Back to Journals » Drug Design, Development and Therapy » Volume 18
Effect of Perioperative Nicorandil on Myocardial Protection in Patients Undergoing Cardiac Surgery with Cardiopulmonary Bypass, a Retrospective Study
Authors Chen Y, Liu Y , Lv H , Li Q, Shen J, Chen W, Shi J, Zhou C
Received 30 August 2023
Accepted for publication 15 January 2024
Published 31 January 2024 Volume 2024:18 Pages 223—231
DOI https://doi.org/10.2147/DDDT.S437801
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Georgios Panos
Yuye Chen,1 Yue Liu,2 Hong Lv,1 Qian Li,1 Jingjia Shen,1 Weiyun Chen,2 Jia Shi,1 Chenghui Zhou3
1Department of Anesthesiology, Fuwai Hospital, Chinese Academy of Medical Sciences&Peking Union Medical College/National Center for Cardiovascular Diseases, Beijing, 100037, People’s Republic of China; 2Department of Anesthesiology, Peking Union Medical College Hospital, Beijing, 100730, People’s Republic of China; 3Center for Anesthesiology, Beijing Anzhen Hospital, Capital Medical University, Beijing, 100029, People’s Republic of China
Correspondence: Chenghui Zhou, Center for Anesthesiology, Beijing Anzhen Hospital, Capital Medical University, No. 2 Anzhen Road, Chaoyang District, Beijing, 100029, People’s Republic of China, Tel +86-137-1889-4919, Email [email protected] Jia Shi, Department of Anesthesiology, Fuwai Hospital, Chinese Academy of Medical Sciences&Peking Union Medical College/National Center for Cardiovascular Diseases, No. 167 North Lishi Road, Xicheng District, Beijing, 100037, People’s Republic of China, Email [email protected]
Background: The potential myocardial protective effect of nicorandil (NICD) in patients undergoing percutaneous coronary intervention has been established. However, its efficacy in the context of cardiac surgery remains controversial. The present study aimed to evaluate the myocardial protective effect of perioperative NICD use in patients undergoing cardiac surgery with cardiopulmonary bypass (CPB).
Methods: We retrospectively gathered data from patients undergoing cardiac bypass surgery between 12/2018 and 04/2021 in Fuwai Hospital. Subsequently, the patients were divided into two groups, NICD group and non-nicorandil (non-NICD) group. A 1, 3 propensity score matching (PSM) was conducted. The primary outcome was the incidence of myocardial injury. The secondary outcomes included the mechanical ventilation (MV) duration, intensive care unit (ICU) length of stay (LOS), hospital LOS, duration of chest drainage, the drainage volume, the total cost, the incidence of acute kidney injury (AKI), and the incidence of acute liver injury (ALI). Subsequently, we divided the entire population into two distinct subgroups based on their administration of NICD, and performed a comprehensive subgroup analysis.
Results: A total of 2406 patients were ultimately included in the study. After PSM, 250 patients in NICD group and 750 patients in non-NICD group were included in the analysis. Perioperative NICD reduced the incidence of myocardial injury (47.2% versus 38.8%, P=0.025). Our subgroup analysis revealed that preoperative NICD administration not only provided myocardial protection benefits (45.7% vs 35.8%, OR 0.66, 95% CI [0.45– 0.97], P=0.041), but also demonstrated statistically significant reduction in ALI, the ICU and hospital LOS, and the duration of chest drainage (all P< 0.05).
Conclusion: The perioperative NICD administration may confer myocardial protection in patients undergoing cardiac surgery with CPB. Furthermore, the preoperative utilization of NICD has the potential to mitigate the incidence of postoperative ALI, a reduction in the ICU and hospital LOS, and the duration of chest drainage.
Keywords: nicorandil, cardiopulmonary bypass, perioperative myocardial injury, acute kidney injury, acute liver injury
Background
Perioperative myocardial injury (PMI) is a significant and prevalent complication across various cardiac surgical procedures.1–3The etiology of perioperative myocardial injury is multifactorial.3,4 Myocardial ischemia-reperfusion injury (I/RI) has been recognized as one of the major contributing mechanisms.2 Despite the various preventive measures in clinical practice including hypothermia cardioplegia, limited CPB duration, and selected anesthetic usage,4–7 the incidence of PMI remains high. Consequently, seeking a novel myocardial protective drug is imperatively needed.
Not only acting as a nitrate analogue, NICD could also function as an agent that opens ATP-sensitive potassium channels. Its structure incorporates both organic nitrate and nicotinamide groups, which confer upon it additional properties as a nitric oxide (NO) donor and antioxidant.8 Thus, NICD induces vasodilation, reduces blood pressure, and exerts cardioprotective effects. These mechanisms elucidate the role of NICD as a safeguard against myocardial damage.
Previous researches have indicated that NICD is purported to induce coronary artery relaxation, prevent coronary spasm, and protect the myocardium.9–11 It has been extensively investigated and utilized in percutaneous coronary intervention (PCI), as evidenced by providing cardioprotection and renoprotection, and improved cardiovascular mortality.12–14 Nevertheless, as a potential cardioprotective drug, the evidence in cardiac surgery is insufficient.15
The objective of this study was to evaluate the myocardial protective effect of perioperative NICD during cardiac bypass surgery.
Methods
This study was a retrospective, single-center study conducted on patients undergoing cardiac surgery with CPB at Fuwai Hospital between 12/2018 and 04/2021. Since this article was a retrospective study, the data was collected retrospectively without any intervention or observation, guaranteeing the absence of harm on the patients. This study was conducted in compliance with the Declaration of Helsinki’s ethical principles. Thereby the Ethics Committee in Fuwai Hospital approved to waive informed consent for patients. During the process of data analysis and processing, strict measures were implemented to ensure the preservation of patient data confidentiality.
The primary inclusion criteria were patients aged 18–70 years who had undergone elective cardiac bypass surgery receiving NICD before or within 48 hours after surgery. Patients with incomplete data were excluded. Surgical procedures, anesthesia, CPB procedures, and postoperative management were all standardized. The timing of NICD administration was recorded.
The study designates the cohort that received NICD either before or within 48 hours following surgery as the NICD group, while the remaining people is referred to as the non-NICD group.
The primary outcome was the incidence of myocardial injury, defined as peak hsTnI levels exceeding 40 times the upper limit of normal within 48 hours post-surgery.16 Secondary outcomes included the MV duration, ICU LOS, the hospital LOS, duration of chest drainage, the drainage volume, the total cost, and the incidence of AKI and ALI. AKI was defined as stage 2 or higher level according to the KDIGO standard.17 The ALI was defined as serum levels of aspartate transferase or alanine transferase exceeding five times the upper limit of normal (with a normal range of 0 to 40 U/l for aspartate transferase and 0 to 50 U/l for alanine transferase) or total serum bilirubin levels of 5 mg/dl or higher within a seven-day postoperative period.18
We used propensity score matching (PSM) to adjust patient baseline characteristics, in order to evaluate the effect of perioperative NICD administration on the study outcomes. The nearest neighbor algorithm was utilized in PSM, with a 1, 3 matching ratio and a caliper distance of 0.2, to mitigate the confounding effects between the two groups. The selected covariates included gender, age, height, weight, baseline heart rate, preoperative NYHA classification, left ventricular ejection fraction, preoperative NT-proBNP, systolic blood pressure, history of diabetes, history of smoking, history of hypertension, and history of stroke, preoperative routine blood test, liver and kidney function, and coagulation status.
In order to gain a comprehensive role of the optimal timing for administering NICD, this study conducted a subgroup analysis by categorizing patients based on whether they received the medication before or after cardiac surgery. The subgroup analysis used a matched database of 1000 people and excluded patients who received NICD after surgery when analyzing preoperative patients. Additionally, a 1, 3 PSM was conducted to ensure consistent performance at baseline characteristics. The postoperative utilization of NICD was analyzed using a similar way.
Within the matching queue, the representation of continuous variables was achieved through the utilization of mean and standard deviation, while categorical variables were represented through counts and percentages (%). The statistical measurement of continuous variables was conducted through the application of Student’s t-test while the categorical variables were accomplished through the implementation of either the Chi-square test or the Fisher exact test.
In our study, R (software version 4.2.0R Foundation for Statistical Computing) was employed for data analysis. The “MatchIt” package was utilized for propensity score matching, while the “compareGroups” package was used for statistical output. Statistical significance was determined by two-sided P < 0.05.
Results
Figure 1 illustrates the retrospective collection of data from 2510 patients undergoing cardiac surgery at Fuwai Hospital between 12/2018 and 04/2021. After the exclusion of 104 cases with missing data, 2406 cases were ultimately included in the analysis. The mean age of the patients was 52.3±12.0 years, with 1512 (62.8%) males and 894 (37.2%) females. The overall incidence of PMI was found to be 50.3%. Among the patients, 250 received NICD perioperatively, with 151 before surgery and 99 within 48 hours after surgery.
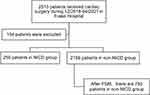 |
Figure 1 Patient flow chart. Abbreviations: PSM, propensity score matching; NICD, Nicorandil. |
Table 1 displays the patient baseline characteristics before and after matching. Before matching, the baseline of patient characteristics exhibited a notable degree of heterogeneity. However, after controlling for covariates such as age, gender, height, weight, baseline heart rate, preoperative NYHA classification, left ventricular ejection fraction, preoperative NT-proBNP, systolic blood pressure, history of diabetes, history of smoking, history of hypertension, history of stroke, preoperative routine blood routine, liver and kidney function, and coagulation, no significant difference in patient baseline characteristics was observed between the two groups. Simultaneously, to make full use of the amassed data and prevent information loss, we opted to conduct a 1, 3 matching. To evaluate the efficacy of the matching, we computed the standardized mean difference (SMD) of the covariates before and after matching. As depicted in Figure 2, the SMD of the aforementioned covariates is below 0.1 after matching, signifying a satisfactory outcome.19
 |
Table 1 Baseline Characteristics Before and After Propensity Score Matching |
Among the observed outcomes, NICD significantly reduced the incidence of PMI (47.2% vs 38.8%, P=0.025). The odds ratio (OR) between the two groups was 0.71[95% confidence interval (CI), 0.53–0.95]. However, no significant differences were observed between the two groups in the secondary outcomes.
Detailed results of the primary and secondary outcomes are presented in Table 2.
 |
Table 2 Propensity Score-Matched Analysis of Primary Outcome and Secondary Outcomes |
Subgroup analysis revealed that preoperative NICD not only provided myocardial protection (45.7% vs 35.8%, OR 0.66, 95% CI[0.45–0.97], P=0.041), but also reduced the risk of ALI (8.83% vs 3.31%, OR0.36, 95% CI [0.12–0.86], P=0.04). Similar results were also observed in hospital LOS (days) (8.38 ±3.35 vs 7.77 ±2.28, OR0.93, 95% CI [0.86–1.00], P=0.012), ICU LOS (days) (2.59±2.08 vs 2.28±1.50, OR 0.91, 95% CI[0.81–1.02], P=0.049), drainage duration (days) (4.25±2.51 vs 3.69±1.65, OR0.87, 95% CI[0.79–0.97], P=0.002). Meanwhile, our study did not observe a statistically significant difference in AKI between the two groups (2.87% vs 0.66%, P=0.207). Furthermore, no significant difference in outcomes was detected in the subset that received NICD post-surgery. These findings of the subgroup analysis are presented in Tables 3 and 4.
 |
Table 3 PSM Analysis of Primary Outcome and Secondary Outcomes When Using Nicorandil Preoperatively |
 |
Table 4 PSM Analysis of Primary Outcome and Secondary Outcomes When Using Nicorandil Postoperatively |
Discussion
The findings of this study exhibit a myocardial protective effect of perioperative NICD administration in Chinese population undergoing cardiac bypass surgery. Nevertheless, with regard to the secondary outcomes, the utilization of NICD during the perioperative period did not yield a statistically significant difference. In recent years, an increasing recognition of PMI following cardiac procedures has emerged. The myocardial protection during such operations have become a subject of interest to a growing number of medical researchers. Currently, various researches about NICD have been focused on PCI, and a proved cardioprotection by NICD has been evidenced.12–14,20–22 However, only several related researches with small sample size have been conducted in cardiac surgery. The previous prospective randomized clinical study conducted in Japan discovered that patients who received NICD during coronary artery bypass graft surgery exhibited lower TnT concentrations.15 Conversely, a separate study involving individuals at high risk for coronary heart disease who underwent non-cardiac surgery revealed that NICD did not decrease 30-day mortality or adverse cardiovascular events.23 Consequently, the efficacy of NICD in surgical procedures remains a topic of debate.
There has always been a concern whether the NICD administrative timing affect the clinical benefits for patients. Previous research has shown that both preoperative and intraoperative administration of NICD could have a positive impact on patient outcomes.23–25 In our study, perioperative use of NICD has shown to exert a myocardial protective effect indeed. In addition, the subgroup analysis with preoperative NICD showed a new role of NICD in liver protection. However, no significant difference in liver protection for these patients who received NICD post-surgery, suggesting a better preventive effect in preoperative usage. This is consistent with the results of the previous small sample-sized prospective cardiac surgery study. The study by Yamamoto, S. et al actually applied NICD after induction, that is, before it struck the heart muscle.15 Hence, the observed cardioprotective impact of NICD implies that its prophylactic administration may yield superior outcomes.
The liver protective effect is noteworthy. Previous animal studies have demonstrated that NICD elevates cGMP levels in rat liver, aorta, and human coronary artery smooth muscle cells in vitro.26 Additionally, a study conducted on pigs revealed that NICD augmented hepatic blood flow and mitigated liver ischemia-reperfusion injury.27 Another animal study has demonstrated that continuous NICD infusion could prevent the progression of liver fibrosis.28 Consequently, it is presumed that NICD’s ability to relax hepatic blood vessels may augment hepatic blood flow, enhance hepatic metabolic capacity, leading to confer a degree of hepatoprotection. Notably, no clinical literature has reported on the hepatoprotective effects of NICD on human body.
In fact, in the investigation of NICD’s organ protective effects, beyond the extensively studied AKI and ALI posited in this research, there exist additional studies that demonstrate the potential for NICD to protect the lungs and brain.29,30 Animal experiments have revealed that NICD could mitigate ischemic reperfusion injury in isolated rat lungs. In addition, another clinical study has demonstrated that NICD could provide lung protection in patients undergoing thoracic surgery who require lung collapse techniques by inhibiting apoptosis. Additionally, an animal study have shown that the administration of NICD during rabbit CPB surgery exerts a protective influence on the heart, brain, and kidney.31 Take together, these findings collectively support the perspective that NICD possesses a multi-organ protective effect, which holds significant clinical relevance in the context of cardiac surgery involving CPB related multiple organ injury.
The findings of this study align with those of previous researches, indicating that NICD offers a degree of myocardial protection. However, this study distinguishes itself from previous investigations by virtue of larger sample size, more comprehensive data collection, and inclusion of a wider category of surgical procedures. Additionally, this study represents the first exploration of NICD’s efficacy in cardiac bypass surgery. The outcome suggests that NICD may be particularly valuable as a preoperative prophylactic measure and offer some degree of liver protection.
This study exhibits several limitations. Firstly, as a retrospective study, the data collection process is still flawed, resulting in the exclusion of numerous cases. Secondly, several data processing methods were utilized to enhance the reliability of the outcomes and the repeated utilization of PSM during the analysis stage to balance the baseline data, which may lead to missing data and selection bias. Thirdly, the study’s design did not consider the differences arising from the timing of NICD administration, thereby restricting the primary conclusion to the perioperative use of NICD providing myocardial protection only. Preoperative use of NICD may reflect a more obvious protective role of NICD in cardiac and liver tissue according to our subgroup analysis. Fourthly, our study also exhibits a deficiency in the exploration of drug administration mode and dosage, and diverse methods of oral administration, intravenous administration, and coronary perfusion may yield varying advantages. Optimizing the timing of NICD administration should be explored to determine the optimal therapeutic window for maximal benefit. Last but not least, the primary outcome of the study was the incidence of myocardial injury, whereas previous studies utilized myocardial perfusion imaging as an evaluative indicator,32 which appeared to be more objective than our definition of myocardial injury based solely on alterations in troponin levels.
Conclusions
Perioperative NICD has been shown to confer myocardial protection in patients undergoing cardiac bypass surgery. Furthermore, the preoperative administration of NICD has the potential to mitigate the incidence of postoperative liver injury and a reduction in the ICU and hospital LOS, and the duration of chest drainage.
Funding
This work was supported by the National Natural Science Foundation of China (No. 81970290).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Hövels-Gürich HH, Vazquez-Jimenez JF, Silvestri A, et al. Production of proinflammatory cytokines and myocardial dysfunction after arterial switch operation in neonates with transposition of the great arteries. J Thoracic Cardiovasc Surg. 2002;124(4):811–820. doi:10.1067/mtc.2002.122308
2. Bosnjak ZJ, Ge ZD. The application of remote ischemic conditioning in cardiac surgery. F1000Research. 2017;6:928. doi:10.12688/f1000research.11018.1
3. Ucak HA, Uncu H. Impact of coronary collateral circulation of perioperative myocardial damage in high-risk patients undergoing coronary artery bypass grafting surgery. Heart Surg Forum. 2019;22(5):E375–e9. doi:10.1532/hsf.2483
4. Wei M, Kuukasjarvi P, Laurikka J, et al. Cytokine responses and myocardial injury in coronary artery bypass grafting. Scand J Clin Lab Invest. 2001;61(2):161–166. doi:10.1080/00365510151097700
5. Nesher N, Zisman E, Wolf T, et al. Strict thermoregulation attenuates myocardial injury during coronary artery bypass graft surgery as reflected by reduced levels of cardiac-specific troponin I. Anesthesia Analg. 2003;96(2):328–335. doi:10.1213/00000539-200302000-00007
6. Uhlig C, Labus J. Volatile versus intravenous anesthetics in cardiac anesthesia, a narrative review. Curr Anesthesiol Rep. 2021;11(3):275–283. doi:10.1007/s40140-021-00466-1
7. Liu Y, Paterson M, Baumgardt SL, et al. Vascular endothelial growth factor regulation of endothelial nitric oxide synthase phosphorylation is involved in isoflurane cardiac preconditioning. Cardiovascul Res. 2019;115(1):168–178. doi:10.1093/cvr/cvy157
8. Chao HH, Hong HJ, Sung LC, Chen JJ, Cheng TH, Liu JC. Nicorandil attenuates cyclic strain-induced endothelin-1 expression via the induction of activating transcription factor 3 in human umbilical vein endothelial cells. Eur J Pharmacol. 2011;667(1–3):292–297. doi:10.1016/j.ejphar.2011.05.062
9. Camm AJ, Maltz MB. A controlled single-dose study of the efficacy, dose response and duration of action of nicorandil in angina pectoris. Am J Cardiol. 1989;63(21):61j–5j. doi:10.1016/0002-9149(89)90207-5
10. Sakata Y, Kodama K, Komamura K, et al. Salutary effect of adjunctive intracoronary nicorandil administration on restoration of myocardial blood flow and functional improvement in patients with acute myocardial infarction. Am Heart J. 1997;133(6):616–621. doi:10.1016/S0002-8703(97)70162-5
11. IONA Study Group. Effect of nicorandil on coronary events in patients with stable angina, the Impact Of Nicorandil in Angina (IONA) randomised trial. Lancet. 2002;359(9314):1269–1275. doi:10.1016/S0140-6736(02)08265-X
12. Wang ZQ, Chen MX, Liu DL, et al. The effect on myocardial perfusion and clinical outcome of intracoronary nicorandil injection prior to percutaneous coronary intervention in ST-segment elevation myocardial infarction. Zhonghua xin xue guan bing za zhi. 2017;45(1):26–33. doi:10.3760/cma.j.issn.0253-3758.2017.01.006
13. Nishimura M, Tokoro T, Nishida M, et al. Oral nicorandil to reduce cardiac death after coronary revascularization in hemodialysis patients, a randomized trial. Am J Kidney Dis. 2009;54(2):307–317. doi:10.1053/j.ajkd.2009.03.025
14. Umemura S, Nakamura S, Sugiura T, et al. Preservation of myocardial viability within the risk area by intravenous nicorandil before primary coronary intervention in patients with acute myocardial infarction. Nuclear med commun. 2008;29(11):956–962. doi:10.1097/MNM.0b013e32830fdde7
15. Yamamoto S, Yamada T, Kotake Y, Takeda J. Cardioprotective effects of nicorandil in patients undergoing on-pump coronary artery bypass surgery. J Cardiothor Vascul Anesth. 2008;22(4):548–553. doi:10.1053/j.jvca.2008.02.011
16. Mauermann E, Bolliger D, Fassl J, et al. Postoperative high-sensitivity troponin and its association with 30-day and 12-month, all-cause mortality in patients undergoing on-pump cardiac surgery. Anesthesia Analg. 2017;125(4):1110–1117. doi:10.1213/ANE.0000000000002023
17. Disease K; Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Polskie Archiwum Medycyny Wewn?trznej. 2013;120(7–8):300–306.
18. Majumder K, Spratt JR, Holley CT, et al. Impact of postoperative liver dysfunction on survival after left ventricular assist device implantation. Ann Thorac Surg. 2017;104(5):1556–1562. doi:10.1016/j.athoracsur.2017.04.048
19. Schober P, Vetter TR. Propensity score matching in observational research. Anesthesia Analg. 2020;130(6):1616–1617. doi:10.1213/ANE.0000000000004770
20. Kim JH, Jeong MH, Yun KH, et al. Myocardial protective effects of nicorandil during percutaneous coronary intervention in patients with unstable angina. Circ J. 2005;69(3):306–310. doi:10.1253/circj.69.306
21. Kim SJ, Kim W, Woo JS, et al. Effect of myocardial protection of intracoronary adenosine and nicorandil injection in patients undergoing non-urgent percutaneous coronary intervention, a randomized controlled trial. Int J Cardiol. 2012;158(1):88–92. doi:10.1016/j.ijcard.2011.01.011
22. Xu L, Wang L, Li K, Zhang Z, Sun H, Yang X. Nicorandil prior to primary percutaneous coronary intervention improves clinical outcomes in patients with acute myocardial infarction, a meta-analysis of randomized controlled trials. Drug Des Devel Ther. 2019;13:1389–1400. doi:10.2147/DDDT.S195918
23. Miyake K, Yoshida S, Seki T, Joo WJ, Takeuchi M, Kawakami K. Effectiveness of intraoperative nicorandil in patients with a history of ischemic heart disease undergoing high-risk noncardiac surgery, a retrospective cohort study. J Anesth. 2023;37(4):562–572. doi:10.1007/s00540-023-03204-5
24. Kaneko T, Hayashida M, Saito Y, Hikawa Y, Yasuda K. 虚血性心疾患危険因子を有する患者の開腹術におけるニコランジルの術中心筋虚血予防効果 [The effect of prophylactic nicorandil infusion to reduce intraoperative myocardial ischemia during abdominal surgery in patients with risk factors of ischemic heart disease]. Masui Japan J Anesthesiol. 2000;49(1):54–59. Japanese.
25. Blanc P, Aouifi A, Bouvier H, et al. Safety of oral nicorandil before coronary artery bypass graft surgery. Br J Anaesth. 2001;87(6):848–854. doi:10.1093/bja/87.6.848
26. Minamiyama Y, Takemura S, Hai S, Suehiro S, Okada S, Funae Y. Nicorandil elevates tissue cGMP levels in a nitric-oxide-independent manner. J Pharmacol Sci. 2007;103(1):33–39. doi:10.1254/jphs.FP0061003
27. Yamazaki H, Oshima K, Sato H, et al. The effect of nicorandil on ischemia-reperfusion injury in a porcine total hepatic vascular exclusion model. J Surg Res. 2011;167(1):49–55. doi:10.1016/j.jss.2009.09.049
28. Mohamed YS, Ahmed LA, Salem HA, Agha AM. Role of nitric oxide and KATP channel in the protective effect mediated by nicorandil in bile duct ligation-induced liver fibrosis in rats. Biochem. Pharmacol. 2018;151:135–142. doi:10.1016/j.bcp.2018.03.003
29. Abe K, Horiguchi T, Enzan K, Masaki Y, Nishikawa T, Kimura T. Nicorandil, a K(ATP) channel opener, attenuates ischemia-reperfusion injury in isolated rat lungs. Lung. 2020;198(2):315–321. doi:10.1007/s00408-020-00339-0
30. Wang C, Wu Z, Li Z, Wang Z, Ke H, Huang X. Beneficial effect of the mitochondrial ATP‑sensitive potassium channel‑specific opener nicorandil on the collapsed lung via inhibition of apoptosis in clinical thoracic surgery. Mole Med Rep. 2023;27(3). doi:10.3892/mmr.2023.12948
31. Peng YW, Major T, Deatrick KB, Mohammed A, Jeakle M, Charpie JR. Nicorandil attenuates ventricular dysfunction and organ injury after cardiopulmonary bypass. Int J Cardiol. 2022;368:62–68. doi:10.1016/j.ijcard.2022.08.030
32. Qian G, Zhang Y, Dong W, et al. Effects of nicorandil administration on infarct size in patients with ST-segment-elevation myocardial infarction undergoing primary percutaneous coronary intervention, the CHANGE trial. J Am Heart Assoc. 2022;11(18):e026232. doi:10.1161/JAHA.122.026232
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

