Back to Journals » Clinical Ophthalmology » Volume 16
Effect of Prophylactic Mitomycin C on Corneal Endothelium Following Transepithelial Photorefractive Keratectomy in Myopic Patients
Authors Al-Mohaimeed MM
Received 28 May 2022
Accepted for publication 11 August 2022
Published 25 August 2022 Volume 2022:16 Pages 2813—2822
DOI https://doi.org/10.2147/OPTH.S375587
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Mansour M Al-Mohaimeed
Department of Ophthalmology, College of Medicine, Qassim University, Qassim, Kingdom of Saudi Arabia
Correspondence: Mansour M Al-Mohaimeed, Email [email protected]
Purpose: This study investigated the effect of prophylactic mitomycin C (MMC) on corneal endothelium to inhibit corneal haze formation post transepithelial photorefractive keratectomy (T-PRK).
Methods: A total of 120 eyes of 60 patients with low, moderate, and high myopia were subjected to T-PRK with intraoperative application of MMC (0.02%) for 30– 50s. Patients’ files were categorized into three groups according to ablation depths (if ≥ 100 μm) during T-PRK as follows: (1) Group A – low myopia without MMC, (2) Group B – low myopia with MMC, and (3) Group C – moderate/high myopia with MMC. Preoperative/surgical parameters and refractive outcomes were documented. Cell density (CD), number of cells (NUM), coefficient of variation, central corneal thickness (CCT), hexagonality (HEX/6A), average cell area (AVG), and its standard deviation (SD) were evaluated using specular microscopy preoperatively and postoperatively.
Results: Overall, 119 out of 120 eyes showed significant prevention of corneal haze. Groups A and C showed no significant changes in endothelial CD and NUM. Group B showed a non-significant reduction in CD. However, all three groups showed significant variations in HEX/6A, CCT, AVG, and SD.
Conclusion: The MMC application did not significantly affect corneal endothelial density or number and can be used safely and effectively to prevent corneal haze following T-PRK in myopia.
Keywords-: ablation depth, corneal endothelium, corneal haze, mitomycin C, transepithelial key photorefractive keratectomy
Introduction
Refractive errors are the most prevalent visual problem worldwide.1 According to the World Health Organization, refractive errors are the primary cause of visual impairment and even vision loss globally affecting all age groups.1 Various types of refractive surgeries have been developed in the past years to correct vision by modifying the shape of the cornea through laser photoablation such as laser-assisted in situ keratomileusis (LASIK), laser-assisted subepithelial keratomileusis (LASEK), and photorefractive keratectomy (PRK).2,3 Creating a flap to photoablate stroma is a critical step in LASIK. However, studies show an increased prevalence of intraoperative flap-related complications.2,4 In LASEK, the alcohol-assisted epithelial removal can potentially be toxic to the corneal epithelial tight junctions.2,5 PRK involves mechanical debridement of epithelium using a blade and followed by surface ablation of the stroma. This method is appropriate for patients with thin corneas who cannot undergo LASIK.3,6 Although safe, effective, and even correct high range refractive errors, the development of postoperative corneal haze is one of the serious complications of PRK due to the ongoing surgery-made aberrant wound healing process in the tissue.6,7
Unlike the above methods, transepithelial photorefractive keratectomy (T-PRK) has advantages as it is a non-touch, gentle laser-corrective method that removes corneal epithelium along with stroma in a single step.8,9 This procedure has demonstrated minimal corneal epithelial defect to ablate stroma resulting in faster re-epithelialization and enhanced visual recovery along with proven safety and efficacy through lower pain scores and shorter healing time.10,11
Following any in-depth laser ablation method, the corneal stromal keratocytes undergo abnormal proliferation causing subepithelial fibrosis, which leads to haze formation.12 Several studies have shown that this corneal haze can be effectively prevented by the application of mitomycin C (MMC) over the surface ablated stroma due to its interference with the stromal wound healing process.13,14 MMC is a known non-cell cycle-specific antineoplastic agent that actively inhibits the proliferation of highly mitotic cells by blocking their DNA synthesis and causing cell cycle arrest. MMC also mediates inhibition of keratocyte activation and differentiation into myofibroblasts and counteracts disorganized deposition of stromal extracellular matrix.13 Thus, the use of MMC has been found to be critical in increasing corneal transparency in high myopic patients treated with higher ablation depths.15 Pharmacokinetic studies elucidate that MMC can be evidenced in the anterior segment (aqueous humor) of the eye even at lower concentrations and after a shorter application time.12,16 This poses a high-risk to corneal endothelium, although MMC is known to mainly attack proliferating cells. A study reported that the MMC acts as a corneal healing modulator during refractive surface ablation procedure. Also, the effect of MMC has been reported to be both concentration and time-dependent. At the time, the long-term effects of MMC remained unclear due to its inducible DNA damage to surviving corneal cells that could lead to corneal thinning or edema after operation in the later period.17 Another study reported regarding the long-term effects of MMC decreased haze formation, showed better results in UDVA for those patients who live in hot or sunny climate as living under high UV environment can also affect the outcomes of PRK. Though there was no significant endothelial loss, decrease in endothelial cell count could be observed upon MMC application.18 There are contradictory studies on the use of MMC and its cytotoxicity, which imply that MMC targets all three cellular layers of the cornea. However, irreversible damage may occur to the corneal endothelium due to its non-regenerative nature.19,20 Thus, the declination of the endothelial cell density/number has become a key concern in using MMC prophylactically to prevent subepithelial scarring during the refractive correction.19
In this study, we investigated the MMC-induced changes in corneal endothelium when used as prophylaxis to inhibit corneal haze formation following T-PRK-based refractive correction of low, moderate, and high myopia. The MMC treatment has been given according to the surface ablation depths of T-PRK.
Materials and Methods
The study was approved by the Committee of Research Ethics, Qassim University (Approval no.: 21–19-03). A signed informed consent about the risks and benefits of the surgery has been obtained from each patient before the surgical procedure. All tenets of the 1964 Helsinki declaration have strictly been followed at each stage of the study.
Study Design
This one-armed, retrospective cohort study recruited 120 eyes of 60 patients with all types of myopia: low myopia (SE (spherical equivalent) ≤ −3.00D), moderate myopia (SE > 3.00D to −6.00D), and high myopia (SE > −6.00 D). All patients underwent T-PRK surgery either with or without MMC treatment. The procedure was performed between 1 December 2020 and 30 February 2021 in a private ophthalmology center in Saudi Arabia. All patients were operated on single-handedly. Demographic variables of patients: age, gender, number of eyes per treatment group were documented (Table 1).
 |
Table 1 Distribution of Demographic Variables of the Study |
Inclusion Criteria
Patients enrolled in the study must be 18 years or older and followed up for at least 3 months after T-PRK. Patients with best-corrected distance visual acuity (BCDVA) 20/20 or greater for both eyes for at least a year prior to the surgery and an estimated postoperative residual stromal corneal bed of thickness >350 μm at the thinnest location were included. The use of contact lenses was discontinued for at least 2–4 weeks prior to preoperative examination.
Exclusion Criteria
Patients with a history of severe eye trauma, history of ocular surgery, corneal dystrophy, retinal disease or any other active ocular diseases, irregular astigmatism or suspected keratoconus, severe dry eye (Schirmer test result <4.0 mm) were excluded. In addition, patients with systemic ailments such as diabetes mellitus, autoimmune diseases, and pregnant or lactating women were also excluded.
Preoperative and Postoperative Assessments
All patients had undergone complete eye examination prior to surgery. Both the uncorrected distance visual acuity (UCDVA) and best-corrected distance visual acuity (BCDVA) were documented. The corneal topography was evaluated both preoperatively and postoperatively at 3 months after surgery using a Pentacam camera (OCULUS-Netzteil Art., Pentacam HR, Germany) and Sirius (SCHWIND eye-tech-solutions, GmbH, Kleinostheim, Germany) to obtain the measurements of keratometry (K1 and K2), central corneal thickness (CCT) and pupillary diameter. Spherical, cylindrical, and spherical equivalent (SE) refractive power in a diopter (D) were documented for each eye. The logarithm of the minimum angle of resolution (LogMAR) notations was recorded for both uncorrected and best-corrected visual acuity. Slit-lamp examination was performed to examine different parts of the eye.
The corneal endothelial cell count was measured using a non-contact specular microscopy (Tomey, EM-3000, Japan) pre- and post-operatively. Endothelial cell count was made using non-contact examination, auto-alignment and auto-measurement mode. The central fixation method was used for the measurement and automatic-analysis of endothelial count was done with Trace method. This auto-alignment technology gave reproducibility of measured area and analyzed values. Sixteen images were captured automatically, and the best endothelial image was taken for the analysis. Trace Method was applied to measure the endothelial parameters such as number of cells (NUM), Cell density (CD) in cells/mm2, Average (AVG) in μm2, Standard Deviation (SD) in μm2, Co-efficient of Variation (CV) in %, Maximum (Max), Minimum (Min), Hexagonality (6A) in % and Central corneal thickness (CCT) in µm. Specular microscopy was performed preoperatively and postoperatively at 1 week and 3 months after surgery.
Surgical Method and Treatment Groups
Bilateral T-PRK was performed on all enrolled patients (120 eyes). The surface ablations were performed using an Amaris 500 Hz excimer laser (SCHWIND eye-tech-solutions, GmbH, Kleinostheim, Germany). Prior to laser ablation, the machine was kept on aberration-free mode, with a 6.5–7.5 mm treatment zone according to the pupillary diameter to prevent uneven ablation. The treatment goal was to retain emmetropia. The application of MMC was chosen based on the surface ablation depth during T-PRK surgery. Generally, an ablation depth of 50–75 µm has been considered a safer cutoff for MMC use in conventional PRK surgeries.3,12,21 However, we have chosen a total ablation depth of 100 µm as a cutoff in this study, as the T-PRK procedure ablates both corneal epithelium and stroma.22,23 Three treatment groups were made based on the above criteria; (1) Group A – low myopia without MMC treatment (ablation depth <100 µm), (2) Group B – low myopia with MMC treatment (ablation depth ≥100 µm) and (3) Group C – moderate and high myopia with MMC treatment (ablation depth ≥100 µm).
For Group A, the cornea was irrigated with a 30 mL balanced salt solution (BSS) im-mediately after ablation. For Group B and C, a prepared sponge soaked with 0.02% MMC (0.2 mg/mL, diluted in BSS) was coated evenly on the ablated cornea using forceps for 30–50 seconds (s). A timer was set by the surgical assistant for MMC application on the residual stromal bed. After application, the cornea was copiously irrigated with BSS. Subsequently, one drop of 0.3% of ofloxacin was instilled, and a soft bandage contact lens (PureVision, Bausch and Lomb) was placed on the cornea. All the operated eyes received 0.3% topical ofloxacin four times daily until the removal of the contact lens, 0.1% dexamethasone drops four times daily and then slowly tapered over 6–8 weeks, and artificial tears four times daily for 3 months.
Statistical Analysis
A pretested data collection form obtained from both patients’ records and different equipment’s software files was used to collect the data for the study. The obtained data from patients were compiled, and a graph was made using Microsoft Excel 2010 and then exported to the data editor page of IBM SPSS version 22.0 (SPSS Inc., Chicago, Illinois, USA). Descriptive statistics like frequency distribution, mean and standard deviations (SD) were calculated. Paired t-test and repeated measures analysis of variance (ANOVA) with post hoc test were performed to compare the paired quantitative variables. A Chi-square test was performed to compare the unpaired qualitative variables. For all tests, p≤ 0.05 was considered statistically significant.
Results
A total of 60 patients who underwent T-PRK bilaterally (n = 120) were included in the study. Among the study participants, 50% were 25 years or younger, followed by 36.6% and 13.3% belonged to 26–35 years and 36 years or older age groups, respectively. Among the study population, 51.7% were females and 48.3% were males. Among the total eyes involved in the study, 32.5% had low myopia and underwent T-PRK without mitomycin C (Group A), 24.2% with low (Group B) and 43.3% eyes with moderate or high (Group C) myopia had undergone T-PRK with mitomycin C, Table 1. The mean and standard deviation of the preoperative study variables such as cornea flat meridian curvature (Keratometry 1), cornea steep meridian curvature (Keratometry 2), and pupil diameter were listed for Group A, B, and C in Table 2 along with the surgical parameters such as surgical transitional zone, optical zone, total ablation zone, and ablation depth. The mean surface ablation depth for the T-PRK procedure was found to be higher in Group C (131.75 ± 17.23 µm) followed by Group B (108.72 ± 8.43 µm) and Group A (86.54 ± 6.67 µm).
 |
Table 2 Preoperative and Surgical Variables of the Studies |
The preoperative and postoperative assessments of the refractive outcomes after the T-PRK procedure among the three treatment groups are summarized in Table 3. At postoperative 3 months, a significant decrease in the refractive powers was noticed in the parameters (p < 0.05); mean sphere, mean cylinder, and mean spherical equivalent in all three groups. A higher mean difference in the refractive outcome was observed among Group C followed by Group A and Group B. An uncorrected visual acuity of 20/20 or better was found to be achieved in 100% of the eyes in Group A and B postoperatively. One participant in Group C achieved 20/25 after the treatment for corneal haze. Overall, a significant improvement in the visual acuity (p < 0.05) was found in low, moderate, and high myopic patients after T-PRK treated with both with and without MMC.
 |
Table 3 Comparison of Refractive Outcomes and Visual Acuity Before and After T-PRK |
A quantitative assessment of different indices of the corneal endothelium was performed preoperatively and postoperatively to critically evaluate the effect of MMC after T-PRK using specular microscopy, Table 4. A significantly high mean number of endothelial cells was found to be maintained in Group A followed by Group B and C at 1-week post-surgery. Although a significant decrease was evidenced temporarily in Group C at postoperative 1 week, the mean number of cells increased significantly at postoperative 3 months (p = 0.021). Thus, there was no difference noticed in the cell number between preoperative and postoperative 3 months. The mean endothelial cell density (CD) did not change significantly at postoperative 1 week in all three groups, but a significant decrease was noted at postoperative 3 months in Group B. However, no reduction in CD in Group A and Group C. (Figure 1) represents the graphical comparison of mean endothelial CD from all the treatment groups.
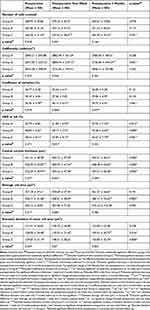 |
Table 4 Differences in the Corneal Endothelial Parameters in Different Treatment Groups |
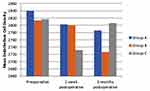 |
Figure 1 Graph representing comparative mean corneal endothelial cell density in myopic patients among different treatment groups with and without MMC during T-PRK. |
The specular microscopic results depict no significance between preoperative and postoperative endothelial cell density in Group A and Group C but in Group B. Group A – Low myopia without MMC treatment (n = 39); Group B – Low myopia with MMC treatment (n = 29) and Group C – Moderate and high myopia with MMC treatment (n = 52); where n refers to the number of eyes distributed into three groups according to their respective conditions and treatment.
In addition, the variation in the endothelial cell size was found to be increased significantly as the coefficient of variation (CV) increased at both 1 week and 3 months follow-ups in Group C (p = 0.001) denoting polymegathism. A significantly high mean CV value was noticed in Group C, followed by Group B and A during both 1 week (p = 0.006) and 3 months (p = 0.047) follow-ups. Similarly, a significant increase in mean average cell area (AVG) denoting polymegathism was observed only in Group B between preoperative to postoperative 1 week and 3 months follow-ups. However, no difference in AVG among the other two treatment groups. Accordingly, a significant increase in the standard deviation of mean cell area (SD) was observed in Group B and also in Group C signifying much of a deviation in the mean cell area between preoperative and postoperative follow-ups.
In concurrence with the variation in the size of the endothelial cells, a significant decrease in the mean number of hexagonal-shaped endothelial cells (HEX/6A) signifying pleomorphism was found in Group C, followed by B and A at postoperative 1 week (p = 0.031). Furthermore, a significant loss in the hexagonal cell shape was noticed in all the three treatment groups from one week to 3 months postoperatively. This difference was also seen in comparison with preoperative data in Group B and C. A significant reduction in the mean central corneal thickness (CCT) which is expected post-surgery was noticed in Group C followed by Group B and A during both the follow-ups.
Cumulatively, Group A showed no significant changes in the endothelial indices except reduced hexagonality and central corneal thickness. A significant reduction in the cell density was noticed in Group B along with a reduction in hexagonality, CCT, and an increase in AVG and SD of cell area. No significant changes in cell number or density but variation in size, shape, and SD of cell area were noticed in Group C postoperatively. The CCT has also been found significantly reduced.
In our study, only one patient was found to have developed postoperative corneal haze in Group C with moderate/high myopia despite the MMC treatment during T-PRK. This patient has been treated with extended dexamethasone drops for 4 weeks more.
Discussion
This is the first clinical study that addresses the effect of MMC on corneal endothelium post-T-PRK from low- to high-order myopia to the best of our knowledge. In this study, the MMC treatment was selectively given based on the ablation depth required during T-PRK to correct myopia. This has been an important criterion to limit the unnecessary exposure of MMC. Previous studies emphasize MMC concentration and duration to prevent any toxic effect on corneal endothelium.12,20,24 Although the ablations were deeper for the moderate/high myopic group using T-PRK (131.75 ± 17.23 µm) in this study, the duration of MMC exposure has been limited to 30–50 s to prevent any consequences. We used the standard dose of 0.02% MMC as this concentration has been found to be safe and effective in minimizing corneal haze and accelerating visual outcome in PRK.25
Generally, the incidence of corneal haze is common among high order refractive corrections due to deeper ablations.23 However, the T-PRK procedure itself reduces haze formation as it is gentle and aberration-free.23 Furthermore, the combined effect of MMC has resulted in significant prevention of corneal haze in 119 out of 120 eyes including the high-order myopic subjects used in the study.
The current study shows the detailed evaluation of the corneal endothelial indices after the intraoperative MMC application during T-PRK in low, moderate, and high myopia. The low myopic group without MMC treatment has shown no differences in the endothelial cell number or density. This could be due to the shallow ablation depths required for T-PRK along with no MMC interference. The MMC application did not show any significant effect on endothelial cell number or density in the moderate/high myopic group, although a transient decrease in number was noticed at 1-week post-surgery. However, a statistically significant reduction in cell density has been noticed without a significant change in the cell number in the low myopic group with MMC. The effects confirm the usage of MMC along T-PRK as a healing modulator and not getting affected negatively. Also, the transient decrease in the number of cells can relate to the use of MMC according to optimal dose and its respective time.26,27 The mean CD has changed from 2813.00 ± 235.53/mm2 preoperatively to 2726.38 ± 244.24/mm2 at postoperative 3 months. This reduction in CD could be due to the outliers in the preoperative endothelial CD measurements. Nevertheless, this minimal loss does not account for clinical significance.28–30
Previous studies show significant pleomorphism and polymegathism in the early postoperative period after myopic PRK with regard to MMC.29,31 However, changes in the hexagonality of endothelial cells were noticed in both treatment groups with and without MMC. Thus, we can speculate that these morphological changes are not related to the application of MMC.
The mean postoperative CCT was found to be significantly highest among Group A (492.51 ± 30.41 µm) followed by Group B (468.48 ± 43.63 µm) and Group C (429.31 ± 36.46 µm). This could be explained by the corresponding ablation depths used in the respective treatment groups. Although statistically significant, the CCT was not critically low for clinical relevance. Moreover, the residual corneal thickness >300 µm was found to be enough to protect the underlying endothelium.20 This finding was consistent with the previous studies using MMC in different refractive correction surgeries.32
Although contradictory findings were observed with respect to significant changes in the different corneal endothelial indices and MMC exposure and application in the literature,20,33 similar prospective studies investigating the effect of MMC on corneal endothelium post-refractive correction reveal no differences in the endothelial indices after 6–12 months postoperatively.20,25 This suggests that we may need longer follow-ups to investigate the long-term safety and efficacy of MMC on corneal endothelium after T-PRK. Apart from the short follow-up, this being a retrospective study with a small number of eyes studied in Group B compared with other groups is another limitation. In addition, the duration of the MMC application was kept constant for all groups irrespective of the severity of myopia. A change in the duration of MMC treatment according to the severity of myopia might have helped to limit any unnecessary exposure in the future.
Despite these limitations, this study holds many strong points. Apart from being the first clinical study investigating MMC-induced changes in corneal endothelium after T-PRK for various degrees of myopia, the study design allows us to assess the effect of MMC on endothelium in different groups according to ablation depth during T-PRK and the severity of myopia. Moreover, the endothelial indices have thoroughly been compared between the early and late follow-up periods.
Conclusions
The current study demonstrates the safety and efficacy of MMC application in reducing corneal haze after T-PRK in low- to high-order myopia. The MMC does not affect the corneal endothelial number or density, especially in moderate and high myopic T-PRK. There were slight differences noted in certain parameters relating to endothelial changes, which were of less clinical significance. Despite the contradictions in results and certain limitations in the study, it can be deduced that this study allows further investigation on long-term effects of MMC on endothelium after T-PRK with the severity of myopia.
Ethics
The study was conducted in accordance with the Declaration of Helsinki and approved by the Committee of Research Ethics, Qassim University (Approval number: 21-19-03). Informed consent was obtained from all subjects involved in the study. The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Disclosure
The author declares that there are no conflicts of interest in this work.
References
1. Hashemi H, Fotouhi A, Yekta A, Pakzad R, Ostadimoghaddam H, Khabazkhoob M. Global and regional estimates of prevalence of refractive errors: systematic review and meta-analysis. J Curr Ophthalmol. 2018;30(1):3–22. doi:10.1016/j.joco.2017.08.009
2. Kuryan J, Cheema A, Chuck R. Laser-assisted subepithelial keratectomy (LASEK) versus laser-assisted in-situ keratomileusis (LASIK) for correcting myopia. Cochrane Database Syst Rev. 2017;2:CD011080. doi:10.1002/14651858.CD011080.pub2
3. Naderi M, Ghadamgahi S, Jadidi K. Photorefractive Keratectomy (PRK) is safe and effective for patients with myopia and thin corneas. Med Hypothesis Discov Innov Ophthalmol. 2016;5(2):58–62.
4. Azar DT, Chang JH, Han KY. Wound healing after keratorefractive surgery: review of biological and optical considerations. Cornea. 2012;31(01):S9–S19. doi:10.1097/ICO.0b013e31826ab0a7
5. Hazarbassanov R, Ben-Haim O, Varssano D, Grinbaum A, Kaiserman I. Alcohol- vs hypertonic saline-assisted la-ser-assisted subepithelial keratectomy. Arch Ophthalmol. 2005;123(2):171–176. doi:10.1001/archopht.123.2.171
6. Wilson SE, Medeiros FW. CHAPTER 7 - refractive surgery – corneal opacity (Haze) after surface ablation. In: Yorio T, Clark AF, Wax MB, editors. Ocular Therapeutics. Academic Press; 2008:133–141. doi:10.1001/archopht.123.2.171
7. Tomas-Juan J, Larranaga AMG, Hanneken L. Corneal regeneration after photorefractive keratectomy: a review. J Optom. 2015;8(3):149–169. doi:10.1016/j.optom.2014.09.001
8. Bakhsh AM, Elwan SA, Chaudhry AA, El-Atris TM, Al-Howish TM. Comparison between transepithelial photorefractive keratectomy versus alcohol-assisted photorefractive keratectomy in correction of myopia and myopic astigmatism. J Ophthalmol. 2018;2018:5376235. doi:10.1155/2018/5376235
9. Kaluzny BJ, Cieslinska I, Mosquera SA, Verma S. Single-step transepithelial PRK vs alcohol-assisted PRK in myopia and compound myopic astigmatism correction. Medicine. 2016;95(6):e1993. doi:10.1097/md.0000000000001993
10. Naderi M, Jadidi K, Mosavi SA, Daneshi SA. Transepithelial photorefractive keratectomy for low to moderate myopia in comparison with conventional photorefractive keratectomy. J Ophthalmic Vis Res. 2016;11(4):358–362. doi:10.4103/2008-322X.194070
11. Alasmari M, Alfawaz A. Transepithelial photorefractive keratectomy to treat mild myopia. Int Ophthalmol. 2021;41(7):2575–2583. doi:10.1007/s10792-021-01816-y
12. Arranz-Marquez E, Katsanos A, Kozobolis VP, Konstas AG, Teus MA. A critical overview of the biological effects of mitomycin c application on the cornea following refractive surgery. Adv Ther. 2019;36(4):786–797. doi:10.1007/s12325-019-00905-w
13. Teus MA, de Benito-Llopis L, Alio JL. Mitomycin C in corneal refractive surgery. Surv Ophthalmol. 2009;54(4):487–502. doi:10.1016/j.survophthal.2009.04.002
14. Hashemi H, Taheri SMR, Fotouhi A, Kheiltash A. Evaluation of the prophylactic use of mitomycin-C to inhibit haze formation after photorefractive keratectomy in high myopia: a prospective clinical study. BMC Ophthalmol. 2004;4(1):12. doi:10.1186/1471-2415-4-12
15. Hofmeister EM, Bishop FM, Kaupp SE, Schallhorn SC. Randomized dose-response analysis of mitomycin-C to prevent haze after photorefractive keratectomy for high myopia. J Cataract Refract Surg. 2013;39(9):1358–1365. doi:10.1016/j.jcrs.2013.03.029
16. Song JS, Kim JH, Yang M, Sul D, Kim HM. Mitomycin-C concentration in cornea and aqueous humor and apoptosis in the stroma after topical mitomycin-C application: effects of mitomycin-C application time and concentration. Cornea. 2007;26(4):461–467. doi:10.1097/ICO.0b013e318030d217
17. Mohammad-Rabei H, Moravej R, Almasi-Nasrabadi M. Effect of mitomycin-C on corneal endothelial cell parameters after refractive surface ablation procedures. Med Hyp Disc Innov Ophthalmol. 2021;10(4):156–164. doi:10.51329/mehdiophthal1434
18. Ouerdane Y, Zaazouee M, Mohamed MA. Mitomycin C application after photorefractive keratectomy in high, moderate, or low myopia: systematic review and meta-analysis. Indian J Ophthalmol. 2021;69(12):3421. doi:10.4103/ijo.IJO_3768_20
19. Nassiri N, Farahangiz S, Rahnavardi M, Rahmani L, Nassiri N. Corneal endothelial cell injury induced by mitomycin-C in photorefractive keratectomy: nonrandomized controlled trial. J Cataract Refract Surg. 2008;34(6):902–908. doi:10.1016/j.jcrs.2008.03.007
20. Zare M, Jafarinasab MR, Feizi S, Zamani M. The effect of mitomycin-C on corneal endothelial cells after photorefractive keratectomy. J Ophthalmic Vis Res. 2011;6(1):8–12.
21. Lacayo GO, Majmudar PA. How and when to use mitomycin-C in refractive surgery. Curr Opin Ophthalmol. 2005;16(4):256–259. doi:10.1097/01.icu.0000172830.41394.7c
22. Lu NJ, Koppen C, Awwad S, Aslanides MI, Aslanides IM, Chen SH. Effect of intraoperative mitomycin-C application on epithelial regeneration after transepithelial photorefractive keratectomy. J Cataract Refract Surg. 2021;47(2):227–232. doi:10.1097/j.jcrs.0000000000000427
23. Aslanides IM, Georgoudis PN, Selimis VD, Mukherjee AN. Single-step transepithelial ASLA (SCHWIND) with mito-mycin-C for the correction of high myopia: long term follow-up. Clin Ophthalmol. 2015;9:33–41. doi:10.2147/OPTH.S73424
24. Thornton I, Puri A, Xu M, Krueger RR. Low-dose mitomycin C as a prophylaxis for corneal haze in myopic surface ablation. Am J Ophthalmol. 2007;144(5):673–681. doi:10.1016/j.ajo.2007.07.020
25. Almosa AA, Fawzy SM. Effect of mitomycin C on myopic versus astigmatic photorefractive keratectomy. J Ophthalmol. 2017;2017:2841408. doi:10.1155/2017/2841408
26. Hassan Mohammad H, Mohamed Tag El-Din AE-M, Al-Sheikh M. Postoperative corneal haze after transepithelial photorefractive keratectomy (TRANS PRK) versus conventional photorefractive keratectomy (PRK). Al-Azhar Med J. 2020;49(3):979–986. doi:10.21608/amj.2020.91622
27. Ellakwa AF, Badawy NM, Mohamad IF. Evaluation of corneal haze after transepithelial photorefractive keratectomy. Menoufia Med J. 2020;33(4):1201. doi:10.4103/mmj.mmj_121_20
28. Özbilen KT, Altinkurt E, Ceylan NA, Bilgin GS, Gözüm N. Effect of myopic femtosecond laser-assisted lasik on an-terior chamber inflammation (Flare Values) and corneal endothelium: a prospective before and after study. J Ophthalmol. 2021;2021:2395028. doi:10.1155/2021/2395028
29. Adel Refai T, Hassanin O. Evaluation of endothelial function after using high concentration and prolonged exposure of mitomycin C following photorefractive keratectomy for high myopia and astigmatism. J Ophthalmol Relat Sci. 2020;4(1):11–20. doi:10.1016/j.ajo.2007.07.020
30. Al Samak AM, Shehab SY, Al Ameedee H. The usage of Mitomycin-C with photorefractive keratectomy in myopic patients and its effect on density of corneal endothelium. Eur J Mol Clin Med. 2020;7(8):928–934.
31. Gharaee H, Zarei-Ghanavati S, Alizadeh R, Abrishami M. Endothelial cell changes after photorefractive keratectomy with graded usage of mitomycin C. Int Ophthalmol. 2018;38(3):1211–1217. doi:10.1007/s10792-017-0584-5
32. Kim BK, Mun SJ, Yang YH, Kim JS, Moon JH, Chung YT. Comparison of anterior segment changes after femtosecond laser LASIK and SMILE using a dual rotating Scheimpflug analyzer. BMC Ophthalmol. 2019;19(1):251. doi:10.1186/s12886-019-1257-0
33. Neamah GAT, Ameedee HA, Samak AMA, Kareem AA. The effect of mitomycin-c on corneal endothelial cell count after photorefractive keratectomy. J Nat Sci Res. 2017;7(24):73–77.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
