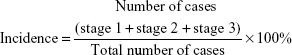Back to Journals » Therapeutics and Clinical Risk Management » Volume 12
Effect of ketamine combined with butorphanol on emergence agitation of postoperative patients with gastric cancer
Authors Lin L, Liu S, Chen Z, Lin S
Received 23 December 2015
Accepted for publication 26 January 2016
Published 4 May 2016 Volume 2016:12 Pages 713—717
DOI https://doi.org/10.2147/TCRM.S103060
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Deyun Wang
Liang Lin, Shuncui Liu, Zhenyi Chen, Shaoli Lin
Department of Anesthesiology, The First Affiliated Hospital of Xiamen University, Xiamen, Fujian, People’s Republic of China
Background: This study aimed to investigate the effect of ketamine combined with butorphanol on emergence agitation (EA) in postoperative gastric cancer patients.
Materials and methods: A total of 150 patients with gastric cancer were included and divided into group B (1 mg butorphanol before anesthesia induction, n=50), group K (1 mg/kg ketamine, n=50), and group C (1 mg butorphanol combined with 1 mg/kg ketamine, n=50). Mean arterial pressure (MAP) and heart rate (HR) at the end of operation, just before extubation (T0) and at 0 minute (T1), 5 minutes (T2), and 30 minutes (T3) after extubation were compared. Statistical analysis of recovery time, extubation time, time in postanesthesia care unit, and EA incidence and adverse reactions were performed.
Results: There were no differences among groups with respect to MAP and HR at T0 and T1 (P>0.05). Compared with patients in group C, significant reduction of MAP and HR were observed in groups K and B at T2 and T3 (P<0.05), while no differences were found between group K and group B (P>0.05). Recovery time, extubation time, time in postanesthesia care unit, and incidence of EA in group C were significantly less than those in groups K and B (P<0.05), but no differences were observed between group K and group B (P>0.05). Total incidence of adverse reactions were significantly increased in group K compared to those in groups C and B (P<0.05).
Conclusion: Injection of ketamine combined with butorphanol before anesthesia induction was more effective than injection of ketamine or butorphanol separately in the prevention of EA.
Keywords: emergence agitation, ketamine, butorphanol, gastric cancer
Introduction
Emergence agitation (EA) is a common phenomenon during recovery from general anesthesia. Behavioral changes in patients, including restlessness, disorientation, and excitation, may easily cause involuntary physical accidental injury. Moreover, restlessness can induce sympathetic excitement, which probably raises blood pressure and heart rate (HR) and increases myocardial oxygen consumption and the risk of cardiovascular and cerebrovascular diseases.1 An effective prevention of EA plays an important role in clinical anesthesia outcomes and evaluation.2
EA in patients recovering from general anesthesia is attributed to complicated causes, and one of the most confounding factors is the postoperative pain caused by reduction of anesthesia effect.3 Ultrashort-acting drugs such as remifentanil have been used for sedation, and have also been combined with other medications for use in general anesthesia.4 Due to the relatively short context-sensitive half-life of remifentanil, the desired blood plasma level can be achieved quickly and recovery occurs faster; postoperative pain in the recovery phase is unbearable, which contributes to the incidence of EA.5 Therefore, it is necessary to take appropriate measures for prevention of EA. Intravenous morphine, fentanyl, and other opioids were used previously to reduce incidence of EA, but those drugs could not effectively ease the postoperative pain, as well as nausea, vomiting, delayed recovery, respiratory depression, and chest wall muscle rigidity.6 Investigating safer and more efficient measures for prevention of EA has become the major challenge faced by clinical anesthesiologists.
Ketamine and butorphanol are effective drugs for analgesia routinely used in the clinical practice. Although preoperative injection of ketamine or butorphanol can reduce the incidence of EA, the combined application of ketamine and butorphanol in practice is not reported yet.7–9 This study aimed to investigate the preventive effect of different drugs (butorphanol, ketamine, and combination of butorphanol and ketamine) on EA in patients with gastric cancer.
Materials and methods
Subjects
This study was carried out among 150 patients with gastric cancer who underwent radical surgery from the First Affiliated Hospital of Xiamen University, People’s Republic of China, between October 2014 and October 2015. All patients met the following criteria. Inclusion criteria were the diagnosis of gastric cancer by gastroscopy or pathological examination, submission of signed informed consent form, and the absence of any surgical contraindication. Exclusion criteria were patients with psychosis, anaphylactic reactions to drugs, severe cardiovascular and cerebrovascular disease, and opiod addiction. Approximately 150 patients were divided into three groups, and no differences were found in characteristics between the groups, as shown in Table 1. This study was approved by the ethics committee of the Affiliated Hospital of Xiamen University. All patients provided written informed consent.
  | Table 1 Characteristics of the study populations |
Anesthesia methods
Patients were assigned to receive intravenous midazolam (0.05–0.10 mg/kg), propofol (1.5–2.0 mg/kg), vecuronium (0.1 mg/kg), and fentanyl (2 μg/kg) as the anesthetic induction agent. General anesthesia was maintained with propofol (4.0–6.0 mg/kg), vecuronium (0.1–0.2 mg/kg), and remifentanil (0.1–0.2 μg/kg). Patients in group B were injected with 1 mg butorphanol as premedication, those in group K were injected with 1 mg/kg ketamine, and the rest of the patients (group C) were injected with 1 mg butorphanol combined with 1 mg/kg ketamine. All individuals were transferred to the postanesthesia care unit (PACU) after operation.
Examination
The following characteristics were measured:
- Vital sign: the mean arterial pressure (MAP) and HR at the end of operation just before extubation (T0), and at 0 minute (T1), 5 minutes (T2), and 30 minutes (T3) after extubation;
- anesthesia recovery: the recovery time, extubation time, and time in the PACU;
- the stage of EA: stage 0: quiet; stage 1: slightly restless to stimuli; stage 2: obviously excited without stimulation; stage 3: delirious and struggling and shouting continuously. for the formula for calculation of incidence of EA is:
- adverse reactions: drowsiness, glossoptosis, respiratory depression, etc.
|
|
Statistical analysis
Statistical analyses were performed using the SPSS statistical package, version 18.0 (SPSS Inc., Chicago, IL, USA). The measurement data were analyzed by analysis of variance and expressed as mean ± standard deviation, the enumeration data were expressed as number of cases. The incidence was determined by χ2 test, with a significance of P<0.05.
Results
Vital sign
In this study, no significant differences were found in multiple comparison among groups with respect to MAP and HR at T0 and T1 (P>0.05). Compared with patients in group C, significant reduction of MAP and HR were observed in groups K and B at T2 and T3 (P<0.05), while no difference were found between group K and group B (P>0.05, Table 2). The results showed that injection of ketamine combined with butorphanol before anesthesia induction was more effective than injection of ketamine or butorphanol separately with regard to the vital sign stability.
Anesthesia recovery
As shown in Table 3, we found that the recovery time, extubation time, and time in the PACU in group C were significantly less than those in groups K and B (P<0.05). However, no differences were observed between group K and group B (P>0.05). The data suggested that injection of concomitant drugs (ketamine and butorphanol) before anesthesia might contribute to improve the quality of anesthesia recovery than injection of each drug separately.
Incidence of EA
In this study, we found the incidences of EA in group C, group K, and group B were 6.0% (3/50), 24.0% (12/50), and 20.0% (10/50), respectively. The incidence of EA in group C was remarkably lower than those in groups K and B (χ2=6.353, 4.332; P<0.05), but the difference between group K and group B did not reach statistical significance (Table 4, χ2=0.233, P>0.05). The results indicated that combined application of ketamine and butorphanol could greatly abate the incidence of EA.
Incidence of adverse reactions
In our data, the incidence of adverse reactions were greatly increased in group K compared with those in groups C and B (Table 5, χ2=5.316, P<0.05), while no statistical significance was found between group C and group B (Table 5, χ2=0.000, P>0.05). It was suggested that using ketamine combined with butorphanol might be beneficial in reducing the incidence of adverse reactions than using each drug separately.
  | Table 5 Comparison of the incidence of adverse reactions among groups |
Discussion
Butorphanol is a morphinan-type synthetic opioid analgesic that exhibits partial agonist and antagonist activity at the μ-opioid receptor, as well as competitive antagonist activity and partial agonist activity at the κ-opioid receptor. The analgesic potency of butorphanol was five to eight times that of morphine, while the respiratory depression was only one-fifth as much as that of the latter.10–14 A previous study reported that butorphanol could reduce the incidence of EA in patients recovering from general anesthesia, but prolong the recovery time of emergence.15
Ketamine is classified as an N-methyl-D-aspartic ammonia acid receptor antagonist, and it is a medication used mainly for starting and maintaining anesthesia. Ketamine is an analgesic that is most effective when used alongside a low-dose opioid; while it does have analgesic effects by itself, the doses required for adequate pain relief when it is used as the sole analgesic agent are considerably higher and far more likely to produce disorienting side effects.16–19 In our study, we found that the incidence of adverse reactions were greatly increased in those injected with 1 mg/kg ketamine before anesthesia induction than those injected with butorphanol (P<0.05). In addition, injection with ketamine combined with butorphanol before anesthesia could efficiently shorten recovery time and reduce incidence of adverse reactions than application of each drug separately. The results indicated that use of concomitant drugs would not cause oversedation and could be used safely. As previously reported, the incidence of EA in patients with gastric cancer was ~30%.14 According to our study, combined application of ketamine and butorphanol could remarkably reduce the incidence of EA to 6% (3/5). This suggested that ketamine combined with butorphanol might have a synergistic action on the analgesic effect because of different pain reflex arc.20
Based on the findings reported from our study, we speculate that there are may be more promise for the use of ketamine and butorphanol in the other clinically significant areas. In the clinic, both ketamine and butorphanol have been proven to be the effective drugs for analgesia.7–9 Mahran and Hassan21 reported that ketamine is effective in postoperative pain management in breast cancer surgery. Minoshima et al22 also proved that ketamine could play an important role postoperation following adolescent idiopathic scoliosis surgery. Moreover, butorphanol was also applied in therapy of some diseases or for surgery.23 Stambaugh and McAdams24 found that butorphanol could act as an effective drug in chronic cancer pain. Kaur and Bajwa25 reported that the butorphanol could also be used in lower abdominal surgery.
In this study, we found the incidences of EA in group C, group K, and group B were 6.0%, 24.0%, and 20.0% (10/50), respectively. These results suggested that the butorphanol combined with ketamine could decrease the rate of complication compared to butorphanol and ketamine used separately. Previous studies26–28 have proved that both ketamine and butorphanol could improve or decrease the EA in the clinic. However, the EA occurrence (side effect) triggered by ketamine or butorphanol is also not acceptable. In this study, we combined ketamine and butorphanol together and discovered that they cooperated with each other to decrease the EA. The specific mechanism for the effects of the ketamine–butorphanol combination on EA should be further investigated.
Conclusion
Our data provide evidence that injection of ketamine combined with butorphanol before anesthesia induction was more effective than injection of ketamine or butorphanol separately in the prevention of EA. Future studies with a larger number of patients should be performed to validate the findings of our study, which had a small sample size.
Disclosure
The authors report no conflicts of interest in this work.
References
Citrome L. Addressing the need for rapid treatment of agitation in schizophrenia and bipolar disorder: focus on inhaled loxapine as an alternative to injectable agents. Ther Clin Risk Manag. 2013;9:235–245. | ||
Kim NY, Kim SY, Yoon HJ, Kil HK. Effect of dexmedetomidine on sevoflurane requirements and emergence agitation in children undergoing ambulatory surgery. Yonsei Med J. 2014;55(1):209–215. | ||
Kim HJ, Kim DK, Kim HY, Kim JK, Choi SW. Risk factors of emergence agitation in adults undergoing general anesthesia for nasal surgery. Clin Exp Otorhinolaryngol. 2015;8(1):46–51. | ||
Lee MH, Chung MH, Han CS, et al. Comparison of effects of intraoperative semolol and ketamine infusion on acute postoperative pain after remifentanil-based anesthesia in patients undergoing laparoscopic chelecystectomy. Korean J Anesthesiol. 2014;66(3):222–229. | ||
Lee WK, Kim MS, Kang SW, Kim S, Lee JR. Type of anaesthesia and patient quality of recovery: a randomized trial comparing proporol-remifentanil total i.v. anaaesthesia with desflurane anaesthesia. Br J Anaesth. 2015;114(4):663–668. | ||
Draskovic B, Stanic D, Uram-Benka A, Fabri I. Stress indicators during general anesthesia with opioid analgesics in children. Turk J Med Sci. 2014;44(6):1095–1102. | ||
Izer JM, Whitcomb TL, Wilson RP. Atipamezole reverses ketamine-dexmedetomidine anesthesia without altering the antinociceptive effects of butorphanol and buprenorphine in female C57BL/6J mice. J Am Assoc Lab Anim Sci. 2014;53(6):675–683. | ||
Bettschart-Wolfensberger R, Stauffer S, Hassig M, Flaherty D, Ringer SK. Racemic ketamine in comparison to S-ketamine in combination with azaperone and butorphanol for castration of pigs. Schweiz Arch Tierheilkd. 2013;155(12):669–675. | ||
Thakur BP, Sharma SK, Sharma A, Kumar A. Clinical evaluation of detomidine-butorphanol-guaifenesin-ketamine as short term TIVA in Spiti ponies. Pak J Biol Sci. 2011;14(11):647–652. | ||
Papoiu AD, Kraft RA, Coghill RC, Yosipovitch G. Butorphanol suppression of histamine itch is mediated by nucleus accumbens and septal nuclei: a pharmacological fMRI study. J Invest Dermatol. 2015;135(2):560–568. | ||
Love EJ, Taylor PM, Murrell J, Whay HR. Effects of acepromazine, butorphanol and buprenorphine on thermal and mechanical nociceptive thresholds in horses. Equine Vet J. 2012;44(2):221–225. | ||
Du BX, Song ZM, Wang K, et al. Butorphanol prevents morphine-induced pruritus without increasing pain and other side effects: a systematic review of randomized controlled trials. Can J Anaesth. 2013;60(9):907–917. | ||
Polson S, Taylor PM, Yates D. Analgesia after feline ovariohysterectomy under midazolam-medetomidine-ketamine anaesthesia with buprenorphine or butorphanol, and carprofen or meloxicam: a prospective, randomised clinical trial. J Feline Med Surg. 2012;14(8):553–559. | ||
Ren BX, Zong J, Tang JC, et al. Effects of intravenous analgesia with combined dezocine and butorphanol on postoperative cognitive function in elderly patients. Genet Mol Res. 2014;14(2):5571–5576. | ||
Becker WM, Mama KR, Rao S, Palmer RH, Egger EL. Prevalence of dysphoria after fentanyl in dogs undergoing stifle surgery. Vet Surg. 2013;42(3):302–307. | ||
Narayanan S, Shannon A, Nandalan S, Jaitly V, Greer S. Alternative sedation for the higher risk endoscopy: a randomized controlled trial of ketamine use in endoscopic retrograde cholangiopancreatography. Scand J Gastroenterol. 2015;50(10):1293–1303. | ||
Grady MV, Mascha E, Sessler DI, Kurz A. The effect of perioperative intravenous lidocaine and ketamine on recovery after abdominal hysterectomy. Anesth Analg. 2012;115(5):1078–1084. | ||
Kramer KJ, Ganzberg S, Prior S, Rashid RS. Comparison of propofol-remifentanil versus propofol-ketamine deep sedation for third molar surgery. Anesth Prog. 2012;59(3):107–117. | ||
Polat R, Aktay M, Ozlu O. The effects of remifentanil, lidocaine, metoclopramide, or ketamine pretreatment on propofol injection pain. Middle East J Anaesthesiol. 2012;21(5):673–677. | ||
Shukry M, Miller JA. Update on dexmedetomidine: use in nonintubated patients requiring sedation for surgical procedures. Ther Clin Risk Manag. 2010;6:111–121. | ||
Mahran E, Hassan ME. Comparison of pregabalin versus ketamine in postoperative pain management in breast cancer surgery. Saudi J Anaesth. 2015;9(3):253–257. | ||
Minoshima R, Kosugi S, Nishimura D, et al. Intra-and postoperative low-dose ketamine for adolescent idiopathic scoliosis surgery: a randomized controlled trial. Acta Anaesthesiol Scand. 2015;59(10):1260–1268. | ||
De la Garza J. Oral butorphanol tartrate for the long-term treatment of out-patients with moderate to severe cancer pain. J Int Med Res. 1981;9(2):124–127. | ||
Stambaugh JE, McAdams J. Comparison of intramuscular dezocine with butorphanol and placebo in chronic cancer pain: a method to evaluate analgesia after both single and repeated doses. Clin Pharmacol Ther. 1987;42(2):210–219. | ||
Kaur J, Bajwa SJ. Comparison of epidural butorphanol and fentanyl as adjuvants in the lower abdominal surgery: a randomized clinical study. Saudi J Anaesth. 2014;8(2):167–171. | ||
Eghbal MH, Taregh S, Amin A, Sahmeddini MA. Ketamine improves postoperative pain and emergence agitation following adenotonsillectomy in children. A randomized clinical trial. Middle East J Anaesthesiol. 2013;22(2):155–160. | ||
Chen JY, Jia JE, Liu TJ, Qin MJ, Li WX. Comparison of the effects of dexmedetomidine, kemine, and placebo on emergence agitation after strabismus surgery in children. Can J Anaesth. 2013;60(4):385–392. | ||
Guzman DS, Drazenovich TL, KuKanich B, Olsen GH, Willits NH, Paul-Murphy JR. Evaluation of thermal antinociceptive effects and pharmacokinetics after intramuscular administration of butorphanol tartrate to American kestrels (Falco sparverius). Am J Vet Res. 2014;75(1):11–18. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.