Back to Journals » Therapeutics and Clinical Risk Management » Volume 19
Effect of Moxibustion on Inflammatory Cytokines for Low Back Pain: A Systematic Review, Meta-Analysis and Meta-Regression
Authors Zhao Z, Li J, Wen J, He Y, Sun Z
Received 3 August 2023
Accepted for publication 3 October 2023
Published 18 October 2023 Volume 2023:19 Pages 811—827
DOI https://doi.org/10.2147/TCRM.S429469
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Deyun Wang
Zhenni Zhao,1 Jiawei Li,2 Jiamin Wen,2 Yanyan He,2 Zhiling Sun2
1Nursing Department, Sir Run Run Shaw Hospital, Zhejiang University School of Medicine, Zhejiang, People’s Republic of China; 2School of Nursing, Nanjing University of Chinese Medicine, Nanjing, People’s Republic of China
Correspondence: Zhiling Sun, School of Nursing, Nanjing University of Chinese Medicine, 138 Xian Lin Road, Nanjing, People’s Republic of China, Tel +86 13813892093, Email [email protected]
Background and Objective: Moxibustion is effective for low back pain (LBP), and inflammatory cytokines may play an important role in the mechanism of moxibustion treatment. The purpose of this meta-analysis was to explore the mechanism of moxibustion in LBP in terms of inflammatory cytokines.
Methods: We searched China National Knowledge Infrastructure, Wanfang database, Cochrane Central Register of Controlled Trials, Ovid MEDLINE, Embase, PubMed, and Web of Science to identify eligible randomized controlled trials (RCTs). There was no restriction on the publication date.
Results: Thirty RCTs measuring interleukin (IL-) 1, IL-1β, IL-6, IL-12, IL-17, IL-23, and tumor necrosis factor (TNF-) α were included in this meta-analysis. Compared to controls: single moxibustion could effectively decrease levels IL-6 and IL-23 (SMD, − 0.71, 95% CI: − 1.25 to − 0.17, p = 0.01; SMD, − 1.61, 95% CI: − 2.20 to − 1.03, p < 0.01, respectively); combined moxibustion had significant effects on IL-1, IL-1β, IL-6, IL-12, IL-17, and TNF-α (p < 0.05). Overall, for LBP, single or combined moxibustion could effectively down-regulate levels of pro-inflammatory cytokines (p = 0.007 and p < 0.00001, respectively). For safety of moxibustion, the incidence rate of side effects was similar to that of controls (RD, − 0.01, 95% CI: − 0.02 to 0.01, p = 0.59). Sensitivity analysis showed that the pooled estimates were robust, and publication bias analysis showed there was a significant small study effect (Egger’s test p = 0.0000). High statistical heterogeneity existed between included RCTs, meta-regression showed there was no potential factor explaining the source of heterogeneity.
Conclusion: For LBP, moxibustion can effectively decrease levels of IL-1, IL-1β, IL-6, IL-12, IL-17, IL-23, and TNF-α to achieve analgesia. Because the side effects of moxibustion are transient, it is relatively safe for clinical use. However, based on high heterogeneity in this meta-analysis, rigorously designed RCTs are required to further confirm the results in this review.
Keywords: low back pain, moxibustion, cytokines, tumor necrosis factors, interleukins
Introduction
Low back pain (LBP) is an extremely common problem worldwide experienced by people of all ages.1 People with LBP often complain of varying degrees of pain. In addition, the recurrence and severity of LBP usually result in dysfunction and poor quality of life,1 which brings great distress to patients. LBP is predominantly caused by intervertebral disc degeneration (IDD),2 and the primary cause of IDD is the production of pro-inflammatory mediators.2 This means inflammatory responses are important events during LBP. Inflammatory responses are induced by inflammatory cytokines, such as interleukin (IL-) 1β, IL-6, IL-17, IL-23, and tumor necrosis factor (TNF-) α, and these cytokines have been proved to be strongly associated with the progression of LBP.3–5 Currently, many Western drugs used in LBP have been proved to take effect by regulating inflammatory cytokines,6–8 however, a range of side effects caused by Western drugs (eg, gastrointestinal and cardiovascular adverse events) remain a concern for patients.9 Therefore, alternative therapies without side effects to treat LBP have been increasingly getting attention.
Traditional Chinese medicine (TCM) as a mainstream alternative therapy has its own characteristics in treating LBP and has various therapeutic forms, of which moxibustion is the most widely used in China.10 Moxibustion is an external therapy based on the theory of TCM. It takes effect by burning of mugwort (moxa, Artemisia argyi) to facilitate healing over specific acupuncture points and meridians.11 Although previous studies have suggested that moxibustion can relieve pain and dysfunction in patients with LBP12 and other related diseases, such as lumbar disc herniation (LDH)13,14 and IDD,15 the mechanism of its analgesic effect remains unclear.
Previous studies have found that moxibustion could relieve pain and dysfunction in rheumatoid arthritis by down-regulating pro-inflammatory cytokines and up-regulating anti-inflammatory cytokines.16 What is more, inflammatory cytokines were also associated with pain intensity and progression of LBP.17 Whether moxibustion is able to treat LBP by the same mechanism (modulating inflammatory cytokines) deserves further research. Not only that, moxibustion has an advantage over acupuncture and Western drugs in regulating pain of LBP,10 but it is unclear if moxibustion still has an advantage in the inflammatory response. Due to inconsistent findings of moxibustion on regulating inflammatory cytokines in LBP,18,19 there is a need for a systematic review to summarize this evidence of moxibustion on inflammatory cytokines in patients with LBP. Thus, the purpose of this study is to systematically assess and meta-analyze the efficacy of moxibustion on inflammatory cytokines in patients with LBP from randomized controlled trials (RCTs).
Methods
This study protocol was registered in PROSPERO, the International Prospective Registry of Systematic Reviews (registration no. CRD42022357108). When conducting and reporting this systematic review, we followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 statement.20
Selection Criteria
We strictly limited the inclusion and exclusion criteria according to PICOS (P-participant, I-intervention, C-comparison, O-outcome, S-study design) framework to ensure clinical homogeneity as far as possible.
For participant: patients with LBP or any other disorders that lead to LBP were included, such as LDH, IDD, lumbar spinal stenosis (LSS), sciatica, ankylosing spondylitis (AS), and failed back surgery syndrome (FBSS).
For intervention: we only included studies that used moxibustion alone or in combination with other treatments; that is, no more than two forms of treatment in the experimental group.
For comparison: there was no restriction on control groups.
For outcome: the primary outcomes considered were inflammatory cytokines, including TNF-α, interferon (IFN-) α, IFN-γ, IL-1, IL-1β, IL-2, IL-4, IL-6, IL-10, IL-17, etc. The secondary outcome was the incidence rate of side effects related to moxibustion.
We excluded studies that LBP was caused by trauma, tumor, or infection. We also excluded studies if moxibustion was combined with other therapies, making it difficult to distinguish the effect of moxibustion; for example, a study comparing moxibustion plus acupuncture with another type of treatment (eg, Chinese herbal medicine). Studies comparing the effectiveness of different types of moxibustion were also excluded (eg, heat-sensitive moxibustion vs manual moxibustion).
Search Strategy
We identified RCTs from an electronic search of several databases: China National Knowledge Infrastructure (CNKI), Wanfang database, Cochrane Central Register of Controlled Trials (CENTRAL), Ovid MEDLINE, Embase, PubMed, and Web of Science. There was no restriction on language or publication date. Besides, a manual search of related reviews and studies’ reference lists was conducted. The search filter was set to limit search results to studies focused on clinical trials. The search process was conducted by two reviewers.
The search term format, guided by the PICO framework, included keywords, terms, and Medical Subject Headings (Mesh) related to LBP (Participant), moxibustion (Intervention), and inflammatory cytokines (Outcome). The search strategy of PubMed is shown in Table 1. A Supplementary Material: Search Key Terms and Strategy describing the comprehensive search term framework is attached.
 |
Table 1 PubMed Search Strategy |
Study Selection Process
All the records retrieved from the databases and websites were first exported to EndNote X9 for removing of duplication. First, titles and abstracts of the records were screened by two independent reviewers for eligibility, and in the absence of an abstract, records were retained for full-text review. Second, the same reviewers assessed the full text of potential studies to determine ultimate inclusion in this review. Finally, in case of any disagreement, a decision would be made by consensus with a senior researcher.
Data Collection
Two reviewers independently extracted information based on preset standards, including the first author, publication year, location, condition, sample size, population characteristics, intervention details, control details, and outcomes. A third reviewer checked these data. If key information was missing from the study report, we would contact the report authors to obtain the information. In case of any disagreement, we would use the Kappa score to assess interrater agreement. As Cochrane described, a Kappa score of 0 to 0.2 was considered a slight agreement, 0.21 to 0.40 as fair agreement, 0.41 to 0.60 as moderate agreement, 0.61 to 0.80 as substantial agreement and 0.81 to 1.00 as almost perfect (https://s4be.cochrane.org/blog/2016/05/13/kappa-value/).
Assessment of Risk of Bias in Included Studies
Two independent reviewers assessed the risk of bias (RoB) according to the guidelines of the Cochrane Back Review Group.21 In case of any disagreement, the authors discussed and reached a consensus. The RoB assessment tool has 13 independent criteria; with a judgement of “yes”, “unsure”, or “no”. As described in the guidelines,21 the types of biases assessed are as follows: selection bias (criteria 1, 2, 9), performance bias (criteria 3, 4, 10, 11), attrition bias (criteria 6, 7), detection (or measurement) bias (criteria 5, 12) and reporting bias (criterion 8). The last criterion, “Other” (criteria 13), is reserved for any type of potential bias that is not detected by the previous items. According to the Cochrane Handbook for Systematic Reviews of Interventions,22 the overall RoB for each study was assessed as follows:23
- Low RoB if the trial was judged to be at low risk of bias for all domains for this result;
- Moderate RoB if the trial was judged to raise some concerns in at least one domain for this result but not to be at high risk of bias for any domain;
- High RoB if the trial was judged to be at high risk of bias in at least one domain for this result, or the trial was judged to have some concerns for multiple domains in a way that substantially lowers confidence in the result.
Data Synthesis and Analysis
We used Review Manager (RevMan) (V.5.3) to perform meta-analyses of clinically homogeneous studies, where we could not combine data for clinical heterogeneity, and a narrative synthesis of results would be presented. Since the studies on moxibustion treatment alone and in combination would be included, we first analyzed the effect of moxibustion used alone on inflammatory cytokines, and then analyzed the joint effect of moxibustion on inflammatory cytokines. Inflammatory cytokines were considered as continuous outcomes, so mean difference (MD) with 95% confidence interval (CI) would be employed to estimate the combined effect sizes. If different measurement units were used to measure the same outcome across separate studies, standardized mean difference (SMD) would be used. For incidence rate of side effects (dichotomous outcome), risk ratio (RR) with 95% CI would be employed to estimate the combined effect sizes. If zero-events existed in dichotomous outcomes, risk difference (RD) with Mantel–Haenszel methods would be employed.24
For any pooled data, we used I² statistics to assess statistical heterogeneity, as described in Cochrane Handbook of Systematic Reviews of Interventions, a rough interpretation of heterogeneity is as follows: 0% to 25%: insignificant heterogeneity; 25% to 50%: low heterogeneity; 50% to 75%: moderate heterogeneity; and 75% to 100%: high heterogeneity. If I² <50%, a fixed-effect model would be used; otherwise, a random-effect model would be performed. When high heterogeneity existed, subgroup analysis would be conducted according to the prosperity of inflammatory cytokines. The robustness of the pooled estimates was assessed by sensitivity analysis. If sufficient data were available, a random-effect meta-regression would be performed to explore the potential sources of heterogeneity, such as settings of subgroups, condition of participants, types of moxibustion, and types of controls. Publication bias and small study effects were assessed with the Egger test. We considered a p-value of 0.05 or less to be statistically significant. Sensitivity analysis, meta-regression, and publication bias would be analyzed with Stata 16.0.
Results
Study Selection
The flow of study selection was according to the PRISMA 2020 statement.20 A total of 500 articles were identified through electronic databases (93 articles from CNKI, 350 from Wanfang, 2 from CENTRAL, 5 from Ovid MEDLINE, 19 from Embase, 12 from PubMed, and 19 from Web of Science). After removing of duplication, 419 records were left. 378 records were excluded on the basis of title and abstract screening. Hence, after assessing full-text articles, only 30 records fulfilled the inclusion criteria. The flow of study selection and reasons for exclusion is presented in Figure 1.
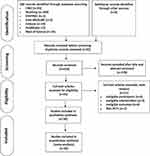 |
Figure 1 Flow diagram of study selection adapted from PRISMA. |
Study Characteristics
30 RCTs14,18,19,25–50 with a total of 2560 participants satisfied our inclusion criteria and were included in this review. The characteristics of the included studies are presented in Table 2. All trials were conducted in Chinese public hospital. The LBP subjects included in this review were chronic non-specific LBP (1 RCT), LDH (14 RCTs), lumbar muscle strain (4 RCTs), ankylosing spondylitis (8 RCTs), LBP (1 RCT), and sciatica (2 RCTs). Except for pure moxibustion, there were five specific types of moxibustion involved in this review: bamboo circle salt moxibustion (1 RCT), long snake moxibustion (13 RCTs), heat-sensitive moxibustion (3 RCTs), seed-sized moxibustion (1 RCT), and vesicular moxibustion (1 RCT). All of these are ancient moxibustion therapies widely used in the treatment of LBP in China. In addition, seven studies18,19,25,29,41,50,51 reported the effects of moxibustion alone on LBP inflammatory cytokines, while others reported the joint effects of moxibustion. Except for Du meridian that is specifically used in long snake moxibustion, the most commonly used acupoints for moxibustion were Shenshu (BL23) and Weizhong (BL40), which were used in nine and eight RCTs, respectively. Five studies used Dachangshu (BL25), Jiaji (EX-B2), Mingmen (GV4), Huantiao (GB30), Yanglingquan (GB34), and Ashi point. The most frequently used acupoints and locations are presented in Table 3.
 |
Table 2 Characteristics of Included Studies |
 |
Table 3 Acupoints Commonly Used for LBP |
In terms of interventions in control groups, western medicine, acupuncture, tuina, cupping therapy, herbal medicine, exercise, catgut embedding and traction were involved in this review. All of the included studies reported outcomes at post-intervention, among them, only two studies reported drop-out rates. In Zhang’s et al study,19 the drop-out reasons were job transfer (1 case) and receiving other treatments for a cold (1 case), and Cui et al, did not report drop-out reasons.26 The outcome measures of inflammatory cytokines reported in the included studies were IL-1, IL-1β, IL-6, IL-12, IL-17, IL-23, and TNF-α. All of these were pro-inflammatory cytokines, and no study reported anti-inflammatory cytokines as outcomes. In addition, eight studies reported incidence of side effects of moxibustion.19,26,27,31,36,42,44,48
Risk of Bias Assessment
The RoB assessment of all the included studies is presented in Table 4. According to the evaluation rules reported in the Methods section, we found 2 RCTs (7%) were classified as low RoB, 20 RCTs (66%) as moderate RoB, and 8 RCTs (27%) as high RoB.
 |
Table 4 Risk of Bias Assessment |
The main methods limitations across the included RCTs were that 8 RCTs (27%) did not report the randomization process, and 29 RCTs (97%) did not use or inform about concealed allocation. In terms of missing outcome data, 28 RCTs (93%) did not inform about missing data, only 2 RCTs reported the details of drop-out and did not use intention-to-treat (ITT) analysis. Because of the nature of interventions, it was difficult to blind therapists and participants, and only 2 RCTs (7%) provided details about blinding. Due to inflammatory cytokines and side effects were observer-reported outcomes not involving judgment, we classified these studies as moderate RoB regarding the influence of the non-blinding of the assessor.
Outcome Analysis
Effects of Single Moxibustion on Inflammatory Cytokines
Six pro-inflammatory cytokines including IL-1, IL-1β, IL-6, IL-17, IL-23, and TNF-α were measured in selected studies, and no anti-inflammatory cytokines were measured. Meta-analyses (see Figure 2) showed that moxibustion alone had a significant effect on pro-inflammatory cytokine level over controls (SMD, −0.42, 95% CI: −0.72 to −0.11, p = 0.007; I² = 81%). Among these pro-inflammatory cytokines, subgroup analysis showed that there was no significance was found between moxibustion group and controls in IL-1 (SMD, −0.00, 95% CI: −0.42 to 0.41, p = 0.99; I² = 24%), IL-1β (SMD, −0.23, 95% CI: −1.18 to 0.72, p = 0.64; I² = 88%), IL-17 (SMD, −0.47, 95% CI: −0.98 to 0.04, p = 0.07; I² = NA), and TNF-α (SMD, −0.08, 95% CI: −0.48 to 0.31, p = 0.68; I² = 59%). However, for inflammatory cytokine IL-6 and IL-23, moxibustion alone had a significant effect over controls (SMD, −0.71, 95% CI: −1.25 to −0.17, p = 0.01; I² = 82%; SMD, −1.61, 95% CI: −2.20 to −1.03, p < 0.01; I² = NA, respectively).
 |
Figure 2 Forest plot for single moxibustion on pro-inflammatory cytokines. |
Joint Effects of Moxibustion on Inflammatory Cytokines
Six pro-inflammatory cytokines including IL-1, IL-1β, IL-6, IL-12, IL-17, and TNF-α were measured in selected studies. Meta analysis (see Figure 3) showed that the combined moxibustion had a significant effect on pro-inflammatory cytokine level over controls (SMD, −1.56, 95% CI: −1.86 to −1.26, p < 0.00001; I² = 93%). Foremove, in subgroup analysis, we found the combined moxibustion had a superior effect on IL-1 (SMD, −0.65, 95% CI: −1.29 to −0.02, p = 0.004; I² = NA), IL-1β (SMD, −0.98, 95% CI: −1.32 to −0.64, p < 0.00001; I² = 48%), IL-6 (SMD, −2.19, 95% CI: −2.85 to −1.53, p < 0.00001; I² = 96%), IL-12 (SMD, −0.67, 95% CI: −1.09 to −0.25, p = 0.002; I² = NA), IL-17 (SMD, −0.62, 95% CI: −1.14 to −0.11, p = 0.02; I² = NA), and TNF-α (SMD, −1.52, 95% CI: −1.90 to −1.15, p < 0.00001; I² = 90%) than controls.
 |
Figure 3 Forest plot for combined moxibustion on pro-inflammatory cytokines. |
Incidence Rate of Side Effects of Moxibustion Vs Controls
Among 30 studies included in this review, eight studies reported side effects of moxibustion and controls, through meta-analysis (see Figure 4), we found the incidence rate of side effects of moxibustion was similar to controls (RD, −0.01, 95% CI: −0.02 to 0.01, p = 0.59; I² = 0%). Side effects of moxibustion reported in Zhang’s et al study were nausea (1 case), gastrointestinal upset (1 case), and local redness (1 case), all of these were transient side effects.
 |
Figure 4 Forest plot for incidence rate of side effects of moxibustion vs controls. |
Sensitivity Analysis
For effects of single (see Figure 5) or combined moxibustion (see Figure 6) on inflammatory cytokines, we performed a sensitivity analysis by deleting individual studies one by one and evaluating the effect of each deletion on the pooled prevalence. Based on the sensitivity analysis results, none of the studies had an impact on the overall effect, indicating that our meta-analysis was statistically stable.
 |
Figure 5 Sensitivity analysis for single moxibustion on pro-inflammatory cytokines. |
 |
Figure 6 Sensitivity analysis for combined moxibustion on pro-inflammatory cytokines. |
Meta-Regression Analysis
More than ten studies evaluated the effects of combined moxibustion on inflammatory cytokines; therefore, we conducted a multivariate meta-regression analysis to explore potential sources of high heterogeneity. And the results of regression showed settings of subgroups, conditions of participants, types of moxibustion, and types of controls were not potential factors (p > 0.05) that could explain the source of high heterogeneity (see Table 5).
 |
Table 5 Multivariate Meta-Regression Analysis for Combined Moxibustion on Pro-Inflammatory Cytokines |
Publication Bias
More than ten studies evaluated the effects of combined moxibustion on inflammatory cytokines; therefore, we assessed publication bias by Egger test. And the results showed there was a significant small study effect in this meta-analysis (Egger’s test p = 0.0000), which might indicate a publication bias of small studies being less likely to be published in moxibustion clinical trials on inflammatory cytokines.
Discussion
Main Findings
The aim of this study was to meta-analyze the effects of moxibustion on inflammatory cytokines for LBP. Thirty RCTs were reviewed with seven pro-inflammatory cytokines measured, including IL-1, IL-1β, IL-6, IL-12, IL-17, IL-23, and TNF-α. The results showed that moxibustion used alone or in combination could effectively decrease overall levels of pro-inflammatory cytokines. The incidence of side effects of moxibustion was similar to that of controls, and only a few transient side effects of moxibustion were reported. In summary, moxibustion could positively down-regulate the levels of pro-inflammatory cytokines, and could be used in clinical practice as an alternative therapy for LBP.
Possible Modern Biological Mechanism of Moxibustion
Among numerous members of pro-inflammatory cytokines, IL-1, IL-1β, and IL-17 have been proved to be major risk factors for IDD.52–54 IDD is the predominant cause of LBP, and during the progression of LBP, IL-1β and TNF-α were considered to be the key mediators.2 High levels of IL-1, IL-1β, IL-6, IL-17, and TNF-α were thought to accelerate the occurrence and progression of LBP.5,55,56 In addition, other pro-inflammatory cytokines (eg, IL-12 and IL-23) have also been shown to be associated with the presence of LBP.4,57
Pro-inflammatory cytokines are sensitive indicators for the progression and regression of LBP, therefore, therapeutic interventions targeting pro-inflammatory cytokines may represent a novel and effective approach for LBP. And in our study, we found that moxibustion takes effect by down-regulating levels of pro-inflammatory cytokines for LBP. Similarly, for other inflammatory diseases, there was evidence that moxibustion could relieve inflammation by down-regulating pro-inflammatory cytokines.58,59 However, the current research on the mechanism of moxibustion therapy is still not systematic and thorough. Inflammatory response is just one part of the complicated mechanism, and we still have a long way to go to explore the mechanism of moxibustion.
Comparison with Previous Systematic Reviews
To our knowledge, this is the first systematic review and meta-analysis evaluating the value of moxibustion on inflammatory cytokines for LBP. Compared to previous study, moxibustion has been proved to be effective for pain and disability for LBP,10 and on that basis, our study further explored the mechanism of moxibustion. Similarly, high heterogeneity was found between the included studies, and the quality of the included studies was generally low. Low quality of moxibustion RCTs published in Chinese journals seems to be a common phenomenon.60
Previous study also conducted a meta-analysis of moxibustion on inflammatory cytokines for rheumatoid arthritis,16 and consistent results were found for moxibustion on pro-inflammatory cytokines. There are some methodological differences that deserve mentioning: firstly, we included human rather than animal models as subjects in our study, relative to human experiments, the results of animal experiments cannot be directly applied and popularized in the human population; secondly, we conducted statistical analysis for sensitivity analysis and publication bias, compared to visual inspection, the former is more objective; finally, although high statistical heterogeneity could not be well addressed, we conducted multivariate meta-regression analysis to explore the potential factors that could explain the sources of high heterogeneity. In brief, although high statistical heterogeneity was found in this meta-analysis, we designed and conducted a rigorous methodological analysis to promote the level of evidence of this meta-analysis.
Recommendation for Research
In terms of methodology, firstly, the included RCTs were generally of low quality due to RoB, and randomization and blinding method are the main sources of high RoB. Therefore, future moxibustion RCTs should follow The Standards for Reporting Interventions in Clinical Trials of Moxibustion (STRICTOM) to reduce RoB.61 Secondly, none of the included studies reported the method of sample size estimate. We recommend that clinical trials related to moxibustion or acupuncture follow the recommendations and guidelines of the Beijing Evidence-Based Center,62 and perform standardized sample size estimation when designing trials.
Although this meta-analysis found the effectiveness of moxibustion on inflammatory cytokines for LBP, the targeting cytokines of moxibustion to take effect remain unclear and still deserve more research. Besides, to deeply explain the mechanism of moxibustion, more researches are still needed to explore whether moxibustion can be widely used in other inflammatory pain.
Implications for Practice
For clinicians, there are several important points of this meta-analysis that need to be mentioned. First of all, this meta-analysis involved different acupoints for LBP, the most frequently used acupoints in moxibustion were Shenshu (BL23), Weizhong (BL40), Dachangshu (BL25), Jiaji (EX-B2), Mingmen (GV4), Huantiao (GB30), Yanglingquan (GB34), and Ashi point which were similar to that in acupuncture treatment.63 Different acupoints belong to different meridians, therapists should individualize the acupoints for treatment according to the specific situation of meridian blockage. Second, for the effects of moxibustion on inflammatory cytokines, there was a difference between single moxibustion and combined moxibustion. Moxibustion alone can reduce the level of pro-inflammatory factors in general, but the effect is diminished on IL-1, IL-1β, IL-17, and TNF-α. Therefore, when the effects of moxibustion used alone are not ideal, the combined use of moxibustion is indicated. Finally, for the safety of moxibustion, only transient side effects of moxibustion were reported in this meta-analysis, in contrast to irreversible side effects of perennial use of Western medicine (eg, NSAIDs and opioid), moxibustion seems to be a safe treatment for LBP. In China, moxibustion can be performed by clients at home, thereby care providers still need to inform clients about side effects of moxibustion, such as scald, redness, and gastric upset. If any side effects occur, moxibustion should be stopped immediately.
Inflammatory cytokines play an important role in the pain and dysfunction caused by LBP, and this meta-analysis proved that moxibustion could take effect by regulating inflammatory cytokines. Therefore, for patients who suffer from inflammatory pain, moxibustion may become an effective alternative therapy. Furthermore, the effectiveness and the relative safety also prompt moxibustion to be widely used, and when Western drugs are not indicated, patients could use moxibustion at home to relieve inflammation and inflammatory pain.
Strengths and Limitations
The present systematic review has several strengths and some limitations that should be mentioned. The strengths are as follows: first, we systematically searched several databases and grey literature, which reduced publication bias to some extent; second, we used a 13-item tool to evaluate the RoB of each included trial, and this tool has been widely used in systematic reviews focused on LBP; last, we assessed sensitivity analysis and publication bias by statistical analysis, and performed meta-regression to explore sources of heterogeneity, and the results of this meta-analysis was robust. As a limitation, due to the high statistical heterogeneity existed in this meta-analysis, the overall quality of evidence was low. High-quality RCTs are needed in the future. In addition, this meta-analysis involved several types of moxibustion, and we did not explore therapeutic differences between different types of moxibustion, and network meta-analysis may be needed in the future. Last but not least, we failed to detect the effectiveness of moxibustion on anti-inflammatory cytokines, more research is needed to overcome the limitations of the existing evidence.
Conclusions
This meta-analysis measured moxibustion on IL-1, IL-1β, IL-6, IL-12, IL-17, IL-23, and TNF-α for LBP. We found that moxibustion used alone or in combination can reduce the overall level of pro-inflammatory cytokines for LBP. Due to the high statistical heterogeneity and limited studies in this meta-analysis, further meticulous RCTs are needed to explore the mechanism and safety of moxibustion for LBP.
Funding
This work was supported by the Postgraduate Research & Practice Innovation Program of Jiangsu Province (No. SJCX23_0735).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Hartvigsen J, Hancock MJ, Kongsted A, et al. What low back pain is and why we need to pay attention. Lancet. 2018;391(10137):2356–2367. doi:10.1016/S0140-6736(18)30480-X
2. Wang Y, Che M, Xin J, Zheng Z, Li J, Zhang S. The role of IL-1beta and TNF-alpha in intervertebral disc degeneration. Bio Pharmacot. 2020;131:110660. doi:10.1016/j.biopha.2020.110660
3. Khan AN, Jacobsen HE, Khan J, et al. Inflammatory biomarkers of low back pain and disc degeneration: a review. Ann N Y Acad Sci. 2017;1410(1):68–84. doi:10.1111/nyas.13551
4. Sanabria-Mazo JP, Colomer-Carbonell A, Carmona-Cervello M, et al. Immune-inflammatory and hypothalamic-pituitary-adrenal axis biomarkers are altered in patients with non-specific low back pain: a systematic review. Front Immunol. 2022;13:945513. doi:10.3389/fimmu.2022.945513
5. van den Berg R, Jongbloed EM, de Schepper E, Bierma-Zeinstra S, Koes BW, Luijsterburg P. The association between pro-inflammatory biomarkers and nonspecific low back pain: a systematic review. Spine J. 2018;18(11):2140–2151. doi:10.1016/j.spinee.2018.06.349
6. Dias QJ, Reis GA, Galdino G, et al. Tnf-alpha, cxcl-1 and il-1 beta as activators of the opioid system involved in peripheral analgesic control in mice. Eur J Pharmacol. 2021;896:173900. doi:10.1016/j.ejphar.2021.173900
7. Fragoulis GE, Siebert S. Treatment strategies in axial spondyloarthritis: what, when and how? Rheumatology. 2020;59(Suppl4):v79–v89. doi:10.1093/rheumatology/keaa435
8. Helm IS, Harmon PC, Noe C, et al. Transforaminal epidural steroid injections: a systematic review and meta-analysis of efficacy and safety. Pain Physician. 2021;24(S1):S209–S232.
9. Knezevic NN, Candido KD, Vlaeyen J, Van Zundert J, Cohen SP. Low back pain. Lancet. 2021;398(10294):78–92. doi:10.1016/S0140-6736(21)00733-9
10. Chen FQ, Ge JF, Leng YF, Li C, Chen B, Sun ZL. Efficacy and safety of moxibustion for chronic low back pain: a systematic review and meta-analysis of randomized controlled trials. Compl Ther Clin Pract. 2020;39:101130. doi:10.1016/j.ctcp.2020.101130
11. Dubois MY, Chen L. Of low back pain and moxibustion. Pain Med. 2014;15(8):1243–1244. doi:10.1111/pme.12513
12. Yao Y, Zhou L, Chen FQ, et al. The effect and safety of thunder-fire moxibustion for low back pain: a meta-analysis of randomized controlled trials. Evid Based Compl Altern Med. 2022;2022:6114417.
13. Hua F, Xiong J, Zhang H, Xiang J, Huang S. Moxibustion therapy on lumbar disc herniation: an evidence-based clinical practice guideline. Medicine. 2021;100(9):e24347. doi:10.1097/MD.0000000000024347
14. Lu T, Zhang J, Lv Y, Wu Y. The effect of warm needle moxibustion on lumbar disc herniation. Am J Transl Res. 2021;13(5):5059–5065.
15. Zhang B, Zhao Q, Li Y, Zhang J. Moxibustion alleviates intervertebral disc degeneration via activation of the HIF-1alpha/VEGF pathway in a rat model. Am J Transl Res. 2019;11(9):6221–6231.
16. Zhong YM, Cheng B, Zhang LL, Lu WT, Shang YN, Zhou HY. Effect of moxibustion on inflammatory cytokines in animals with rheumatoid arthritis: a systematic review and meta-analysis. Evid Based Complement Altern Med. 2020;2020:6108619. doi:10.1155/2020/6108619
17. Djuric N, Lafeber G, Vleggeert-Lankamp C. The contradictory effect of macrophage-related cytokine expression in lumbar disc herniations: a systematic review. Eur Spine J. 2020;29(7):1649–1659.
18. Hu X, Deng C, Huang H, et al. Impact of long snake-like moxibustion on pain symptoms and serum IL-1β, TNF-α levels in the lumbago patients with cold-dampness syndrome. Chin Med Mod Distan Educ Chin. 2020;18(10):92–95.
19. Zhang M. Clinical Observation of Bamboo Circle Salt Moxibustion in the Treatment of Chronic Non-Specific Low Back Pain with Kidney-Yang Deficiency. Fujian University of Traditional Chinese Medicine; 2021. doi:10.27021/d.cnki.gfjzc.2021.000085
20. Page MJ, Mckenzie JE, Bossuyt PM, et al. The prisma 2020 statement: an updated guideline for reporting systematic reviews. BMJ Br Medl Jl. 2021;372:n71. doi:10.1136/bmj.n71
21. Furlan AD, Malmivaara A, Chou R, et al. 2015 updated method guideline for systematic reviews in the Cochrane Back and Neck Group. Spine. 2015;40(21):1660–1673. doi:10.1097/BRS.0000000000001061
22. Higgins JPT. Cochrane handbook for systematic reviews of interventions version 6.3. Cochrane; 2022. Available from: http://www.training.cochrane.org/handbook.
23. Cashin AG, Folly T, Bagg MK, et al. Efficacy, acceptability, and safety of muscle relaxants for adults with non-specific low back pain: systematic review and meta-analysis. BMJ Br Medl Jl. 2021;374:n1446. doi:10.1136/bmj.n1446
24. Xu C, Furuya-Kanamori L, Zorzela L, Lin L, Vohra S. A proposed framework to guide evidence synthesis practice for meta-analysis with zero-events studies. J Clin Epidemiol. 2021;135:70–78. doi:10.1016/j.jclinepi.2021.02.012
25. Cao X. The Research of the Influence of du-Moxibustion and Acupuncture to Il-1, Il-6 and Tnf-Α Inflammatory Cytokines in Treating as. Henan University of Chinese Medicine; 2013.
26. Cui J. Therapeutic effect of warming needle moxibustion on lumbar disc herniation and its influence on serum β-endorphin and inflammatory factors. J Cervicodynia Lumbodynia. 2019;40:2.
27. Fan Y. Effect of governor vessel long-snake moxibustion on immune status and quality of life of patients with ankylosing spondylitis. Med J Air Force. 2019;35(04):338–341.
28. Gong P, Dai W, Yu R. Effect of thermal moxibustion combined with tuina on levels of txb2 and pge2 and its influence to lumbar function in patients with ldh. J Clin Acupunct Moxibustion. 2020;36(11):17–21.
29. Jiang X, Huang P. Clinical observation on long snake-like moxibustion therapy in the treatment of lumbar disc herniation. Chin Med Mod Distan Educ Chin. 2020;18(24):98–100.
30. Kong L. The Research of Influence About du-Moxibustion on Inflammatory Indicators of Ankylosing Spondylitis (the Kidney Yang Deficiency Syndrome). Henan University of Chinese Medicine; 2014.
31. Li X. Effects of needle warming moxibustion combined with acupuncture on patients with lumbar disc herniation. Med J Chin People Health. 2021;33:22.
32. Li X, Luo G, Wu Y. Clinical study of seed-seized moxibustion on lumbago (kidney deficiency type. Asia Pacific Tradit Med. 2019;15(02):144–146.
33. Li Z, Chen H, Wen X. Effect of moxibustion on the quality of life in patients with ankylosing spondylitis with deficiency of kidney and yang. Chin Foreign Med Treat. 2020;39(23):177–179.
34. Liu H. Effect of warm acupuncture and moxibustion on pge2 and il-6 in the treatment of cold-dampness and stasis sciatica. Guang J Chin Med. 2020;35(20):3250–3252.
35. Lyu M, Hu X, Gen L, Gao J, Zhu X. Clinical observation of long snake moxibustion on ankylosing spondylitis. Guang J Chin Med. 2018;33(14):2086–2088.
36. Lyu S. Effect of thermal moxibustion in the treatment of LDS and its influence to immune system. J Clin Acupunct Moxibustion. 2018;34(04):26–29.
37. Lyu Z. Clinical study on lumbar muscle strain of cold-dampness type treated by needle warming moxibustion. J N Chin Med. 2019;51(08):247–249.
38. Niu L, Qin H, Kong Y. Clinical effect of governor vessel moxibustion combined with lumbar core muscle strength training in the treatment of lumbar disc herniation. J Pract Tradit Chin Med. 2022;38(02):280–282.
39. Qin H, Li Y, Guo N. Clinical study on governor vessel moxibustion combined with traction therapy for lumbar disc herniation. J N Chin Med. 2021;53(18):135–138.
40. Qin Q. Temperature acupuncture treatment of sciatica and effect on pge2, il一6 influence. Guizhou University of Traditional Chinese Medicine; 2012.
41. Xu J, Lin R, Niu Z, Zhang Y, Wu Y. Therapeutic effect of guanyuan point moxibustion on 30 cases of lumbar disc herniation. J Gansu Univ Chin Med. 2012;29(06):55–58.
42. Xu K, Gao H, Qin X, Zeng K. Clinical research on needle-warming moxibustion treating lumbar disc herniation. Chinese J Rehabilitation Med. 2013;12:1.
43. Yan Z, Huang W, Li S, Zhang W, Hu Y, Liu C. Impact of huolong moxibustion on tnf-α and pain degree in the patient of discogenic low back pain. Chin Acupunct Moxibust. 2015;35(11):1121–1123.
44. Yang Y, Su L, Hu N, Zhao X, Tian S. Clinical observation on the treatment of lumbar muscle strain with walking cupping and moxibustion. Chin Manipulat Rehabilitat Med. 2020;11(22):62–64.
45. Yao B, Yang Y, Li Y. Clinical study of herbal medicine moxibustion on mingmen eight array points for lumbar muscle strain of kidney deficiency type. Shanghai J Acupunct Moxibust. 2019;38(09):1035–1038.
46. Yin J. Clinical Study on Treatment of Lumbar Disc Herniation with Warm Acupuncture and Moxibustion. Hubei University of Chinese Medicine; 2008.
47. Zhang G, Yang Y, Li H. Clinic research of heat-sensitive moxibustion therapy and Chinese herbal medicine in treatment of wind-cold-damp type of lumber disc herniation. Chin J Exper Tradit Med Formul. 2012;18(08):255–257.
48. Zhao Y, Tian Y. Clinical study on treatment of 53 cases of ankylosing spondylitis with zhoutian infrared moxibustion combined with catgut embedding at acupoints. Jiangsu J Tradit Chin Med. 2021;53(03):57–60.
49. Zhu X, Guo R, Yu X, Hu X. Clinical observation on long-snake-like moxibustion therapy in treating ankylosing spondylitis. Chin Med Mod Distan Educ Chin. 2019;17(11):97–99.
50. Zuo Z, Liu Z, Yuan K, Wang Y, Dong K. Effects and mechanism of the long-snake moxibustion on ankylosing spondylitis based on th17/treg/th1 immune imbalance. Chin Acupunct Moxibust. 2018;38(10):1053–1057.
51. Hong K, Zhu Y, Wan T, Gong D. 52 cases of lumbar disc herniation with yang deficiency and cold coagulation treated by vesiculation moxibustion. Fujian J Tradit Chin Med. 2016;47(05):1–2.
52. Yang W, Yu XH, Wang C, et al. Interleukin-1beta in intervertebral disk degeneration. Clin Chim Acta. 2015;450:262–272. doi:10.1016/j.cca.2015.08.029
53. Gruber HE, Hoelscher GL, Ingram JA, Norton HJ, Hanley EJ. Increased il-17 expression in degenerated human discs and increased production in cultured annulus cells exposed to il-1ss and tnf-alpha. Biotech Histochem. 2013;88(6):302–310.
54. Tan JH, Li ZP, Liu LL, Liu H, Xue JB. Il-17 in intervertebral disc degeneration: mechanistic insights and therapeutic implications. Cell Biol Int. 2022;46(4):535–547.
55. Canli K, Billens A, Van Oosterwijck J, Meeus M, De Meulemeester K. Systemic cytokine level differences in patients with chronic musculoskeletal spinal pain compared to healthy controls and its association with pain severity: a systematic review. Pain Med. 2022;23(12):1947–1964. doi:10.1093/pm/pnac091
56. Teodorczyk-Injeyan JA, Triano JJ, Injeyan HS. Nonspecific low back pain: inflammatory profiles of patients with acute and chronic pain. Clin J Pain. 2019;35(10):818–825.
57. Shamji MF, Setton LA, Jarvis W, et al. Proinflammatory cytokine expression profile in degenerated and herniated human intervertebral disc tissues. Arthritis Rheum. 2010;62(7):1974–1982. doi:10.1002/art.27444
58. Bao CH, Wang CY, Li GN, et al. Effect of mild moxibustion on intestinal microbiota and nlrp6 inflammasome signaling in rats with post-inflammatory irritable bowel syndrome. World J Gastroenterol. 2019;25(32):4696–4714. doi:10.3748/wjg.v25.i32.4696
59. Xu X, Yang H, Chen JN, et al. Moxibustion attenuates inflammation and alleviates axial spondyloarthritis in mice: possible role of apoe in the inhibition of the wnt pathway. J Tradit Complement Med. 2022;12(5):518–528. doi:10.1016/j.jtcme.2022.04.002
60. Ma B, Chen ZM, Xu JK, et al. Do the consort and stricta checklists improve the reporting quality of acupuncture and moxibustion randomized controlled trials published in Chinese journals? A systematic review and analysis of trends. PLoS One. 2016;11(1):e147244.
61. Cheng CW, Fu SF, Zhou QH, et al. Extending the consort statement to moxibustion. J Integr Med. 2013;11(1):54–63. doi:10.3736/jintegrmed2013009
62. Hu J, Li B, Zhang HN, Liu WH, Feng S. Sample size estimation in acupuncture and moxibustion clinical trials. Zhongguo Zhen Jiu. 2021;41(10):1147–1152. doi:10.13703/j.0255-2930.20201020-0002
63. Berman BM, Langevin HM, Witt CM, Dubner R. Acupuncture for chronic low back pain. N Engl J Med. 2010;363(5):454–461. doi:10.1056/NEJMct0806114
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
