Back to Journals » Vascular Health and Risk Management » Volume 18
Effect of Sequential Nephron Blockade versus Dual Renin-Angiotensin System Blockade Plus Bisoprolol in the Treatment of Resistant Hypertension, a Randomized Controlled Trial (Resistant Hypertension on Treatment - ResHypOT)
Authors Cestario EDES , Vilela-Martin JF , Cosenso-Martin LN , Rubio TA, Uyemura JRR, da Silva Lopes V , Fernandes LAB , Bonalume Tacito LH, Moreno Junior H , Yugar-Toledo JC
Received 29 July 2022
Accepted for publication 20 October 2022
Published 15 December 2022 Volume 2022:18 Pages 867—878
DOI https://doi.org/10.2147/VHRM.S383007
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Harry Struijker-Boudier
Elizabeth do Espirito Santo Cestario,1,* Jose Fernando Vilela-Martin,1,* Luciana Neves Cosenso-Martin,1 Tatiane Azevedo Rubio,1 Jessica Rodrigues Roma Uyemura,1 Valquiria da Silva Lopes,1 Letícia Aparecida Barufi Fernandes,1 Lucia Helena Bonalume Tacito,2 Heitor Moreno Junior,3 Juan Carlos Yugar-Toledo1,*
1Hypertension Clinic, Internal Medicine Department, Medical School in São José Do Rio Preto (FAMERP), São Paulo, Brazil; 2Endocrinology Division, Internal Medicine Department, Medical School in São José Rio Preto (FAMERP), São Paulo, Brazil; 3Cardiovascular Pharmacology Laboratory, Faculty of Medical Sciences, State University of Campinas (UNICAMP), São Paulo, Brazil
*These authors contributed equally to this work
Correspondence: Jose Fernando Vilela-Martin, Ave Brig Faria Lima 5416, Sao Jose do Rio Preto, São Paulo, SP, 15090-000, Brazil, Tel +55 17 32015727, Email [email protected]
Introduction: Hypertension is the most important modifiable risk factor for cardiovascular disease and a leading public health concern.
Objectives: The primary aim was to compare sequential nephron blockade (SNB) versus dual renin-angiotensin system blockade (DRASB) plus bisoprolol in patients with resistant hypertension to observe reductions in systolic and diastolic blood pressure (SBP and DBP) levels after 20 weeks of treatment.
Material and Methods: This trial was an open-label, prospective, randomized, parallel-group, clinical study with optional drug up-titration. Participants were evaluated during five visits at 28-day intervals.
Results: The mean age was 55.5 years in the SNB and 58.4 years in the DRASB + bisoprolol group (p=NS). Significant office BP reductions were observed in both groups. SNB group, SBP decreased from 174.5± 21.0 to 127.0± 14.74 mmHg (p< 0.0001), and DBP decreased from 105.3± 15.5 to 78.11± 9.28 mmHg (p< 0.0001). DRASB group, SBP decreased from 178.4± 21.08 to 134.4 ± 23.25 mmHg (p< 0.0001) and DBP decreased from 102.7± 11.07 to 77.33± 13.75 mmHg (p< 0.0001). Ambulatory blood pressure monitoring (ABPM) showed also significant SBP and DBP reductions in both groups (p< 0.0001).
Conclusion: In patients with RHTN adherent to treatment, SNB and DRASB plus bisoprolol showed excellent therapeutic efficacy, although SNB was associated with earlier SBP reduction.
Keywords: resistant hypertension, natriuretic agents, dual blockade of the renin-angiotensin system, bisoprolol
Introduction
Hypertension (HTN) is the most important modifiable risk factor for cardiovascular disease and a leading public health concern. This condition is associated with functional and structural damage to target organs (heart, brain, kidneys, and blood vessels)1,2 along with metabolic abnormalities, increasing the risk of fatal and nonfatal cerebrocardiovascular events.3
Resistant HTN (RHTN) is defined as the maintenance of BP values persistently above the recommended target values despite the use of three antihypertensive agents of different classes, including one renin-angiotensin system (RAS) blocker (angiotensin-converting enzyme inhibitor [ACEI] or angiotensin II receptor blocker [ARB]), one long-acting calcium-channel blocker (CCB), and one long-acting thiazide diuretic (TZD) at the maximum recommended and tolerated doses, administered with appropriate frequency and with proven adherence.4,5 The definition above includes a subgroup of patients with RHTN whose BP is controlled with four or more antihypertensive medications, known as controlled RHTN (C-RHTN).6
In a meta-analysis of studies evaluating individuals with treated hypertension, Achelrod et al7 found a prevalence of RHTN of 13.72% in 20 observational studies and 16.32% in four randomized controlled trials. In Brazil, a multicenter study (ReHOT), using the ambulatory BP monitoring (ABPM), reported a prevalence of RHTN of 11.7%.8
Management of RHTN is further challenged by patients’ failure in adhering to treatment. Medical inertia hampers the physician’s ability to adjust medications due to the interference of pharmacokinetics and pharmacodynamic factors hindering treatment effectiveness.9
The pathophysiological mechanisms of RHTN remain uncertain but are clearly multifactorial. According to Taler et al, persistent fluid retention, increased sodium sensitivity, excessive salt intake, hyperaldosteronism, renal dysfunction, and sympathetic hyperactivity are common underlying causes contributing to the hypervolemic state found in patients with RHTN.10–14
In patients with RHTN, the addition of mineralocorticoid receptor blockers to hypertension therapy reduces BP and volume overload, further supporting the claim that fluid retention is a major contributor to the RHTN pathophysiology.15
Additional mechanisms involved in the RHTN pathophysiology include vascular smooth muscle tone, intensified sympathetic system activity, and RAS hyperactivity.16,17
Bobrie et al18 compared the efficacy and safety of two stepped care strategies, namely, sequential nephron blockade (SNB) and dual RAS blockade (DRASB) added to triple standardized antihypertensive therapy of TZD, ARB, and CCB during 3 months in patients with RHTN.
Considering the above and the lack of Brazilian studies in patients with RHTN undergoing treatment with SNB or DRASB, the aims of the present study were to demonstrate the therapeutic efficacy of SNB compared with DRASB plus bisoprolol in patients with RHTN and to assess the side effects and adherence to treatment over 20 weeks.
Materials and Methods
Study Design
Detailed information about the study protocol was published previously.19 Briefly, the ResHypOT (Resistant Hypertension on Treatment) trial of SNB versus DRASB plus bisoprolol in the treatment of resistant hypertension was an open-label, prospective, randomized, and parallel-group study with optional drug up-titration (ClinicalTrials.gov, identifier, NCT02832973, registered on 18 July 2016). Two therapeutic regimens for RHTN were compared, namely, SNB versus DRASB plus bisoprolol. Initially, all patients received standardized triple antihypertensive treatment. This research followed the ethical guidelines of the 1975 Declaration of Helsinki, and the study protocol was evaluated and approved by the Research Ethics Committee of the institution (CAAE no 33943014.6.0000.5415, no 870.093). All participants signed a written informed consent before random allocation to the SNB or the DRASB plus bisoprolol group.
Participants
The study, conducted between September 2016 and September 2019, included 72 patients with RHTN undergoing treatment with losartan (100–200 mg), chlorthalidone (25 mg), and amlodipine (5 mg) at the Hypertension Outpatient Clinic of the university hospital. The inclusion criteria were patients with RH on treatment with three antihypertensive drug classes at maximum tolerated doses for at least 6 months, after excluding causes of pseudo resistance, and both genders aged between 18 and 75 years. The exclusion criteria were chronic renal failure with dialysis or creatinine clearance <40 mL/min, coronary artery disease including unstable angina or recent myocardial infarction, atrial fibrillation or atrioventricular block, secondary hypertension, contraindication or intolerance to the drugs used in the study, and refusal or failure to follow the treatment regimen. The First Brazilian position on resistant hypertension20 and the Scientific Statement of American Heart Association5 were used to define RHTN.
Two comparison groups were generated using simple randomization and equal allocation ratio based on a table of random numbers. The study coordinator organized, and sequentially numbered opaque and sealed envelopes allocated to the patients in order of enrollment. The allocation process was developed and monitored to preserve concealment, and the envelopes were opened sequentially after irreversibly assigned to the participant. The flowchart in Figure 1 shows the process of selection of the participants. Participants in both groups were evaluated during five visits at 28-day intervals over 20 weeks. In addition to maintaining the baseline therapy, the participants in the SNB group (n=35) received sequentially (A) spironolactone 25 mg, (B) spironolactone 25 mg plus furosemide 20 mg, (C) spironolactone 25 mg plus furosemide 40 mg, and (D) spironolactone 25 mg plus furosemide 40 mg plus amiloride 5 mg, while participants in the DRASB group (n=37) received sequentially (A) ramipril 5 mg, (B) ramipril 10 mg, (C) ramipril 10 mg plus bisoprolol 5 mg, and (D) ramipril 10 mg plus bisoprolol (10 mg).
Randomization and Follow-Up
Protocol
The flowchart in Figure 1 shows the process of randomization and treatment follow-up of the participants.
Measurement of Office Blood Pressure and 24-Hour Ambulatory Blood Pressure Monitoring
Office BP was measured by the indirect method using an automatic electronic device (Omron HEM-711DLX, Omron Healthcare Inc., Bannockburn, Illinois, USA) in the office during follow-up visits, according to the VI Brazilian Guidelines on Hypertension.21 The mean level of three measurements was considered. ABPM and was additionally carried out to investigate true RHTN, white-coat effect, and masked hypertension. ABPM was performed using the Mobil-O-Graph NG (IEM, Stolberg, Germany) according to technical norms by the V Guidelines for Ambulatory Blood Pressure Monitoring.22
Anthropometric Measurements
Weight and height, measured by anthropometric scales, were used to calculate body mass index (BMI) according to the formula weight (kg)/height squared (m²). Abdominal circumference (waist) was measured and values ≤80 cm and ≤94 cm were considered appropriate for women and men, respectively. We also measured hip circumference and calculated the participants’ waist/hip ratio.
Biochemical and Imaging Tests
Blood samples were drawn from all patients at the first and last visits after 12-hour fasting for measurement of serum total cholesterol (TC), high-density lipoprotein cholesterol (HDLc), triglycerides (TG), glucose, insulin, creatinine, sodium, and potassium. Sodium and potassium were evaluated monthly. A diagnosis of diabetes was confirmed by the presence of two blood glucose measurements ≥126 mg/dL after at least 8-hour fasting. The low-density lipoprotein cholesterol (LDLc) fraction was calculated using the formula LDLc = TC - HDLc - TG/5 (for TG <400 mg/dL). Electrocardiography, echocardiography, Doppler ultrasonography of the carotids and renal arteries, and treadmill stress test were performed in all patients.
Compliance
Compliance was assessed by pill counting. The drugs were delivered at each appointment, and the patients were asked to return empty bottles at the following appointment. Patients were considered adherent when consuming above 80% of the prescribed medication. Adherence was calculated after the last visit using the Litt & Cuskey criterion, which defines adherence as the difference between pills consumed versus those that should have been consumed according to the prescription.22
Primary Outcome Measures
The primary outcome was the average of three office-measured systolic BP (SBP) and diastolic BP (DBP) levels at week 20, measured using an oscillometric device (time point, week 20).
Secondary Outcome Measures
The secondary efficacy outcome measures included the average of three office-measured mean BP (MBP) and pulse pressure (PP) levels at week 20, determined with an oscillometric device (time point, week 20), and the mean 24-hour SBP and DBP at week 20, measured with an ABPM device (time point, week 20). Safety and tolerability were also secondary outcome measures (time frame, throughout the study). During the study, BP levels were evaluated every 4 weeks by office-measured BP measurement to detect hypotension (time frame, every 4 weeks).
Sample Size Calculation
Sample size was calculated using Stata version 13.0 (StataCorp, College Station, TX, USA). The calculation was based on the detection of a difference ≥12 mmHg in SBP considering an alpha error of 5%, statistical power of 80%, and standard deviation (SD) of 8 mmHg, resulting in 36 patients per group (SNB versus DRASB + bisoprolol).
Statistical Analysis
Descriptive statistics were used for data analyses. Continuous variables are expressed as means ± 1 SD. Categorical variables are described as absolute and relative frequencies. The differences in the effects of the intensifying diuretic therapy with SNB and the DRASB intervention between baseline and 20 weeks were evaluated using paired Student’s t-test. This same test was used to compare the means of normally distributed continuous variables for related and independent samples. Nonparametric tests were used to compare data without normal distribution. The analyses were performed using the software GraphPad Prism, version 6 (GraphPad Software, Inc., San Diego, CA, USA) and Stata SE, version 13.0 (Stata Corp LLC, Texas, USA). P values <0.05 were considered statistically significant.
Results
A total of 135 participants were invited and 80 were screened for the study. Of these, 72 met the criteria for inclusion in the trial and were randomly assigned to treatment with SNB (n=35) versus DRASB + bisoprolol (n=37). All the patients completed the study in both groups (Figure 2).
 |
Figure 2 Flowchart of study. |
The baseline demographic and clinical characteristics of all subjects divided in each treatment group are shown in Table 1. No significant differences in baseline clinical characteristics were observed between subjects randomized to SNB or DRASB + bisoprolol. Distribution of risk factors (diabetes, dyslipidemia, sedentary lifestyle) between groups was also similar, although the SNB group had a higher prevalence of smoking (p<0.05). Significant reductions in weight (p=0.008), BMI (p=0.007), and waist (p=0.04) and hip circumference (p=0.03) were observed at week 20 compared with baseline in the SNB group, while the only significant difference in the DRASB group at week 20 compared with baseline was a reduction in waist circumference (p=0.04) (date no showed). In addition, no significant difference was observed in biochemical parameters into each group before, and after the treatment and between the groups at the end of treatment (Table 2).
 |
Table 1 Baseline Demographic and Clinical Characteristics in Two Groups of the Study |
 |
Table 2 Comparison of the Biochemical Data Within of Each Group and Between Groups (SNB and DRASB + Bisoprolol) at Baseline and After Treatment |
Primary Outcome Measures
Office Blood Pressure
Patients in the SNB group had mean decreases from baseline to 20 weeks of 47.5 mmHg (174.5±21.08 to 127.0±14.74 mmHg, respectively, p<0.0001) in SBP and 24.6 mmHg (105.3±15.5 to 78.11±9.28 mmHg, respectively, p<0.0001) in DBP. In the DRASB + bisoprolol group, the corresponding mean reductions were 43.9 mmHg (178.4±21.08 to 134.4±23.25 mmHg, p<0.0001) in SBP and 28.0 mmHg (102.7±11.07 to 77.33±13.75 mmHg, p<0.0001) in DBP from baseline to final visit. No significant difference was found between the two groups for mean SBP (p=0.113) and mean DBP (p=0.779) at the final visit (Table 3).
 |
Table 3 Office Blood Pressure Results at Baseline (Pre-Intervention), Twelve Weeks and After Treatment (Post-Intervention) in SNB Group and DRASB Group |
Both groups presented significant reductions in pulse pressure (ΔPP) from baseline to 20 weeks, 22.29 mmHg (71.48 to 49.01 mmHg, respectively, p<0.0001 in the SNB group and 15.92 mmHg (73.05 to 57.13 mmHg, respectively, p<0.0001) in the DRASB + bisoprolol group. The ΔPP was greater in the SNB compared with the DRASB + bisoprolol group (p=0.019).
Treatment discontinuation due to drug-related adverse events was not observed in any of the groups.
Secondary Outcome
Results of Ambulatory Blood Pressure Monitoring
The Tables 4 and 5 show the SBP and DBP values during 24-hour ABPM in both groups. The BP values in SNB group were 151.9±18.4 / 93.9 ± 12 mmHg in pre-intervention phase versus 127.3±17.8 / 77.5±10.6 mmHg in post-intervention phase (p<0.0001) (Table 4). The BP values in DRASB + bisoprolol group were 153.3±16.2 / 92.9±13.8 mmHg in pre-intervention phase versus 134.1±26.5 / 74.9±16.3 mmHg in post-intervention phase (p<0.0001) (Table 5).
 |
Table 4 Ambulatory Blood Pressure Monitoring Results at Baseline (Pre-Intervention) and After Treatment (Post-Intervention) in SNB Group |
 |
Table 5 Ambulatory Blood Pressure Monitoring Results at Baseline (Pre-Intervention) and After Treatment (Post-Intervention) in DRASB + Bisoprolol Group |
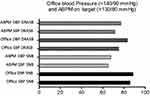
In the DRASB + bisoprolol group, the office values of SBP <140 mmHg and DBP <90 mmHg were achieved in 85.7% and 88.6% of the participants, respectively. The 24-hour ABPM values of SBP <130 mm Hg and DBP <80 mm Hg were achieved in 64.9% and 67.6% in the DRASB + bisoprolol group, respectively. In the SNB group, office SBP and DBP was achieved in 75.0% and 83.6%, respectively, and the ABPM values in 71.4% and 77.1% of the individuals, respectively (Figure 3).
Discussion
The present results of the ResHypOT trial show that patients with RHTN who fail to control BP levels with standard triple antihypertensive therapy present significant reductions in SBP and DBP 20 weeks after randomization to SNB or DRASB + bisoprolol group.
Despite standard triple antihypertensive therapy, many patients with RHTN fail to have adequate BP control, and new associations of antihypertensive drugs – some with relative contraindications – are necessary to treat these patients with high cardiovascular risk.24 The challenge set by the present study was to analyze strategies to increase natriuresis by combining different diuretics that act at various nephron sites, rather than using the usual approach of increasing the dose of a single diuretic that acts only at one site or changing the diuretic class.25
Both TZDs and furosemide are effective in reducing BP levels, but high doses of these drugs are poorly tolerated. Other strategies include mineralocorticoid receptor blockade, especially in patients with RHTN, to produce substantial BP reduction when added to other antihypertensive drugs.26,27 Some studies have shown that diuretics comprise the basis for RHTN treatment, especially mineralocorticoid receptor antagonists, which added to TZDs, have a summative effect in reducing blood pressure.28–30
The rationale for SNB using low/moderate doses of diuretics acting at different nephron sites is to neutralize the effects of intrarenal counterregulatory mechanisms that are triggered by the use of diuretics acting at a single site when administrated in high doses for a long time. The expression, transfer, and number of apical sodium transporters located in upstream or downstream segments of the nephron are modified towards stimulation of tubular sodium reabsorption, reducing the long-term efficacy of the diuretic.31 The doses of each diuretic used in the SNB arm of this trial were low/moderate to avoid risks due to natriuresis, orthostatic hypotension, fatigue, cramps, sexual dysfunction, electrolytic disturbances, and functional renal insufficiency. We selected a low dose of spironolactone (25 mg/day), as used in other RHTN studies.26
Several studies addressing DRASB in patients with non-resistant HTN and diabetes have shown a significant reduction in BP levels and microalbuminuria. However, no additional benefit of DRASB compared to the use of ACEI or ARBs alone was observed in studies including patients with high cardiovascular risk but no RHTN and, in some cases, a significant increase in adverse events was observed.32–38
Bobrie et al have shown that the sequential addition of low doses of diuretics acting at different nephron segments increased BP control in 30–58% of the patients in the SNB arm at 12 weeks. In contrast to SNB, DRASB allowed BP control in only 20% of the patients with RHTN. The rationale for using DRASB was to neutralize another counterregulatory mechanism – renin release – which is triggered by the use of combined ARBs plus TZDs and may limit the antihypertensive efficacy of these agents. A systematic review has shown an additional BP-lowering effect (3–5 mmHg) with dual RAS blockade using ACEIs and ARBs.39
Different from their study, our RepHypOT trial extended to 20 weeks. During the initial 1–3 visits, we observed no significant differences in SBP and DBP decrease in the SNB and DRASB + bisoprolol groups. Nonetheless, we found similar results at 12 weeks compared with those reported by Bobrie et al. Significant SBP reduction was observed in the SNB group compared with the DRASB + bisoprolol group at 12 weeks (140.1±16.20 versus 151.1±21.45, p=0.015), but the final measurements at 20 weeks showed no significant difference in SBP and DBP levels between both study groups.
Our results show that a progressive reinforcement of sodium depletion by the SNB strategy offers the possibility of early SBP control, reducing cardiovascular risk. In contrast, DRASB + bisoprolol group achieved the goal after 20 weeks of treatment, suggesting that patients with RHTN are more sensitive to the reinforcement of sodium depletion compared with reinforcement of RAS blockade.
Study Limitations
Even though the ResHypOT trial was not a multicenter study and our data with a small number of patients we showed the impact of SNB on early control of SBP and adds to current knowledge of this new strategy for BP control. Future multicentric studies are necessary to determine the potential positive effects of both strategies in relation to cardiovascular events, mortality, target-organ damage, and biomarkers like pulse wave velocity, arterial stiffness, carotid intima-media thickness, and renal dysfunction.
Conclusion
In patients with RHTN adherent to treatment, both SNB and DRASB plus bisoprolol showed excellent therapeutic efficacy, although SNB showed earlier and greater SBP reduction.
Abbreviations
ABPM, ambulatory blood pressure monitoring; ACEI, Angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; BP, Blood Pressure; CCB, Calcium-channel blocker; C_RHTN, controlled RHTN; DBP, diastolic blood pressure; DRASB, dual renin-angiotensin system blockade; SBP, systolic blood pressure; MAP, Mean arterial pressure; HDLc, high density lipoprotein cholesterol; HR, Heart Rate; HTN, Hypertension; LDLc, low density lipoprotein cholesterol; PP, Pulse Pressure; RAS, Renin-angiotensin system; Resistant Arterial Hypertension; ReHOT, The ReHOT Randomized Study (Resistant Hypertension Optimal Treatment).; ResHypOT, Sequential Blockade vs Dual Blockade Renin-angiotensin System + Bisoprolol in; RHTN, Resistant Hypertension; SNB, sequential nephron blockade; TC, Total Cholesterol; TZD, thiazide diuretic.
Academic Link
This study is part of the Doctoral’s thesis by Cestario EES.
Data Sharing Statement
The datasets generated and analyzed during the current study are available from the corresponding author on a reasonable request.
Consent Statement
Approval was obtained from the Research Ethics Committee of the State Medical School at Sao Jose do Rio Preto (FAMERP) according to national and international guidelines. The authors declare that this research complies with the privacy of the participants, with the data maintained anonymous and confidential. The current study was performed according to the ethical standards of the Helsinki Declaration.
Acknowledgments
The authors would like to thank the patients and all other health care professionals who participated in this study.
Funding
The present study had no external funding sources.
Disclosure
The authors declare no conflict of interest.
References
1. Kannel WB. Blood pressure as a cardiovascular risk factor, prevention and treatment. JAMA. 1996;275(20):1571–1576. doi:10.1001/jama.1996.03530440051036
2. Kannel WB. Risk stratification in hypertension, new insights from the Framingham study. Am J Hypertens. 2000;13(1 Pt 2):3S–10S. doi:10.1016/S0895-7061(99)00252-6
3. Karmali KN, Lloyd-Jones DM. Global risk assessment to guide blood pressure management in cardiovascular disease prevention. Hypertension. 2017;69(3):e2–e9. doi:10.1161/HYPERTENSIONAHA.116.08249
4. Calhoun DA, Jones D, Textor S, et al. Resistant hypertension, diagnosis, evaluation, and treatment. A scientific statement from the American heart association professional education committee of the council for high blood pressure research. Hypertension. 2008;51(6):1403–1419. doi:10.1161/HYPERTENSIONAHA.108.189141
5. Carey Robert M, Calhoun David A, Bakris George L, et al. resistant hypertension, detection, evaluation, and management, a scientific statement from the American Heart Association. Hypertension. 2018;72(5):e53–e90. doi:10.1161/hyp.0000000000000084
6. Yugar-Toledo JC, Brunelli V, Vilela-Martin JF, Fattori A, Moreno H. Controlled versus uncontrolled resistant hypertension, are they in the same bag? Curr Hypertens Rep. 2018;20(3):26. doi:10.1007/s11906-018-0825-7
7. Achelrod D, Wenzel U, Frey S. Systematic review and meta-analysis of the prevalence of resistant hypertension in treated hypertensive populations. Am J Hypertens. 2015;28(3):355–361. doi:10.1093/ajh/hpu151
8. Krieger EM, Drager LF, Giorgi DMA, et al. Spironolactone versus clonidine as a fourth-drug therapy for resistant hypertension, the ReHOT randomized study (resistant hypertension optimal treatment). Hypertension. 2018;71(4):681–690. doi:10.1161/HYPERTENSIONAHA.117.10662
9. Daugherty SL, Powers JD, Magid DJ, et al. Incidence and prognosis of resistant hypertension in hypertensive patients. Circulation. 2012;125(13):1635–1642. doi:10.1161/CIRCULATIONAHA.111.068064
10. Taler SJ, Textor SC, Augustine JE. Resistant hypertension, comparing hemodynamic management to specialist care. Hypertension. 2002;39(5):982–988. doi:10.1161/01.hyp.0000016176.16042.2f
11. Pimenta E, Gaddam KK, Oparil S, et al. Effects of dietary sodium reduction on blood pressure in subjects with resistant hypertension, results from a randomized trial. Hypertension. 2009;54(3):475–481. doi:10.1161/HYPERTENSIONAHA.109.131235
12. Agarwal R. Resistant hypertension and the neglected antihypertensive, sodium restriction. Nephrol Dial Transplant. 2012;27(11):4041–4045. doi:10.1093/ndt/gfs384
13. Shimosawa T. Salt, the renin-angiotensin-aldosterone system and resistant hypertension. Hypertens Res. 2013;36(8):657–660. doi:10.1038/hr.2013.69
14. Eirin A, Textor SC, Lerman LO. Emerging concepts for patients with treatment-resistant hypertension. Trends Cardiovasc Med. 2016;26(8):700–706. doi:10.1016/j.tcm.2016.05.004
15. Liu G, Zheng XX, Xu YL, Lu J, Hui RT, Huang XH. Effect of aldosterone antagonists on blood pressure in patients with resistant hypertension, a meta-analysis. J Hum Hypertens. 2015;29(3):159–166. doi:10.1038/jhh.2014.64
16. Sim JJ, Bhandari SK, Shi J, et al. Characteristics of resistant hypertension in a large, ethnically diverse hypertension population of an integrated health system. Mayo Clin Proc. 2013;88(10):1099–1107. doi:10.1016/j.mayocp.2013.06.017
17. Tsioufis C, Kordalis A, Flessas D, et al. Pathophysiology of resistant hypertension, the role of sympathetic nervous system. Int J Hypertens. 2011;2011:642416. doi:10.4061/2011/642416
18. Bobrie G, Frank M, Azizi M, et al. Sequential nephron blockade versus sequential renin-angiotensin system blockade in resistant hypertension, a prospective, randomized, open blinded endpoint study. J Hypertens. 2012;30(8):1656–1664. doi:10.1097/HJH.0b013e3283551e98
19. Cestario EDES, Fernandes LAB, Giollo-Junior LT, et al. Resistant Hypertension On Treatment (ResHypOT): sequential nephron blockade compared to dual blockade of the renin-angiotensin-aldosterone system plus bisoprolol in the treatment of resistant arterial hypertension – study protocol for a randomized controlled trial. Trials. 2018;19(1):101. doi:10.1186/s13063-017-2343-3
20. Alessi A, Brandao AA, Coca A, et al. First Brazilian position on resistant hypertension. Arq Bras Cardiol. 2012;99(1):576–585. doi:10.1590/s0066-782x2012000700002
21. Brandão AA, Rodrigues CI, Consolim-Colombo F. [VI Brazilian Guidelines on Hypertension]. VI Diretrizes Brasileiras de hipertensao. Arq Bras Cardiol. 2010;95(1Suppl):1–51.
22. Dohmann HF. One decade of stem cell therapy for bone marrow, what is missing? Arq Bras Cardiol. 2011;97(1):1–2. doi:10.1590/S0066-782X2011000900001
23. de Souza WA, Sabha M, de Faveri Favero F, Bergsten-Mendes G, Yugar-Toledo JC, Moreno H. Intensive monitoring of adherence to treatment helps to identify “true” resistant hypertension. J Clin Hypertens. 2009;11(4):183–191. doi:10.1111/j.1751-7176.2009.00102.x
24. Alvarez-Alvarez B, Abad-Cardiel M, Fernandez-Cruz A, Martell-Claros N. Management of resistant arterial hypertension, role of spironolactone versus double blockade of the renin-angiotensin-aldosterone system. J Hypertens. 2010;28(11):2329–2335. doi:10.1097/HJH.0b013e32833d4c99
25. Ernst ME, Moser M. Use of diuretics in patients with hypertension. N Engl J Med. 2009;361(22):2153–2164. doi:10.1056/NEJMra0907219
26. Williams B, MacDonald TM, Morant S, et al. Spironolactone versus placebo, bisoprolol, and doxazosin to determine the optimal treatment for drug-resistant hypertension (PATHWAY-2), a randomised, double-blind, crossover trial. Lancet. 2015;386(10008):2059–2068. doi:10.1016/S0140-6736(15)00257-3
27. Williams B, MacDonald TM, Morant SV, et al. Endocrine and haemodynamic changes in resistant hypertension, and blood pressure responses to spironolactone or amiloride, the PATHWAY-2 mechanisms substudies. Lancet Diabetes Endocrinol. 2018;6(6):464–475. doi:10.1016/S2213-8587(18)30071-8
28. Yugar-Toledo JC, Modolo R, de Faria AP, Moreno H. Managing resistant hypertension, focus on mineralocorticoid-receptor antagonists. Vasc Health Risk Manag. 2017;13:403–411. doi:10.2147/VHRM.S138599
29. de Souza F, Muxfeldt E, Fiszman R, Salles G. Efficacy of spironolactone therapy in patients with true resistant hypertension. Hypertension. 2010;55(1):147–152. doi:10.1161/HYPERTENSIONAHA.109.140988
30. Dudenbostel T, Calhoun DA. Use of aldosterone antagonists for treatment of uncontrolled resistant hypertension. Am J Hypertens. 2017;30(2):103–109. doi:10.1093/ajh/hpw105
31. Na KY, Oh YK, Han JS, et al. Upregulation of Na+ transporter abundances in response to chronic thiazide or loop diuretic treatment in rats. Am J Physiol Renal Physiol. 2003;284(1):F133–43. doi:10.1152/ajprenal.00227.2002
32. Mogensen CE, Neldam S, Tikkanen I, et al. Randomised controlled trial of dual blockade of renin-angiotensin system in patients with hypertension, microalbuminuria, and non-insulin dependent diabetes, the candesartan and lisinopril microalbuminuria (CALM) study. BMJ. 2000;321(7274):1440–1444. doi:10.1136/bmj.321.7274.1440
33. Bakris GL, Ruilope L, Locatelli F, et al. Treatment of microalbuminuria in hypertensive subjects with elevated cardiovascular risk, results of the IMPROVE trial. Kidney Int. 2007;72(7):879–885. doi:10.1038/sj.ki.5002455
34. Fernandez Juarez G, Luno J, Barrio V, et al. Effect of dual blockade of the renin-angiotensin system on the progression of type 2 diabetic nephropathy, a randomized trial. Am J Kidney Dis. 2013;61(2):211–218. doi:10.1053/j.ajkd.2012.07.011
35. Mehdi UF, Adams-Huet B, Raskin P, Vega GL, Toto RD. Addition of angiotensin receptor blockade or mineralocorticoid antagonism to maximal angiotensin-converting enzyme inhibition in diabetic nephropathy. J Am Soc Nephrol. 2009;20(12):2641–2650. doi:10.1681/ASN.2009070737
36. Investigators O, Yusuf S, Teo KK, et al. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med. 2008;358(15):1547–1559. doi:10.1056/NEJMoa0801317
37. Schmieder RE, Nitschmann S. [Optimal therapy of diabetic nephropathy, AVOID Study (Aliskiren in the evaluation of proteinuria in diabetes)]. Therapieoptimierung bei diabetischer Nephropathie, AVOID-Studie (“Aliskiren in the evaluation of proteinuria in diabetes”). Internist. 2009;50(7):895–896. doi:10.1007/s00108-009-2391-1
38. Parving HH, Brenner BM, McMurray JJ, et al. Baseline characteristics in the Aliskiren Trial in Type 2 Diabetes Using Cardio-Renal Endpoints (ALTITUDE). J Renin Angiotensin Aldosterone Syst. 2012;13(3):387–393. doi:10.1177/1470320311434818
39. Doulton TW, He FJ, MacGregor GA. Systematic review of combined angiotensin-converting enzyme inhibition and angiotensin receptor blockade in hypertension. Hypertension. 2005;45(5):880–886. doi:10.1161/01.HYP.0000161880.59963.da
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.