in EXACT-RS scores from baseline with GLY 25 μg BID over the duration of the study (Figure S3A). In the CB group, improvements in EXACT-RS total scores were noted with time in both the GLY 25 μg BID and placebo treatment groups (Figure S3A). In the non-CB group, modest improvements in EXACT-RS scores with GLY 25 μg BID were observed throughout the study, while scores were higher than baseline (indicating worse health status) or near baseline in the placebo group (Figure S3A). In the CB group, modest improvements in EXACT-RS scores were observed in the Cough and Sputum domain, in both GLY 25 μg BID and placebo treatment groups, whereas in the non-CB group, these scores remained at or near baseline levels in both treatment groups (Figure S3B).
At Week 12, the odds of being an EXACT-RS responder rate (defined as ≥2 unit reduction in total score) in the GLY treatment group were significantly greater than placebo in the CB group (OR [95% CI]: 1.72 [1.19, 2.5], p<0.01), but not in the non-CB group (OR [95% CI]: 1.16 [0.66, 2.04], p>0.05; Figure S4). In the CB group, the odds of being an EXACT-RS responder in the GLY treatment groups were significantly greater than placebo at every time point tested; in the non-CB group, the odds were significant only at Week 2 (Figure S4). The odds of being an EXACT-RS responder were similar with GLY 50 μg BID and placebo (Table S1).
Safety
Overall, GLY was generally well-tolerated regardless of CB status at baseline (Tables 3 and S2). The most common AEs across treatment groups were worsening of COPD, cough, dyspnea and wheezing. In both CB and non-CB groups, overall incidence of any AEs, SAEs and AEs leading to study drug withdrawal was lower in GLY 25 µg treatment groups compared with placebo (Table 3). Incidence of any AEs was similar in the placebo and GLY 50 μg BID treatment groups in both CB and non-CB arms; incidence of any SAEs was similar with placebo and GLY 50 μg BID in the CB group, but was lower with GLY 50 μg BID in the non-CB group. Incidence of AEs leading to study withdrawal was lower with GLY 50 μg compared with placebo in both CB and non-CB groups (Table S2).
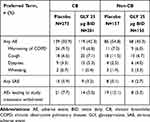 |
Table 3 Summary of AEs, SAEs, and AEs Leading to Treatment Discontinuation, Including Individual AEs with Incidence ≥3% with Placebo or GLY 25 μg BID, by Baseline CB Status (Safety Population) |
Discussion
In this post hoc analysis of pooled data from the GOLDEN 3 and 4 studies, we utilized the SGRQ-derived definition for CB to understand the impact of CB at baseline on bronchodilator response in patients with COPD. Treatment with nebulized GLY 25 μg BID for 12 weeks led to significant improvements in FEV1 compared with placebo, regardless of baseline CB status, although greater improvements were observed in the non-CB group compared with the CB group (Figure 1A). In both CB and non-CB groups, changes from baseline in trough FEV1 of ≥77.5 mL were observed with GLY 25 μg BID through Weeks 2–12, compared to –28.7–8.9 mL with placebo (Figure 1B). This suggests that treatment with GLY 25 μg BID can show lung function improvements as early as 2 weeks, regardless of baseline CB status. Similar results were observed in changes with FEV1 with GLY 50 μg BID, with greater improvements in the non-CB group compared with the CB group (Table S1).
The overall prevalence of CB at baseline among patients with COPD varies widely. While population-based studies estimate that the prevalence of CB in patients with COPD is between 14 and 30%, clinical studies have shown a higher prevalence of 27.3–74.1%.6,10–12 The prevalence of CB was also higher among patients with greater COPD severity, increasing from ~30% among GOLD Stage II to ~40% among GOLD Stage IV.21 In general agreement with these studies, we observed a CB prevalence of ~65% in patients enrolled in the GOLDEN 3 and 4 studies (inclusion criteria for patients was moderate-to-severe COPD), using an SGRQ-based definition of CB that is more sensitive than the classic definition.13 Consistent with previous studies,6,12 we observed a higher prevalence of current smokers (CB: 65%; non-CB: 32%) and younger patients (CB: 62.5 years; non-CB: 67 years) in the CB+ group compared to the CB– group.
In the CB group, there were greater improvements both in SGRQ and EXACT-RS scores with GLY 25 μg BID and 50 μg BID compared with placebo. Improvements in SGRQ total scores with GLY 25 μg BID and 50 μg BID compared with placebo were significant in patients irrespective of their baseline CB status (Figure 2A). However, significant improvements in all SGRQ domain scores with GLY 25 μg BID were observed only in the CB group. Baseline SGRQ scores were generally higher (indicating worse health status) in the CB group compared to the non-CB group; the higher scores at baseline may have provided an opportunity for greater relative SGRQ improvements among patients in the CB group. It is important to note that the CB classification for this study was based on the Symptoms domain of SGRQ and may explain the ~30-point difference at baseline between the CB and non-CB groups in this domain (Table 2). Additionally, while lung function improvements were greater in the non-CB group, improvements in SGRQ were greater in the CB group; this may be an outcome of the patient grouping based on SGRQ scores, which made this readout more sensitive for differences in the CB phenotype between patients. Other factors that may have influenced this discrepancy between lung function and SGRQ outcomes include the objective vs subjective nature of the assessments, respectively, as well as the baseline disease differences in the level of cough and sputum between the groups which adversely impacts lung function.
For EXACT-RS, significant improvements in total scores with GLY 25 μg BID compared with placebo were noted only in the CB group (Figure 3); in contrast, there were no significant differences in the improvements from baseline in EXACT-RS between GLY 50μg BID and placebo (Table S1). At every time point tested in this study, the odds of being an EXACT-RS responder were significantly greater with GLY compared with placebo only in the CB group (Figure S4). In the CB group, we also observed improvements in EXACT-RS scores with placebo treatment (Figure S3). The improvement with GLY 25 μg BID at Week 12 was greater than the minimum clinically important difference of 2 units; however, the high placebo responses in the CB group may have reduced the magnitude of placebo-adjusted changes from baseline in EXACT-RS scores. Such “placebo effects” have been observed in non-COPD clinical trials and can lead to trial failure.22 While the exact reasons behind the high placebo response in the CB group is unclear, it is possible that the nebulization process itself (with either the drug or placebo) offers some relief from the dyspnea and cough among these patients.
Cholinergic pathways play a dominant role in mucus secretion in humans.23 Anticholinergics, such as GLY, inhibit the muscarinic receptors in the airways to induce bronchodilation.24 Muscarinic receptors are localized to smooth muscles of all human airways and are highest in the large airways.25,26 Muscarinic receptors are also expressed on airway epithelium and submucosal glands, consistent with cholinergic control of mucus secretion.25 Therefore, it is likely that inhaled anticholinergics, in addition to acting as bronchodilators, may also inhibit mucus hypersecretion. However, the impact of anticholinergics on airway mucus secretion has rarely been studied in COPD. The effect of anticholinergics on mucus secretion may be difficult to demonstrate in short-term studies, due to mucus accumulation and plugging in the airways. In addition, it may be important to deposit the anticholinergic medications deep into the lungs to have a substantive inhibition of mucus secretion. In in vitro studies, the e-Flow® CS nebulizer generated GLY aerosols with a mean mass median aerodynamic diameter of 3.7 µm and a fine particle fraction of 72%;27 this may facilitate more peripheral drug deposition and may explain the differential effects on SGRQ and EXACT-RS in patients with CB.
To our knowledge, this is one of the first analyses to assess SGRQ-derived baseline CB and its impact on bronchodilator response in patients with COPD. The 1-year, double-blind, placebo-controlled REACT study assessed changes from baseline in post-bronchodilator FEV1 and HRQoL (COPD Assessment Test [CAT]) in 1945 patients with severe COPD and CB, following once-daily treatment with oral roflumilast 500 µg or placebo, together with fixed-dose ICS-LABA combination.15 Improvements in post-bronchodilator FEV1 of 56 mL were observed with roflumilast, compared with placebo (p<0.0001); however, no improvements in CAT scores were noted in the study.15 In the current post hoc analysis, higher placebo-adjusted improvements in FEV1 were observed with GLY 25 μg BID among patients with COPD and CB (CB group: 77.1 mL; p<0.0001 vs placebo), compared to that previously observed with roflumilast (Figure 1). However, in contrast to the REACT study, significant improvement in SGRQ scores were observed with GLY 25 μg BID, compared with placebo, irrespective of CB status at baseline; improvements were also observed with EXACT-RS scores in both the CB and non-CB groups (Figures 2 and 3).
Some limitations of this study include the post hoc nature of the patient stratification and the lack of adjustment for multiplicity. The definition of CB by SGRQ is limited by patient self-reporting and assessment of their symptoms, which may be inconsistent across the patient population. In addition, a greater proportion of patients in the CB group experienced exacerbations in the 12 months prior to the study, compared with the non-CB group; while exacerbations were not assessed in this analysis, the exacerbation history may have impacted patient symptoms and reflects disease severity, all of which may have contributed to differences in treatment outcomes between the CB and non-CB groups. Finally, in our study SGRQ scores were assessed at baseline and Week 12. Since SGRQ measures HRQoL over the prior 4 weeks, SGRQ changes through the 12 weeks of study were not monitored. However, it has been reported that an assessment of cough and sputum over the preceding 4 weeks has a greater prognostic value when compared to measurements over the past 2 years.28 Hence, it is likely that the SGRQ scores at Week 12 are reflective of patient HRQoL during the complete study period.
GLY was well tolerated in both CB and non-CB groups, with no differences in safety outcomes, including a lower incidence of overall AEs, SAEs and AEs leading to discontinuation among patients treated with GLY 25 μg BID compared with those receiving placebo. These results are consistent with the characterized safety profile of nebulized GLY 25 μg BID and support its use in patients, regardless of their baseline CB status.
Conclusions
In conclusion, results from this post hoc analysis show that patients treated with GLY 25 μg BID had significant improvements in lung function compared with placebo, following 12 weeks of treatment, regardless of CB status at baseline. Patients in the CB group showed significant improvements in SGRQ (total and domain) scores and EXACT-RS total scores with GLY 25 μg BID at 12 weeks. Additionally, responder analysis showed a consistent and clinically meaningful improvement in both SGRQ and EXACT-RS total scores with GLY compared with placebo in the CB group. GLY was generally well tolerated across both treatment groups with a low incidence of AEs and SAEs. Despite some of the limitations of this post hoc analysis, the data presented here support the safety and efficacy of GLY 25 μg BID in patients with moderate-to-very-severe COPD, regardless of CB status. Furthermore, these results demonstrate the impact of CB on bronchodilator therapy and highlight the need to explore the CB phenotype in prospective randomized clinical trials.
Data Sharing Statement
Sunovion Pharmaceuticals Inc. is part of a clinical trial data sharing consortium that facilitates access for qualified researchers to selected anonymized clinical trial data. For up-to-date information on data availability, please visit: https://www.clinicalstudydatarequest.com/Study-Sponsors.aspx and click on Sunovion.
Ethics Statement
The GOLDEN 3 (SUN101–301: project approval number 28,481) and GOLDEN 4 (SUN101–302: project approval number 28,482) study protocols were approved by Quorum Review IRB North American (US and Canadian) Board (Panel II) prior to patient enrollment, and were conducted in accordance with the protocols, International Council for Harmonization Good Clinical Practice guidelines, and the Declaration of Helsinki. All patients provided written informed consent.
Acknowledgments
This post hoc analysis was supported by funding from Sunovion Pharmaceuticals Inc. The authors would like to thank Shane Hornibrook from Sarepta Therapeutics, Inc., Diane Hall from Sunovion Pharmaceuticals Inc., and Rajeshwari Sammishetty from Sage Therapeutics, Inc. for support with statistical analyses performed. Medical writing support was provided by Dhivya Ramalingam, PhD of Ashfield MedComms, an Ashfield Health Company, and funded by Sunovion Pharmaceuticals Inc.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data, took part in drafting the article or revising it critically for important intellectual content, agreed to submit to the current journal; gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
DPT declares that he has served as a consultant and speaker for Sunovion, AstraZeneca, Innoviva/Theravance, Mylan, and Boehringer-Ingelheim. AOG was an employee of Sunovion Pharmaceuticals Inc at the time of the study and is currently an employee of Alexion Pharmaceuticals. SSh and SSa are employees of Sunovion Pharmaceuticals Inc. The authors report no other conflicts of interest in this work.
References
1. Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management and prevention of COPD. Available from: http://goldcopd.org/.
2. American Lung Association Epidemiology and Statistics Unit. Trends in COPD (Chronic bronchitis and emphysema): morbidity and mortality 2013. Available from: https://www.lung.org/getmedia/4f74781e-481f-4f10-9255-8dfc9dc56974/copd-trend-report.pdf.pdf.
3. Kim V, Criner GJ. Chronic bronchitis and chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2013;187(3):228–237. doi:10.1164/rccm.201210-1843CI
4. Rogers DF. The role of airway secretions in COPD: pathophysiology, epidemiology and pharmacotherapeutic options. COPD. 2005;2(3):341–353. doi:10.1080/15412550500218098
5. Saetta M, Turato G, Baraldo S, et al. Goblet cell hyperplasia and epithelial inflammation in peripheral airways of smokers with both symptoms of chronic bronchitis and chronic airflow limitation. Am J Respir Crit Care Med. 2000;161(3 Pt 1):1016–1021. doi:10.1164/ajrccm.161.3.9907080
6. Kim V, Han MK, Vance GB, et al. The chronic bronchitic phenotype of COPD: an analysis of the COPDGene Study. Chest. 2011;140(3):626–633. doi:10.1378/chest.10-2948
7. Braman SS. Chronic cough due to chronic bronchitis: ACCP evidence-based clinical practice guidelines. Chest. 2006;129(1 Suppl):104S–115S. doi:10.1378/chest.129.1_suppl.104S
8. Rogers DF, Barnes PJ. Treatment of airway mucus hypersecretion. Ann Med. 2006;38(2):116–125. doi:10.1080/07853890600585795
9. Choi JY, Yoon HK, Shin KC, et al. CAT score and SGRQ definitions of chronic bronchitis as an alternative to the classical definition. Int J Chron Obstruct Pulmon Dis. 2019;14:3043–3052. doi:10.2147/COPD.S228307
10. Burgel PR, Nesme-Meyer P, Chanez P, et al. Cough and sputum production are associated with frequent exacerbations and hospitalizations in COPD subjects. Chest. 2009;135(4):975–982. doi:10.1378/chest.08-2062
11. Lahousse L, Seys LJM, Joos GF, Franco OH, Stricker BH, Brusselle GG. Epidemiology and impact of chronic bronchitis in chronic obstructive pulmonary disease. Eur Respir J. 2017;50:2. doi:10.1183/13993003.02470-2016
12. de Oca MM, Halbert RJ, Lopez MV, et al. The chronic bronchitis phenotype in subjects with and without COPD: the PLATINO study. Eur Respir J. 2012;40(1):28–36. doi:10.1183/09031936.00141611
13. Kim V, Crapo J, Zhao H, et al. Comparison between an alternative and the classic definition of chronic bronchitis in COPDGene. Ann Am Thorac Soc. 2015;12(3):332–339. doi:10.1513/AnnalsATS.201411-518OC
14. Vestbo J, Edwards LD, Scanlon PD, et al. Changes in forced expiratory volume in 1 second over time in COPD. N Engl J Med. 2011;365(13):1184–1192. doi:10.1056/NEJMoa1105482
15. Martinez FJ, Calverley PM, Goehring UM, Brose M, Fabbri LM, Rabe KF. Effect of roflumilast on exacerbations in patients with severe chronic obstructive pulmonary disease uncontrolled by combination therapy (REACT): a multicentre randomised controlled trial. Lancet. 2015;385(9971):857–866. doi:10.1016/S0140-6736(14)62410-7
16. Sunovion Pharmaceuticals Inc. Lonhala Magnair (Glycopyrrolate) Inhalation Solution: [Package Insert]. Marlborough, MA: Sunovion Pharmaceuticals, Inc, 2019.
17. Kerwin E, Donohue JF, Goodin T, Tosiello R, Wheeler A, Ferguson GT. Efficacy and safety of glycopyrrolate/eFlow® CS (nebulized glycopyrrolate) in moderate-to-very-severe COPD: results from the glycopyrrolate for obstructive lung disease via electronic nebulizer (GOLDEN) 3 and 4 randomized controlled trials. Respir Med. 2017;132:238–250. doi:10.1016/j.rmed.2017.07.011
18. Kerwin EM, Murray L, Niu X, Dembek C. Clinically important deterioration among patients with Chronic Obstructive Pulmonary Disease (COPD) treated with nebulized glycopyrrolate: a post hoc analysis of pooled data from two randomized, double-blind, placebo-controlled studies. Int J Chron Obstruct Pulmon Dis. 2020;15:2309–2318. doi:10.2147/COPD.S267249
19. Jones PW. St. George’s respiratory questionnaire: MCID. COPD. 2005;2(1):75–79. doi:10.1081/copd-200050513
20. Leidy NK, Murray LT, Monz BU, et al. Measuring respiratory symptoms of COPD: performance of the EXACT- Respiratory Symptoms tool (E-RS) in three clinical trials. Respir Res. 2014;15:124. doi:10.1186/s12931-014-0124-z
21. Agusti A, Calverley PM, Celli B, et al. Characterisation of COPD heterogeneity in the ECLIPSE cohort. Respir Res. 2010;11(1):122. doi:10.1186/1465-9921-11-122
22. Rutherford BR, Roose SP. A model of placebo response in antidepressant clinical trials. Am J Psychiatry. 2013;170(7):723–733. doi:10.1176/appi.ajp.2012.12040474
23. Rogers DF. Pharmacological regulation of the neuronal control of airway mucus secretion. Curr Opin Pharmacol. 2002;2(3):249–255. doi:10.1016/s1471-4892(02)00146-7
24. Kerwin E, Ferguson GT. An overview of glycopyrrolate/eFlow(R) CS in COPD. Expert Rev Respir Med. 2018;12(6):447–459. doi:10.1080/17476348.2018.1476853
25. Barnes PJ. Distribution of receptor targets in the lung. Proc Am Thorac Soc. 2004;1(4):345–351. doi:10.1513/pats.200409-045MS
26. Ikeda T, Anisuzzaman AS, Yoshiki H, et al. Regional quantification of muscarinic acetylcholine receptors and beta-adrenoceptors in human airways. Br J Pharmacol. 2012;166(6):1804–1814. doi:10.1111/j.1476-5381.2012.01881.x
27. Pham S, Ferguson GT, Kerwin E, Goodin T, Wheeler A, Bauer A. In vitro characterization of the eFlow closed system nebulizer with glycopyrrolate inhalation solution. J Aerosol Med Pulm Drug Deliv. 2018;31(3):162–169. doi:10.1089/jamp.2017.1384
28. Kim V, Zhao H, Regan E, et al. The St. George’s respiratory questionnaire definition of chronic bronchitis may be a better predictor of COPD exacerbations compared with the classic definition. Chest. 2019;156(4):685–695. doi:10.1016/j.chest.2019.03.041
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



