Back to Journals » Drug Design, Development and Therapy » Volume 18
Effect of High-Fat Meal on the Pharmacokinetics and Safety of Valsartan/Amlodipine Fixed Dose Combination Tablets in Healthy Subjects
Authors Song H, Qiu B, Sun X, Guo C, Hu Y, Bai W , Dong Z
Received 16 August 2023
Accepted for publication 29 December 2023
Published 11 January 2024 Volume 2024:18 Pages 43—51
DOI https://doi.org/10.2147/DDDT.S423374
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Georgios Panos
Haojing Song, Bo Qiu, Xue Sun, Caihui Guo, Yiting Hu, Wanjun Bai, Zhanjun Dong
Department of Medicine, Hebei General Hospital, Shijiazhuang, 050051, People’s Republic of China
Correspondence: Wanjun Bai; Zhanjun Dong, Department of Medicine, Hebei General Hospital, Shijiazhuang, 050051, People’s Republic of China, Tel +86-0311-85988326 ; +86-0311-85988604, Fax +86-0311-85988604, Email [email protected]; [email protected]
Background: The objective of this study was to evaluate the effect of a high-fat meal on the pharmacokinetics and safety of 80/5 mg valsartan/amlodipine tablets in healthy subjects.
Subjects and Methods: These results were derived from a bioequivalence trial where subjects were randomly assigned to take valsartan/amlodipine 80/5mg under fed conditions or after a high-fat meal contained 978.6 kilocalories (54.6% from fat). The blood samples were collected and plasma concentrations of valsartan/amlodipine were measured using high-performance liquid chromatography-mass spectrometry. The non-compartmental module of Phoenix WinNonlin Version 8.2 was used to calculate pharmacokinetic parameters. The BE module of WinNonLin was used to analyze the statistics of the maximum plasma concentration (Cmax), the area under the concentration-time curve from zero to the last quantifiable time point (AUC0-t), and the area under the concentration-time curve from zero to infinity(AUC0–∞) in plasma. 88 healthy subjects were enrolled and divided into in a fasted group and a fed group.
Results: The Cmax, AUC0–t, and AUC0–∞ of valsartan in plasma under fed conditions were 51%, 56%, and 57% lower, respectively, than those under fasted conditions, and the 90% confidence interval (90% CI) were outside the 80.00– 125.00% range. All the pharmacokinetic parameters for amlodipine under fed conditions were similar to those observed under fasted conditions, and the 90% CIs were within the 80.00– 125.00% range. The incidence of treatment emergent adverse events (TEAE) was similar between the fasted group and the fed group, while adverse drug reaction (ADR) was more frequent in the fasted group which may be related to the higher blood concentrations of valsartan, but all were mild.
Conclusion: The result indicated that the high-fat meal had a significant effect on the pharmacokinetics of valsartan, but no effect on amlodipine. All treatments were safe and well tolerated in healthy subjects under fed and fasted conditions.
Keywords: amlodipine, valsartan, high-fat meal, pharmacokinetics
Introduction
Over one billion people worldwide suffer from hypertension, one of the most important risk factor for cardiovascular diseases, leading to 9.4 million deaths annually. Furthermore, it is reported that suboptimal blood pressure is the most important risk factors for death all over the world.1 In spite of the wide availability of medications, hypertension is not well managed, even though it can be controlled. It may be beneficial for many patients to use combination therapy to control their blood pressure2 and nearly two-thirds of the patients take multiple antihypertensive medications.3 The most effective combination therapies include renin-angiotensin-aldosterone blockers, such as angiotensin-II receptor blockers (ARBs) or angiotensin-converting enzyme inhibitors (ACEIs), administered together with calcium channel blockers (CCBs) if blood pressure levels need it combination for lowering BP.4
Although single-drug therapy may be effective for some patients, active treatment with two or more antihypertensive drugs targeting different physiologic pathways provides an alternative method for most patients to achieve blood pressure goals. Amlodipine (calcium channel blocker) and valsartan (angiotensin receptor blocker) are antihypertensive drugs available as single-drug therapy.5–8
Given the need for multiple antihypertensive drugs in a large number of patients, to make use of the complementary mechanism of action, valsartan/amlodipine dual-fixed combined tablets have been developed to treat patients with moderate to severe hypertension. Furthermore, fixed-dose combination therapy is expected to improve patients’ compliance by reducing the number of dosage forms and simplifying the treatment plan.9
Following oral administration of valsartan/amlodipine fixed dose combination tablets in normal healthy adults, peak plasma concentrations of valsartan and amlodipine are reached in 3 and 6 to 8 hours, respectively. The rate and extent of absorption of valsartan and amlodipine from valsartan/amlodipine fixed-dose combination tablets are the same as when administered as individual tablets.10
Food can increase or decrease drug exposure through various mechanisms, and formulation factors can also change the effect of food.11–13 There are no significant food effects reported when amlodipine tablets/capsules monotherapies are administered with food in the studies.14–16 In contrast, valsartan Cmax and AUC decreased by 50% and 40%, respectively, when administered with food.17
Results of the study showed that following the administration of a fixed combination tablet containing valsartan/amlodipine 160/10 mg, the bioavailability of amlodipine and valsartan were consistent between the fed and fasted conditions.18
Considering that fixed-dose combination tablets are new formulations, it is important to investigate the effect of food on the bioavailability of active drug components of these tablets. As these drugs are intended for chronic administration, it is imperative to obtain this data so that dosing instructions can be supported.
Based on the difference in the effect of food on the bioavailability of valsartan in two studies,17,18 the purpose of this study was to assess the effect of a high-fat, high-calorie meal on the pharmacokinetics of valsartan/amlodipine and to evaluate the safety of oral administration of valsartan/amlodipine 80/5mg in healthy subjects.
Subjects
Healthy subjects in China were screened for eligibility about 10 days before drug administration. Eligibility criteria included healthy Chinese adults, aged 18 and above, body mass index (BMI) of 19–26 kg/m2, and a minimum weight of 45 kg (female) and 50 kg(male). There was no history of endocrine, cardiovascular, neurological, metabolic, hepatic, gastrointestinal, pulmonary, immunological, infectious, or psychiatric diseases in the subjects. All Subjects with a history of alcoholism, smoking or drug dependence, or under concomitant treatments defined as using any drugs that inhibit or induce liver metabolizing enzymes within 28 days, or having undergone surgery in the past 3 months were excluded from the study. In addition, female subjects who were pregnant, planned to be pregnant, or used oral contraceptives were excluded from this study. During the study, taking other drugs was not allowed. Anyone could freely withdraw from the study at any time.
A bioequivalence clinical trial conducted at the clinic drug clinical trial Institution of Hebei General Hospital provided the study population. The ethical meeting time of the bioequivalence clinical trial was Oct. 22, 2020, and the clinical trial protocol and amendments were approved by the Medical Ethics Committee of Hebei General Hospital on Nov. 05, 2020 (approval No. 2020-11-01).
The bioequivalence clinical trial was registered on the Chinese Clinical Trial website (http://www.chinadrugtrials.org.cn/index.html #CTR20210214, date: Feb 04, 2021). The time of the kick-off meeting for the clinical trial is Feb. 23, 2021, and the time for the first subject to sign informed consent is Mar. 08, 2021.
The Chinese Clinical Trial website is commonly used, but it is unfortunately not recognized by the WHO. So the bioequivalence clinical trial was retrospectively registered at the Chinese Clinical Trial Registry which is recognized by the WHO [https://www.chictr.org.cn/] (number: ChiCTR2300073300, date: July 6, 2023). All individuals gave their consent in writing for the clinical trial. This study was conducted in compliance with the Guidelines for Good Clinical Practice recommended by NMPA, the International Conference on Harmonization Good Clinical Practice Guidelines, and the ethical principles of the Declaration of Helsinki. The participants were free to withdraw from the study at any time.
Study Design and Procedures
The bioequivalence clinical trials were randomized, open-label, cross-over, three-period, three-sequence, single-center studies. The periods were separated by a 14-day washout period, and the analyses of the plasma concentrations of valsartan and amlodipine were conducted with all related information concealed.
The bioequivalence clinical trial consisted of two independent trials: a fasted trial and a fed trial. The participants in the fasted trial were randomly assigned into groups A (TRR), B (RTR), or C (RRT) of 15 subjects each. The participants in the fed trial were randomly assigned into groups D (TRR), E (RTR), or F (RRT) of 15 subjects each. The fasted and fed trials each enrolled 45 subjects. Valsartan/amlodipine tablet (I) which was produced and provided by Shijiazhuang No.4 Pharmaceutical Co.Ltd, and valsartan/amlodipine tablet (I) which is marketed under the brand name EXFORGE® and produced by Novartis Pharma Schweiz, were used as the T and R formulations.
The purpose of this study is to evaluate the influence of food on the bioavailability of valsartan and amlodipine, so all the pharmacokinetic parameters for valsartan and amlodipine of the fasted trial and the fed trial were compared and analyzed.
The pharmacokinetic parameters of all subjects who took R preparation for the first time of the fasted trial and the fed trial were selected to evaluate the influence of food on the bioavailability of valsartan and amlodipine in this study. The fasted and fed trials each enrolled 45 subjects and one subjects in each study did not take R preparation due to withdrawing from the trial. Figure 1 shows the screening and inclusion distributions of the participants in the two groups.
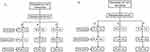 |
Figure 1 Subjects flow chart. Flow chart of the subjects in the fasted state (A). Flow chart of the subjects in the fed state (B). N: the number of subjects. |
We analyzed the main pharmacokinetic parameter of healthy volunteers after a single oral dose administration of 80/5mg valsartan/amlodipine tablets under fasted and fed conditions, and valsartan/amlodipine tablets were purchased from Novartis Farmaceutica S.A. (Switzerland, batch number: BNH61).
In the study, 88 healthy male and female subjects were enrolled, and the subjects were divided into two following groups. Fasted group: valsartan/amlodipine tablets of 80/5mg was administered at least 10 hours after fasting, fed group: valsartan/amlodipine tablets of 80/5mg were administered half an hour after a high-fat meal (timed from the start of the meal), and the high-fat meal contained 800 to 1000 kilocalorie (about 50% from fat) (meal composition was detailed Table 1).
 |
Table 1 The Ingredient List of High-Fat Meal |
Pharmacokinetic Evaluations
Blood samples for pharmacokinetic (PK) evaluation were collected at 0 h before the initiation of dosing (pre-dose) and at 0.5, 1.0, 1.5, 2.0, 2.5, 3.0, 3.5, 4.0, 4.5, 5.0, 5.5, 6.0, 7.0, 8.0, 9.0, 10.0, 11.0, 12.0, 24.0, 48.0 and 72.00h after dosing. Blood samples were centrifuged at 2600g for 10 min at 4 °C. Centrifugation was completed within 60 min after sample collection. The plasma was stored at −80°C for further analysis. Plasma concentrations of valsartan and amlodipine were determined using liquid chromatography-mass spectrometry (LC-MS/MS).
Safety Evaluations
All participants in the study were included in the safety analysis. Safety was assessed by vital signs, physical examination, electrocardiogram(ECG), laboratory examination, adverse events (AEs), and combined medication. According to the NCI CTCAE v5.0, during the trial all AEs were classified into mild, moderate, and severe levels.
Statistical Analysis
Phoenix WinNonlin Version 8.0 (Pharsight Corporation, Sunnyvale, CA, USA) software was used to calculate the pharmacokinetic parameters (Tmax, Cmax, AUC0–t, AUC0–∞, t1/2, V/F, CL/F) of valsartan and amlodipine using a non-compartmental method (NCA module).The Linear Up-Log-Down trapezoidal method was used for AUC calculation. The BE module of WinNonLin was used to analyze AUC0–t, AUC0–∞, and Cmax of valsartan and amlodipine under fasted and fed conditions after logarithmic conversion. The adjusted mean difference (Fed/Fasted) and its 90% confidence interval estimated by the model, were taken as the negative number to obtain the corresponding PK parameter geometric mean ratio (Fed/Fasted), to estimate its 90% confidence interval, and to evaluate the effect of a high-fat meal on the pharmacokinetics of valsartan and amlodipine.
Bioanalytical Methods
The concentrations of valsartan and amlodipine in plasma in healthy subjects were measured by a newly developed and validated LC-MS/MS method. ExionLC AD (AB SCIEX, USA) and a TRIPLE QUAD 5500 Mass Spectrometer with ESI source (AB SCIEX, USA) were used. Briefly, valsartan-d3 and amlodipine-d4 were added as internal standard, and protein was precipitated from the plasma samples. Then 4 μL of the supernatant was injected and separated on an ACQUITY UPLC BEH C18 (1.7μm,2.1×50mm) column with gradient elution at a flow rate of 0.5 mL/min (mobile phase A, 0.2% formic acid in water; mobile phase B, 0.2% formic acid in acetonitrile). The column temperature was maintained at 40 °C. Four transitions, m/z 436.30/291.20 for valsartan, m/z 439.30/294.20 for valsartan-d3, 409.300/238.000 for amlodipine, and 413.30/238.0 for amlodipine-d4, were recorded for quantification. The optimal instrument condition was set as follows: Heater temperature at 500 °C; ion spray voltage as 4500 V; declustering potential as 116 V for both valsartan and valsartan-d3 (IS), and 80 V for amlodipine and amlodipine-d4 (IS); collision energy as 50 V for both valsartan and valsartan-d3 (IS), and 15 V for amlodipine and amlodipine-d4 (IS). Curtain gas was set as 35 psi, and gas 1 and gas 2 were both set as 50 psi.
The linear range is 20.00 ng/mL to 6000.00 ng/mL for valsartan, and 0.10 ng/mL to 10.00 ng/mL for amlodipine. The lower limits of quantitation (LLOQ) of valsartan and amlodipine were 20.00 and 0.10 ng/mL, respectively. The intra-batch and inter-batch precision in plasma samples were less than 3.9% and 4.1% for valsartan, 4.4% and 7.3% for amlodipine, respectively. The intra-batch and inter-batch accuracy was in the range of −0.5% to 6.5% and 2.0% to 2.8% for valsartan, 0.0% to 9.0% and 2.0% to 6.5% for amlodipine.
Results
Subjects
This study was conducted between October 2020 and July 2021. 88 subjects were invited to participate in the study and were assigned to a fasted group or fed group (n=44 each). 28 male subjects and 16 female subjects were in the fasted group, and 29 male subjects and 15 female subjects were in the fed group. Age, height, weight, and BMI of the subjects were 32.84±8.09 years, 166.53±8.15 cm, 63.25±7.98 kg, and 22.76±1.89 kg/m2, respectively. The demographic and baseline characteristics of all subjects are presented in Table 2. 88 subjects were included in pharmacokinetics evaluations of fasting and the food effect on valsartan and amlodipine pharmacokinetics.
 |
Table 2 Demographic Information and Baseline Information of Subjects in Groups |
Pharmacokinetic Evaluations
The mean plasma concentration versus time profiles of valsartan and amlodipine in healthy Chinese subjects receiving a single oral dose of valsartan/amlodipine tablet (80/5mg) are shown in Figure 2.
The Cmax, AUC0–t, AUC0–∞, and the secondary pharmacokinetic endpoints (Tmax, t1/2, V/F, CL/F, and MRT) of valsartan and amlodipine are shown in Table 3. Mean (± standard deviation) Cmax, AUC0–t, AUC0–∞ of valsartan in plasma after a high-fat meal (1411.14±536.64 ng/mL, 8937.32±3356.51ng×h/mL, 9296.81±3430.08 ng×h/mL respectively) were lower than under fasted conditions (2888.34±1426.15ng/mL, 16,115.53±6503.42 ng×h/mL, 16,547.62±6663.39 ng×h/mL, respectively). V/F, CL/F, and MRT of valsartan in plasma after a high-fat meal (82,351.00±51,729.98 mL, 9774.90±3508.89 mL/h, 7.65±1.54 h, respectively) were higher than that under fasted conditions (44,452.13±20,825.24mL, 5748.59±2423.80mL/h, 6.49±1.05 h, respectively).
 |
Table 3 Mean Pharmacokinetic Parameter Values for Valsartan and Amlodipine |
Mean (± standard deviation) Cmax, AUC0–t, AUC0–∞, V/F, CL/F, and MRT of amlodipine in plasma after a high-fat meal (3.72±0.92 ng/mL; 122.40±28.92 ng×h/mL; 184.89±54.87 ng×h/mL, 1,819,424.12±411,380.42 mL, 29,138.51±7637.61 mL/h, 29.83±1.87 h, respectively) were similar with under fasted conditions (3.86±0.83ng/mL, 119.95±27.67 ng×h/mL, 174.78±53.43 ng×h/mL, 1,799,681.41±555,747.93 mL, 32,564.22±17,163.63 mL/h, 28.47±1.88 h, respectively).
Effect of a High-Fat Meal on the Pharmacokinetics
The least-square geometric mean (LSGM) ratio of valsartan and amlodipine and their 90% confidence interval (CI) between fasted and fed conditions are shown in Table 3.
Valsartan. The Cmax, AUC0–t, and AUC0–∞ of valsartan in plasma after a high-fat meal were 51%, 56%, and 57% lower, respectively than those under the fasted conditions. The 90% CIs of Cmax, AUC0–t, and AUC0–∞ between fasted and fed conditions were 44.18 to 55.90, 44.87 to 64.65, and 49.76 to 65.52, respectively, which were outside the 80.00–125.00% range. This indicated that a high-fat meal had a significant effect on the pharmacokinetics of valsartan. CL/F for valsartan increased 73% as a result of decreased bioavailability with a high-fat meal compared with under fasted conditions. Tmax was delayed by one hour with a high-fat meal compared with under fasted conditions. T1/2 was not effected by the administration of a high-fat meal. Considering the fact that the t1/2 was not effected by food intake, the differences observed in valsartan exposure under the fed versus the fasted conditions were most likely caused by an increased bioavailability in the fed conditions.
Amlodipine. All the pharmacokinetic parameters for amlodipine with a high-fat meal were similar to that observed under fasted conditions, which indicated no effect of a high-fat meal on the pharmacokinetics of amlodipine, and the 90% CIs were within the 80.00–125.00% range.
The data analyzed in the study was from the reference product of the bioequivalence study, and the data of the test product was assessed to confirm the effect of a high-fat meal on valsartan/amlodipine. The result showed that the effect of a high-fat meal on the test product of valsartan/amlodipine was consistent with the reference product. The Cmax, AUC0–t, and AUC0–∞ of valsartan of the test product after a high-fat meal were 49%, 53%, and 54% lower, respectively than those under the fasted conditions. The 90% CIs of Cmax, AUC0–t, and AUC0–∞ between fasted and fed conditions were 43.31 to 60.52, 45.80 to 65.77, and 46.56 to 63.16, respectively, which were outside the 80.00–125.00% range. Also, no effect of a high-fat meal was found on the amlodipine of the test product.
Safety Evaluations
There were no AEs or serious AEs that led to discontinuation of the study drug. Administration of a single oral dose of valsartan/amlodipine tablet (80/5mg) was found to be safe and well tolerated in healthy subjects.
In the fasted group, there were 27 cases of AEs (61.4%) and 18 (40.9%) were related to the study drug. In the high-fat meal group, there were 19 cases of AEs (43.2%) and 8 (18.2%) were related to the study drug. The AEs related to the drug included hypotension, Systemic response (headache, dizziness, weakness, and nausea), and mild laboratory abnormalities (alanine aminotransferase, aspartate aminotransferase, myoglobin and Creatine kinase slightly increased). The most common adverse event was hypotension, and all adverse events were of mild intensity and transient in nature. Overall, adverse events were significantly reduced in the high-fat meal group compared to the fasted group, which may be related to the high-fat meal and lower blood concentrations of valsartan in the high-fat meal group.
Discussion
Food is usually considered to be a factor that can alter the PK profiles of drugs, leading to variation in systemic exposure. However, it is not clear that foods affect the PK profile of 80/5mg valsartan/amlodipine tablets in a Chinese population.
Valsartan/amlodipine is formulated for oral administration in patients with hypertension. As with any orally administered drug, it is important to evaluate the effect of food on its bioavailability in order to provide dosing guidance with regard to food intake. In the study, the effect of a high-fat meal on the oral bioavailability of valsartan/amlodipine tablets was examined. In general, foods effect on the bioavailability of drugs is evaluated using meal conditions that are expected to have maximal effects on the pharmacokinetics of drugs; hence, a high-fat and high-calorie meal is recommended by regulatory agencies.
This study evaluated the effect of a high-fat, high-calorie meal on the PK of oral valsartan/amlodipine using fixed-dose combination tablets at a therapeutic 80/5mg dose. The result showed that the bioavailability of amlodipine was comparable, implying the oral absorption of amlodipine was not effected by meal. Given the high solubility of amlodipine, the food effect was not anticipated. For valsartan, the AUC and Cmax were decreased by 50% when the valsartan/amlodipine tablets was administered with a high-fat meal, and there were significant differences in Cmax, AUC0–t, and AUC0–∞ of valsartan (p<0.05) between fasting and a high-fat meal.
The fixed-dose combination tablets containing amlodipine 5 mg and valsartan 80 mg of the study did not significantly differ in pharmacokinetic profiles compared with the literature of amlodipine tablets and valsartan capsules in healthy Chinese volunteers.19,20 These changes in the pharmacokinetics of valsartan in the presence of food are similar to those observed when valsartan was administered alone.21
However, the difference results of the effect of food on the bioavailability of valsartan has been observed across different formulations in combination with other antihypertensives.18,22,23 Previously, Phase I studies in Caucasians have investigated the effect of food on the PKs of valsartan/amlodipine and amlodipine/valsartan/ hydrochlorothiazide fixed dose combination tablets. The healthy Caucasian subjects received valsartan/amlodipine or amlodipine/valsartan/hydrochlorothiazide fixed dose combination tablets under fasted and fed conditions, respectively, but no significant effect of food on the primary PK parameters of valsartan and amlodipine was observed.18 Besides, phase I study in Japanese have investigated the effect of food on the PKs of sacubitril/valsartan fixed dose combination tablets, the results showed that the AUC of valsartan was decreased by 33% when sacubitril/valsartan was administered with low-fat meals in healthy Japanese, whereas it was only slightly decreased with a high-fat meal.22,23 Those studies show that the meal had different effects on the pharmacokinetic parameters of valsartan which may be influenced by ethnic differences.
Dietary intake could cause physiological changes (such as changes in bile production, stomach acidity, and gastrointestinal motility) that could effect drug absorption and bioavailability. The physiological parameters of the gastrointestinal tract after the high-fat meal were significantly different from those during fasting.24 Eating could increase gastric juice pH level, thus increasing the dissociation degree of the weakly acidic drug valsartan which is not conducive to its absorption in the stomach. The food slowed the gastric emptying which increased the retention time of valsartan in the stomach and reduced its absorption in the small intestine.
We believe that the changes in the pharmacokinetics of valsartan/amlodipine analytes in the presence of food may not be considered clinically relevant because of the following reasons. Despite the food effect on the pharmacokinetics, the efficacy of valsartan is unaltered. Considering the high intrasubject variability of valsartan pharmacokinetics (CV%: ~ 40%) and the lack thereof on its blood pressure lowering effects, administration of valsartan alone is recommended regardless of meals.21 However, the pharmacokinetics of valsartan decreased significantly after a high-fat meal, therefore, patients with hypertension should take valsartan under a fixed condition (fasted or after meals) instead of changing the condition at will to prevent adverse reactions.
The incidence of TEAE was similar between the fasted group and the high-fat meal group (P>0.05), while ADR was more frequent in the fasted group (P<0.05). ADR was significantly reduced in the high-fat meal group compared to the fasted group, which may be related to the lower blood concentrations of valsartan in the high-fat meal group. However, it is uncertain whether the safety of the drug after a high-fat meal is better than that after fasting due to the small sample size. TEAEs including mild hypotension, and mild laboratory abnormalities are similar to most of the adverse reactions in hypertension.
Our study presents several limitations. First, we only examined the effects of food on the PK characteristics of valsartan/amlodipine fixed dose combination tablets after a single administration. Second, the effects of food on the PK characteristics of valsartan/amlodipine fixed dose combination tablets in hypertension patients were not explored and remained unknown in the study, which might be different from healthy subjects. Therefore, further studies investigating the effects of food on its PK characteristics after repeated administration in hypertension patients are needed, especially the effect on antihypertensive efficacy should be emphasized and noticed. We are now actively planning another study to address these important questions.
Conclusion
In conclusion, administration of valsartan/amlodipine with meals decreased the rate and extent of absorption of valsartan with no impact on systemic exposure to amlodipine. All treatments were safe and well tolerated in healthy subjects under both fed and fasted conditions.
Data Sharing Statement
The data set used during the current study is available from the corresponding author (Wanjun Bai) upon reasonable request.
Informed Consent
Informed consent was written by all individual participants included in the study.
Acknowledgments
We are grateful to Dr Haowei Song at MilliporeSigma, who assist with the English.
Disclosure
The authors have declared that no competing interests exist.
References
1. Ettehad D, Emdin CA, Kiran A, et al. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet. 2016;387(10022):957–967. doi:10.1016/S0140-6736(15)01225-8
2. Mallat SG, Itani HS, Tanios BY. Current perspectives on combination therapy in the management of hypertension. Integr Blood Press Control. 2013;17:69–78. doi:10.2147/IBPC.S33985
3. Gu Q, Burt VL, Dillon CF, et al. Trends in antihypertensive medication use and blood pressure control among United States adults with hypertension: the National Health And Nutrition Examination Survey, 2001 to 2010. Circulation. 2012;126(17):2105–2114. doi:10.1161/CIRCULATIONAHA.112.096156
4. James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311(5):507–520. doi:10.1001/jama.2013.284427
5. Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood PressureThe JNC 7 Report. JAMA. 2003;289(19):2560–2572. doi:10.1001/jama.289.19.2560
6. Murdoch D, Heel RC. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic use in cardiovascular disease. Drugs. 1991;41(3):478–505. doi:10.2165/00003495-199141030-00009
7. Markham A, Goa KL. A review of its pharmacology and therapeutic use in essential hypertension. Drugs. 1997;54(2):299–311. doi:10.2165/00003495-199754020-00009
8. Meyer MC, Whyatt PL. Hydrochlorothiazide. J Am Pharm Assoc. 1976;16(1):47–50.
9. Sever PS, Franz HM. Hypertension management 2011: optimal combination therapy. Eur Heart J. 2011;32(20):2499–2506. doi:10.1093/eurheartj/ehr177
10. Novartis Pharmaceuticals Corporation. EXFORGE®(Amlodipine Besylate/Valsartan) Tablets [Prescribing Information]. Switzerland: Novartis Pharmaceuticals Corporation; 2021.
11. Melander A. Influence of food on the bioavailability of drugs. Clin Pharmacokinet. 1978;3(5):337–351. doi:10.2165/00003088-197803050-00001
12. Yu LX, Straughn AB, Faustino PJ, et al. The effect of food on the relative bioavailability of rapidly dissolving immediate-release solid oral products containing highly soluble drugs. Mol Pharm. 2004;1(5):357–362. doi:10.1021/mp0499407
13. Marasanapalle VP, Crison JR, Ma J, Li X, Jasti BR. Investigation of some factors contributing to negative food effects. Biopharm Drug Dispos. 2009;30(2):71–80. doi:10.1002/bdd.647
14. Faulkner JK, Hayden ML, Chasseaud LF, Taylor T. Absorption of amlodipine unaffected by food. Solid dose equivalent to solution dose. Arzneimittelforschung. 1989;39(7):799–801.
15. Chung M, Calcagni A, Glue P, Bramson C. Effect of food on the bioavailability of amlodipine besylate/atorvastatin calcium combination tablet. J Clin Pharmacol. 2006;46(10):1212–1216. doi:10.1177/0091270006291097
16. Williams RL, Mordenti J, Upton RA, et al. Effects of formulation and food on the absorption of hydrochlorothiazide and triamterene or amiloride from combination diuretic products. Pharm Res. 1987;4(4):348–352. doi:10.1023/a:1016409606936
17. Israili ZH. Clinical pharmacokinetics of angiotensin II (AT1) receptor blockers in hypertension. J Hum Hypertens. 2000;14(S1):S73–S86. doi:10.1038/sj.jhh.1000991
18. Sunkara G, Jiang X, Reynolds C, et al. Effect of food on the oral bioavailability of amlodipine/valsartan and amlodipine/valsartan/hydrochlorothiazide fixed dose combination tablets in healthy subjects. Clin Pharmacol Drug Dev. 2014;3(6):487–492. doi:10.1002/cpdd.131
19. Wang T, Wang Y, Lin S, et al. Evaluation of pharmacokinetics and safety with bioequivalence of Amlodipine in healthy Chinese volunteers: bioequivalence Study Findings. J Clin Lab Anal. 2020;34(6):e23228. doi:10.1002/jcla.23228
20. Wu Q, Wang X, Chen Q, et al. Pharmacokinetics and Bioequivalence of Two Formulations of Valsartan 80 mg Capsules: a Randomized, Single Dose, 4-Period Crossover Study in Healthy Chinese Volunteers Under Fasting and Fed Conditions. Drug Des Devel Ther. 2020;14:4221–4230. doi:10.2147/DDDT.S253078
21. Novartis Pharmaceuticals Corporation. Diovan® (Valsartan) Tablets [Prescribing Information]. Switzerland: Novartis Pharmaceuticals Corporation; 2017.
22. Ayalasomayajula S, Langenickel TH, Chandra P, et al. Effect of food on the oral bioavailability of the angiotensin receptor-neprilysin inhibitor sacubitril/valsartan (LCZ696) in healthy subjects. Int J Clin Pharmacol Ther. 2016;54(12):1012–1018. doi:10.5414/CP202604
23. Akahori M, Ayalasomayajula S, Langenickel T, et al. Pharmacokinetics After Single Ascending Dose, Food Effect, and Safety of Sacubitril/Valsartan (LCZ696), an Angiotensin Receptor and Neprilysin Inhibitor, in Healthy Japanese Subjects. Eur J Drug Metab Pharmacokinet. 2017;42(3):407–416. doi:10.1007/s13318-016-0354-1
24. Deng J, Zhu X, Chen Z, et al. A review of food drug interactions on oral drug absorption. Drugs. 2017;77(17):1833–1855. doi:10.1007/s40265-017-0832-z
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

