Back to Journals » Drug Design, Development and Therapy » Volume 12
Effect of intrathecal dexmedetomidine on preventing shivering in cesarean section after spinal anesthesia: a meta-analysis and trial sequential analysis
Authors Miao S , Shi M , Zou L , Wang G
Received 29 June 2018
Accepted for publication 9 October 2018
Published 2 November 2018 Volume 2018:12 Pages 3775—3783
DOI https://doi.org/10.2147/DDDT.S178665
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sukesh Voruganti
Shuai Miao,* Mengzhu Shi,* Lan Zou, Guanglei Wang
Department of Anesthesiology, The Affiliated Hospital of XuZhou Medical University, XuZhou, JiangSu, China
*These authors contributed equally to this work
Objective: Intrathecal dexmedetomidine (DEX) has been used to prevent shivering in patients undergoing cesarean section. The aim of this meta-analysis was to evaluate whether intrathecal DEX could prevent shivering in cesarean section after spinal anesthesia.
Methods: We searched PubMed, Embase, Web of Science, and the Cochrane Library for randomized controlled trials (RCTs) comparing intrathecal DEX in cesarean section after spinal anesthesia with placebo and reporting on shivering, postoperative nausea and vomiting (PONV), hypotension, and bradycardia. Trial sequential analysis (TSA) was also carried out for RCTs comparing DEX with placebo. This meta-analysis has been registered on PROSPERO, and the registration number is CRD42017071640.
Results: Six randomized clinical trials comparing 360 patients were included in this study. Compared with placebo, intrathecal DEX significantly reduced the incidence of shivering (risk ratio [RR]=0.40; 95% CI [0.26, 0.62]; P<0.0001). No significant difference was found in the incidence of PONV (RR=1.34; 95% CI [0.82, 2.18]; P=0.24), hypotension (RR=1.09; 95% CI [0.84, 1.42]; P=0.50), or bradycardia (RR=1.55; 95% CI [0.54, 4.42]; P=0.42). However, no firm conclusions can be made based on the results of all outcomes according to the TSA.
Conclusion: This meta-analysis found that intrathecal DEX could prevent shivering in cesarean section after spinal anesthesia and did not induce PONV, hypotension, or bradycardia. However, firm conclusions cannot be made until more studies are conducted.
Keywords: dexmedetomidine, cesarean section, spinal anesthesia, meta-analysis, trial sequential analysis, adverse effect
Introduction
Spinal anesthesia has been widely used to provide anesthesia and analgesia for cesarean section, which allows a patient to remain awake for the birth of her baby while avoiding the risks of general anesthesia.1–3 Even though the risk of spinal anesthesia is lower than that of general anesthesia, the adverse events caused by spinal anesthesia, such as shivering, are still present during surgical procedures, causing discomfort for the patients.4,5
The incidence of shivering is up to 55% according to a previously published study.6 Shivering may lead to increased oxygen consumption and negative effects on pulse, oxygen saturation, and blood pressure. Severe adverse effects may occur if the patient has cardiopulmonary insufficiency.6,7
Dexmedetomidine (DEX), as a highly selective α-2 adrenoreceptor agonist, can provide some beneficial effects when administered through the spinal route, such as sedation, analgesia, antianxiety effects, and increased threshold of shivering.8,9 Some randomized controlled trials (RCTs) have been conducted to estimate the effects of intrathecal DEX on shivering. However, the effect of intrathecal DEX on shivering in cesarean section after spinal anesthesia remains controversial. Therefore, we conducted this meta-analysis from relevant studies to evaluate the effects of intrathecal DEX on shivering in cesarean section after spinal anesthesia. Additionally, trial sequential analysis (TSA) was carried out for RCTs comparing DEX with placebo.
Subjects and methods
Protocol and registration
We followed the recommendations of the Cochrane Handbook for Systematic Reviews of Interventions10 and the PRISMA recommendations to conduct the present meta-analysis.11 The protocol has been registered in the PROSPERO international database under number CRD42017071640.
Search strategy
Two authors searched PubMed, Embase, Web of Science, and the Cochrane Library using search terms (“dexmedetomidine”, “cesarean section”, “cesarean delivery”, “spinal anesthesia”) up to March 31, 2018, without language restriction. We also manually searched for the references of the identified trials and systematic reviews to identify any potentially relevant trials. Titles, abstracts, and full texts of potentially relevant articles were screened after excluding duplicated ones.
Eligibility criteria
The following were the eligibility criteria:
- Participants: adult patients undergoing cesarean section after spinal anesthesia.
- Interventions: any study was considered if DEX was delivered via intrathecal route.
- Comparisons: patients who were exposed to placebo.
- Outcomes: at least one of the following outcomes was reported in the studies: incidence of shivering, postoperative nausea and vomiting (PONV), hypotension, or bradycardia.
- Study design: only RCTs were regarded as eligible for this meta-analysis.
Study selection and data extraction
Two authors independently identified the studies for inclusion according to the eligibility criteria. Any disagreement was resolved by consensus. The following data were extracted from each included study: first author, country, year of publication, number of participants, intervention details, and trial characteristics. The primary outcome was shivering, and the secondary outcomes included PONV, hypotension, and bradycardia.
Assessment of study quality
Two authors independently assessed the risk of bias in the included studies. The following factors were assessed according to the Cochrane risk of bias tool for each study:12 1) random sequence generation; 2) allocation concealment; 3) blinding of participants and personnel; 4) blinding of outcome assessors; 5) incomplete outcome data; and 6) selective outcome reporting and other bias. Trials were classified as having low, unclear, or high risk of bias on each domain. Trials were considered to have a high risk of bias if one or more of these domains were scored as unclear or high risk of bias.
Statistical analyses
All the statistical analyses were conducted using Review Manager, version 5.3 (The Nordic Cochrane Center, The Cochrane Collaboration, Copenhagen, 2014). Dichotomous outcomes were reported by the risk ratio (RR) and 95% CI. Heterogeneity was assessed with the I2 statistic.13 We selected the random-effects model to calculate pooled effects, regardless of whether I2 was ≥50%.
Subgroup analysis and sensitivity analysis were performed on factors that may have contributed to the heterogeneity. Subgroup analysis was carried out according to the drug dosage (5 and 10 μg). Sensitivity analysis was performed by using both the random-effects and fixed-effects analysis models.14 Sensitivity analysis was also performed by removing one study at a time to assess the effect of each study on the overall effect size.
TSA
The reliability and conclusiveness of the available evidence were examined by TSA, which can reduce false-positive results caused by multiple testing and sparse data.15,16 We calculated the required information size (RIS) and the trial sequential monitoring boundaries for all outcomes. The variance was calculated from the data obtained from the included studies. If the cumulative Z curve crossed the TSA boundary, a sufficient level of evidence for the anticipated intervention effect may have been reached and no further studies are needed. However, if the Z curve failed to cross the TSA boundaries and the RIS has not been reached, evidence to reach a conclusion is insufficient. We used two-sided tests with a type I error of 5%, a power of 80%, and a relative risk reduction of 20% to calculate the RIS.17
Results
Trial selection
Figure 1 shows a summary of the study selection process. We identified 104 studies through database searching. After excluding duplicate references and reviewing titles and abstracts, we selected 13 studies for full-text evaluation. Of these, seven trials did not meet the inclusion criteria or the exclusion criteria. The main reasons for exclusion included that the study was not randomized (n=1), that DEX was not given by intrathecal route (n=5), or that the study did not report outcomes that we needed (n=1). Finally, six studies were included.18–23
  | Figure 1 PRISMA flowchart of the included studies. |
Characteristics and quality of included studies
Table 1 shows the details of all the studies included in the meta-analysis. All studies used bupivacaine as the local anesthetic. However, the dose of DEX was different between the studies. In all, 10 μg of DEX was used in two studies and 5 μg of DEX was used in the other four trials. Two authors independently evaluated the quality of all the RCTs. All the six studies were classified as low risk of bias, and details of the risk of bias for all of the included studies are shown in Figure 2.
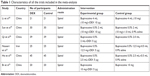  | Table 1 Characteristics of all the trials included in the meta-analysis |
  | Figure 2 The risk of bias of all the included trials. |
Outcomes of the meta-analysis
Shivering
All studies reported the incidence of shivering. Data extracted from relevant studies indicated that the addition of DEX significantly reduced the incidence of shivering (RR=0.40, 95% CI [0.26, 0.62]; Figure 3) according to conventional meta-analysis. The TSA indicated that the Z curve failed to cross the TSA boundary and did not reach the RIS but crossed the conventional boundary (Figure 4).
  | Figure 3 Forest plot for the incidence of shivering with and without DEX. |
  | Figure 4 TSA evaluating the incidence of shivering with and without DEX. |
Subgroup analysis showed that the only the application of DEX 5 μg (RR=0.41, 95% CI [0.26, 0.63]) was significantly more effective than that of placebo for the prevention of shivering, while no difference was found between the two groups when DEX 10 μg was injected.
Heterogeneity was found in the incidence of shivering; therefore, sensitivity analysis was performed. By removing one study at a time, the sensitivity analysis had no effect on the result.
PONV
All six studies reported PONV. It was found that the addition of DEX did not reduce the risk of PONV (RR=1.34, 95% CI [0.82, 2.18]; Figure 5). The TSA indicated that the Z curve failed to cross the conventional boundary or the TSA boundary (Figure 6).
  | Figure 5 Forest plot for the incidence of PONV with and without DEX. |
Hypotension
Data from five studies involving 320 participants showed that the risk of hypotension was similar in the two groups (RR=1.09, 95% CI [0.84, 1.42]; Figure 7). The TSA indicated that the Z curve failed to cross the conventional boundary or the TSA boundary (Figure 8).
  | Figure 7 Forest plot for the incidence of hypotension with and without DEX. |
  | Figure 8 TSA evaluating the incidence of hypotension with and without DEX. |
Bradycardia
Five studies reported data about the occurrence of bradycardia, which showed that the rate of bradycardia in the DEX group was similar to that in the control group (RR=1.55, 95% CI [0.54, 4.42]; Figure 9). There was too little information to conduct the TSA boundary.
  | Figure 9 Forest plot for the incidence of bradycardia with and without DEX. |
Discussion
For the present study, we retrieved six RCTs to investigate the effect of DEX as a neuraxial adjuvant in cesarean section. We found that spinal administration of DEX could effectively prevent perioperative shivering but did not increase the incidence of PONV or induce bradycardia and hypotension.
DEX is a highly selective α2-adrenergic receptor agonist and can provide many beneficial effects, such as sedation, analgesia, perioperative sympatholysis, and synesthetic sparing, without depressing respiration.24,25 Because of these beneficial effects, DEX is widely used clinically, including for cesarean section.18,26 However, when administered by intravenous route, DEX can induce some negative side effects, such as bradycardia and hypotension.27 To decrease side effects, some clinicians choose to decrease the dose or change the administration route. Several studies demonstrated that epidural administration of DEX could provide some beneficial effects, including sedation, analgesia, and antianxiety effects.24,25
Shivering is one of the common complications of neuraxial anesthesia and causes discomfort and dissatisfaction in patients undergoing cesarean section. It was reported6 that the incidence of shivering after regional anesthesia was up to 55% and the incidence in cesarean section may be even higher.28 Shivering increases the consumption of oxygen and production of CO2 and may also intensify postoperative pain.29 The reasons for shivering may include suppression of spinal reflex activity, decrease in sympathetic activity, and inhibition of adrenal function.30 Many studies reported that DEX could effectively reduce the incidence of shivering during neuraxial anesthesia in patients undergoing cesarean section, without any associated respiratory depression as with other antishivering drugs, such as meperidine.18–22 The present meta-analysis showed the same results reported in previous clinical trials. Some studies demonstrated that DEX could increase the temperature range without triggering thermoregulatory defenses by stimulating central α2-adrenergic receptors, thereby decreasing the central thermoregulatory threshold for shivering.31–33 The antishivering effect of intrathecal DEX may be caused by decreasing the shivering threshold.34 Additionally, DEX could prevent postoperative shivering by attenuating the hyperadrenergic response to perioperative stress.35 The data from four studies showed that the incidence of shivering was significantly lower than that in placebo when given DEX 5 μg, revealing that the low dosage of DEX may be more efficacious.
The present meta-analysis showed that, unlike opioids, DEX did not increase the incidence of PONV caused by cesarean section. PONV is a common complication of cesarean section. The causes for PONV during cesarean section include hypotension, visceral traction, and vagal stimulation, and among them, hypotension may be the most common cause.36,37 It was reported that compared with intravenous infusion, intrathecal DEX seldom induced hypotension,38 which is in accordance with the meta-analysis and why spinal administration of DEX would not increase the incidence of PONV. Opioids, which are the most common neuraxial adjuvants to local anesthetics, have many disadvantages, including PONV. Several studies reported that the usage of DEX could reduce the demand for opioids during cesarean section.18,19,30 This means that DEX may not only increase the incidence of PONV but also reduce the incidence of PONV during cesarean section.
There are some potential limitations associated with the included studies that should be considered when interpreting the results of our study. First, the number of included RCTs and the sample size were small, and thus, our conclusions are still based on a relatively small number of studies. Second, since we only included published literature, the search strategy could have also affected the results of this study. Third, the doses of DEX in the retrieved trials are varied. This demonstrated that the clinical use of DEX is not strictly regulated. Fourth, most of the studies included in this meta-analysis were conducted in China, and more studies conducted in different countries are still needed to confirm our findings. Additionally, further studies should be designed to demonstrate the effect of DEX on the neonatus.
Conclusion
This study showed that intrathecal DEX reduced the risk of shivering in patients undergoing cesarean section after spinal anesthesia and did not increase the incidence of PONV, hypotension, or bradycardia. However, the TSA indicated that further trials are still needed to confirm the abovementioned findings.
Disclosure
The authors report no conflicts of interest in this work.
References
Carrie LE. Extradural, spinal or combined block for obstetric surgical anaesthesia. Br J Anaesth. 1990;65(2):225–233. | ||
Buvanendran A, Mccarthy RJ, Kroin JS, Leong W, Perry P, Tuman KJ. Intrathecal magnesium prolongs fentanyl analgesia: a prospective, randomized, controlled trial. Anesth Analg. 2002;95(3):661–666. | ||
Braga AF, Braga FS, Potério GM, Pereira RI, Reis E, Cremonesi E. Sufentanil added to hyperbaric bupivacaine for subarachnoid block in Caesarean section. Eur J Anaesthesiol. 2003;20(8):631–635. | ||
Lussos SA, Bader AM, Thornhill ML, Datta S. The antiemetic efficacy and safety of prophylactic metoclopramide for elective cesarean delivery during spinal anesthesia. Reg Anesth. 1992;17(3):126–130. | ||
Kranke P, Eberhart LH. Possibilities and limitations in the pharmacological management of postoperative nausea and vomiting. Eur J Anaesthesiol. 2011;28(11):758–765. | ||
Crowley LJ, Buggy DJ. Shivering and neuraxial anesthesia. Reg Anesth Pain Med. 2008;33(3):241–252. | ||
Roy JD, Girard M, Drolet P. Intrathecal meperidine decreases shivering during cesarean delivery under spinal anesthesia. Anesth Analg. 2004;98(1):230–234. | ||
Graham AC, Mcclure JH. Quantitative assessment of motor block in labouring women receiving epidural analgesia. Anaesthesia. 2001;56(5):470–476. | ||
Bajwa SJ, Gupta S, Kaur J, Singh A, Parmar S. Reduction in the incidence of shivering with perioperative dexmedetomidine: A randomized prospective study. J Anaesthesiol Clin Pharmacol. 2012;28(1):86–91. | ||
Higgins JPT, Green S. Cochrane handbook for systematic reviews of interventions version 5.1.0 [updated March 2011]. The Cochrane Collaboration; 2011. | ||
Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8(5):336–341. | ||
Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. | ||
Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. | ||
Dersimonian R, Kacker R. Random-effects model for meta-analysis of clinical trials: an update. Contemp Clin Trials. 2007;28(2):105–114. | ||
Brok J, Thorlund K, Gluud C, Wetterslev J. Trial sequential analysis reveals insufficient information size and potentially false positive results in many meta-analyses. J Clin Epidemiol. 2008;61(8):763–769. | ||
Brok J, Thorlund K, Wetterslev J, Gluud C. Apparently conclusive meta-analyses may be inconclusive – Trial sequential analysis adjustment of random error risk due to repetitive testing of accumulating data in apparently conclusive neonatal meta-analyses. Int J Epidemiol. 2009;38(1):287–298. | ||
Wetterslev J, Thorlund K, Brok J, Gluud C. Trial sequential analysis may establish when firm evidence is reached in cumulative meta-analysis. J Clin Epidemiol. 2008;61(1):64–75. | ||
Li Z, Tian M, Zhang CY, et al. A Randomised Controlled Trial to Evaluate the Effectiveness of Intrathecal Bupivacaine Combined with Different Adjuvants (Fentanyl, Clonidine and Dexmedetomidine) in Caesarean Section. Drug Res. 2015;65(11):581–586. | ||
Sun Y, Xu Y, Wang GN. Comparative Evaluation of Intrathecal Bupivacaine Alone, Bupivacaine-fentanyl, and Bupivacaine-dexmedetomidine in Caesarean Section. Drug Res. 2015;65(9):468–472. | ||
Qi X, Chen D, Li G, et al. Comparison of Intrathecal Dexmedetomidine with Morphine as Adjuvants in Cesarean Sections. Biol Pharm Bull. 2016;39(9):1455–1460. | ||
Nasseri K, Ghadami N, Nouri B. Effects of intrathecal dexmedetomidine on shivering after spinal anesthesia for cesarean section: a double-blind randomized clinical trial. Drug Des Devel Ther. 2017;11:1107–1113. | ||
He L, Xu JM, Liu SM, Chen ZJ, Li X, Zhu R. Intrathecal Dexmedetomidine Alleviates Shivering during Cesarean Delivery under Spinal Anesthesia. Biol Pharm Bull. 2017;40(2):169–173. | ||
Bi YH, Cui XG, Zhang RQ, Song CY, Zhang YZ. Low dose of dexmedetomidine as an adjuvant to bupivacaine in cesarean surgery provides better intraoperative somato-visceral sensory block characteristics and postoperative analgesia. Oncotarget. 2017;8(38):63587–63595. | ||
Mantz J, Josserand J, Hamada S. Dexmedetomidine: new insights. Eur J Anaesthesiol. 2011;28(1):3–6. | ||
Wagner DS, Brummett CM. Dexmedetomidine: as safe as safe can be. Seminars in Anesthesia, Perioperative Medicine and Pain. 2006;25(2):77–83. | ||
Shaikh SI, Mahesh SB. The efficacy and safety of epidural dexmedetomidine and clonidine with bupivacaine in patients undergoing lower limb orthopedic surgeries. J Anaesthesiol Clin Pharmacol. 2016;32(2):203–209. | ||
Yq L, Feng YY, Yd L. Comparation of effect of dexmedetomidine and propofol in patients with caesarean section. Practical Pharmacy and Clinical Remedies. 2015;18:920–922. | ||
Hong JY, Lee IH. Comparison of the effects of intrathecal morphine and pethidine on shivering after Caesarean delivery under combined-spinal epidural anaesthesia. Anaesthesia. 2005;60(12):1168–1172. | ||
Park SM, Mangat HS, Berger K, Rosengart AJ. Efficacy spectrum of antishivering medications: meta-analysis of randomized controlled trials. Crit Care Med. 2012;40(11):3070–3082. | ||
Bao Z, Zhou C, Wang X, Zhu Y. Intravenous dexmedetomidine during spinal anaesthesia for caesarean section: A meta-analysis of randomized trials. J Int Med Res. 2017;45(3):924–932. | ||
Doufas AG, Lin CM, Suleman MI, et al. Dexmedetomidine and meperidine additively reduce the shivering threshold in humans. Stroke. 2003;34(5):1218–1223. | ||
Bicer C, Esmaoglu A, Akin A, Boyaci A. Dexmedetomidine and meperidine prevent postanaesthetic shivering. Eur J Anaesthesiol. 2006;23(2):149–153. | ||
Elvan EG, Oç B, Uzun S, Karabulut E, Coşkun F, Aypar U. Dexmedetomidine and postoperative shivering in patients undergoing elective abdominal hysterectomy. Eur J Anaesthesiol. 2008;25(5):357–364. | ||
Paris A, Tonner PH. Dexmedetomidine in anaesthesia. Curr Opin Anaesthesiol. 2005;18(4):412–418. | ||
Talke P, Tayefeh F, Sessler DI, Jeffrey R, Noursalehi M, Richardson C. Dexmedetomidine does not alter the sweating threshold, but comparably and linearly decreases the vasoconstriction and shivering thresholds. Anesthesiology. 1997;87(4):835–841. | ||
Heesen M, Klimek M, Hoeks SE, Rossaint R. Prevention of spinal anesthesia-induced hypotension during cesarean delivery by 5-hydroxytryptamine-3 receptor antagonists: a systematic review and meta-analysis and meta-regression. Anesth Analg. 2016;123(4):977–988. | ||
Langesaeter E, Rosseland LA, Stubhaug A. Continuous invasive blood pressure and cardiac output monitoring during cesarean delivery: a randomized, double-blind comparison of low-dose versus high-dose spinal anesthesia with intravenous phenylephrine or placebo infusion. Anesthesiology. 2008;109(5):856–863. | ||
Yousef AA, Salem HA, Moustafa MZ. Effect of mini-dose epidural dexmedetomidine in elective cesarean section using combined spinal-epidural anesthesia: a randomized double-blinded controlled study. J Anesth. 2015;29(5):708–714. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

