Back to Journals » Patient Preference and Adherence » Volume 15
Effect of Evidence-Based Pharmacy Care on Satisfaction and Cognition in Patients with Non-Valvular Atrial Fibrillation Taking Rivaroxaban
Authors Sun J, Chen GM, Huang J
Received 20 April 2021
Accepted for publication 15 July 2021
Published 25 July 2021 Volume 2021:15 Pages 1661—1670
DOI https://doi.org/10.2147/PPA.S316008
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Johnny Chen
Jie Sun,1,2 Guo-Mei Chen,1,2 Ji Huang1,2
1Department of Pharmacy, The First People’s Hospital of Taicang, Taicang, People’s Republic of China; 2The Affiliated Taicang Hospital of Suzhou University, Taicang, People’s Republic of China
Correspondence: Guo-Mei Chen
The First People’s Hospital of Taicang, The Affiliated Taicang Hospital of Suzhou University, No. 58 Changsheng Road, Taicang, 215400, People’s Republic of China
Tel/Fax +86-512-53101356
Email [email protected]
Purpose: We aimed to determine the effects of using evidence-based pharmacy care on satisfaction and cognition among patients with non-valvular atrial fibrillation (NVAF) and taking rivaroxaban.
Patients and Methods: Between July 2018 and June 2019, 200 consecutive hospitalized patients taking oral rivaroxaban, who were diagnosed with NVAF and registered in the hospital information management system, were randomly assigned to a control group (n=100) and a study group (n=100) in a single-blind manner. The control group received pharmaceutical care based on the general pharmaceutical care model whereas the study group received care based on an evidence-based pharmaceutical care model. Patients’ satisfaction and cognition were evaluated regularly using questionnaires. The follow-up time was 1 year. We compared differences in satisfaction and cognition between the two groups after pharmaceutical-related care administered by clinical pharmacists.
Results: The study group had higher satisfaction scores than the control group after the EBP intervention (14.58± 0.88 vs.13.81± 1.01, p< 0.01); cognition scores were also higher in the study group (22.58± 2.19 vs 20.80± 3.02, p< 0.01) after the intervention. In the study group, satisfaction was increased from a score of 10.15± 1.33 before the EBP intervention. Cognition also increased after the intervention in the study group, from a score of 9.88± 4.09 pre-intervention. In the control group, satisfaction was 10.04± 1.29 before the traditional pharmaceutical care intervention, smaller than the 13.81± 1.01 after the intervention (p< 0.01). Cognition in the control group was 9.83± 3.51 before traditional pharmaceutical care, smaller than the 20.80± 3.02 after the intervention (p< 0.01).
Conclusion: The care model based on evidence-based pharmacy care can improve patient satisfaction and cognition, providing more comprehensive safety and efficacy of subsequent medication.
Keywords: clinical pharmacist, evidence-based pharmacy, non-valvular atrial fibrillation, rivaroxaban, satisfaction, cognition
Introduction
Atrial fibrillation is a common supraventricular arrhythmia with uncoordinated atrial point activation.1 In 2016, the estimated incidence of atrial flutter or atrial fibrillation in adults was approximately 2%–4%. However, this rate may be underestimated up to 2.3-fold owing to increased longevity in the population and improved ability to detect undiagnosed atrial fibrillation.2–5 An epidemiological survey in China revealed that the age-adjusted prevalence of atrial fibrillation was 0.65% and was as high as 7.5% in individuals over 80 years old.6 The proportion of these patients who develop ischemic stroke is approximately 5% per year, and 20–30% of patients with ischemic stroke were diagnosed with atrial fibrillation before, during, or after the event.7–9 Because the mortality from stroke is considerably ameliorated by the use of anticoagulant therapy, anticoagulants should be administered in most patients with atrial fibrillation. In several large studies, novel oral anticoagulants have been proven to be effective in reducing the complications of stroke and thromboembolism and significantly reducing the risk of intracranial hemorrhage.10–13 Rivaroxaban is a direct inhibitor of factor Xa, with rapid absorption and high oral bioavailability. Furthermore, this drug is less affected by foods and other drugs and is convenient to administer, with no need for routine monitoring of blood clotting indexes.
Evidence-based pharmacy (EBP) is an extension and expansion of evidence-based medicine in the field of pharmacy. EBP is a process of clinical practice in which drug treatment decisions are made after systematically collecting and evaluating the scientific evidence, evaluating the role of the drug in clinical treatment plans, and fully considering the needs and wishes of the patient.14–16 In the past, very few approaches to pharmaceutical care have used evidence-based methods, and amongst them, some only provide short-term care. Although EBP offers pharmaceutical care that is more suitable for individual patients on the basis of scientific evidence, constant improvement in the awareness and understanding of a disease and improving the effect of treatment takes time, as all problems cannot be solved at once. In this study, participants were randomized to receive evidence-based pharmaceutical care for an extended duration of 1 year. Subsequently, we explored whether pharmaceutical care according to EBP was superior to traditional pharmaceutical care in terms of satisfaction and cognition, from the perspective of patients.
Methods
Study Participants
From July 2018 to June 2019, 200 hospitalized patients who were diagnosed with non-valvular atrial fibrillation (NVAF) and treated with oral rivaroxaban were randomly divided into a control group and a study group (n=100 patients in each group) and followed up for 1 year. This study was approved by the Ethics Committee of The First People’s Hospital of Taicang (KY-2019-21), and all patients provided their informed consent prior to commencement of the study.
Inclusion and Exclusion Criteria
The inclusion criteria were: (1) age ≥ 18 years; (2) confirmation by electrocardiogram and echocardiography of NVAF (atrial fibrillation without the following conditions: ① artificial mechanical heart valves and ② moderate to severe mitral stenosis [mostly associated with rheumatism];17,18 (3) patients with clear anticoagulant indications and taking oral rivaroxaban (Bayer Medical Care Co., Ltd., National Drug License J20180075, 10 mg/tablet); and (4) agreeing to participate in the study and having signed the informed consent form.
The exclusion criteria were: (1) patients with atrial fibrillation, except for valvular atrial fibrillation; (2) patients with abnormal coagulation function and severe bleeding tendencies; (3) patients undergoing dialysis; and (4) patients taking anticoagulants other than rivaroxaban.
All enrolled patients met the anticoagulant treatment criteria for NVAF according to the European Society of Cardiology (ESC) 2020 thromboembolism risk assessment of atrial fibrillation: CHA2DS2-VASC score (S2 and A2 represent previous stroke, transient ischemic attack, or thromboembolism and age ≥75 years, respectively. These two factors multiply a patient’s risk of thromboembolism and are major risk factors for thromboembolism in patients with atrial fibrillation; therefore, the score for these two factors is 2 points each. A, H, C, D, Sc, and V represent age 65–74 years, hypertension, congestive heart failure, diabetes mellitus, sex [female], and vascular disease; these factors each represent 1 point. The highest score is 9, and oral anticoagulants are recommended [or should be considered] for patients with CHA2DS2-VASC scores ≥1 for men and ≥2 for women. No risk factors [ie, a score of 0] indicates no need for antithrombotic therapy). After inclusion, all patients were assessed for the risk of bleeding following antithrombotic therapy using the HAS-Bled score (hypertension, abnormal renal/liver function, stroke, bleeding history or predisposition, labile international normalized ratio [INR], older age [age ≥65 years], concomitant use of drugs/alcohol), and the dosage of rivaroxaban was determined or adjusted according to the scoring results. According to ESC guidelines,1 the initial dose is 20 mg without any special conditions. If there are special conditions such as advanced age or renal insufficiency, the dose will be reduced appropriately, such as 10–15 mg/d.
Methods
Patients were divided into a study group and a control group using a randomized, controlled, single-blind method (by a random number table). Patients in both groups were not informed regarding the type of pharmaceutical care received. However, because of the nature of the study, the researchers were not blinded to the care method. Patients in the two groups were provided with different pharmaceutical care interventions before, during, and after the treatment regimen. Using online (telephone and short messaging service) and offline (hospital visit for consultation) methods, we regularly evaluated patients’ satisfaction (including the degree of problem resolution, service attitude, professional level, detail of response, and response time) and cognition (including of disease, drug indications, drug dosage and administration, drug effectiveness, drug safety, and monitoring indicators) using a questionnaire. Patients were evaluated at admission and followed up in outpatient visits, and the relevant findings were documented.
The study group received care based on the evidence-based pharmaceutical model, as follows. According to each patient’s medical history and signs, relevant clinical indicators, and drug use, clinical pharmacists fully consider patients’ wishes and preferences according to the questions raised by patients and their families. Pharmacists search the relevant literature, evaluate the level of evidence according to the literature type, use meta-analysis-based methods to screen for effective and reasonable evidence, then sort and analyze the evidence. After systematic analysis and comprehensive evaluation, clinical pharmacists provide objective and reasonable suggestions to patients, using appropriate language, according to the relevant evidence (Table 1 and Figure 1).1,19
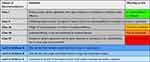 |
Table 1 Classes of Recommendations and Levels of Evidence |
The control group received care based on the general or traditional pharmaceutical care model, as follows. Patients are monitored according to the clinical experience of pharmacists, medication instructions, and individual case reports.
Evaluation Criteria and Indicators
Prior to implementing the two types of pharmaceutical care, participants in both groups were required to complete a questionnaire survey regarding their satisfaction and cognition. The relevant indicators were monitored monthly and all concerns, questions, and outcomes were recorded during the intervention period. After the intervention, a questionnaire survey was again conducted for each patient to compare the degree of satisfaction and cognition between the two groups following the respective interventions. The questionnaire was designed after researching the literature and in joint research conducted by anticoagulant experts. The Cronbach’s α coefficient of the questionnaire was 0.860, indicating good internal consistency.20,21 The average content validity index of all items was 0.90, indicating that the questionnaire had good content validity.22 Additionally, the result of the Kaiser–Meyer–Olkin test was >0.6 in a Bartlett’s sphericity test (P<0.01), indicating that the questionnaire had good structural validity.23 The degrees of satisfaction and cognition were assigned points during data processing. “Very satisfied or highly aware” received 3 points, “satisfied or moderately aware” received 2 points, and “dissatisfied or low awareness” received 1 point. In this study, the advantages and disadvantages of the two monitoring or care models were compared in real-world settings.
Statistical Analysis
IBM SPSS version 22.0 statistical software was used for data analysis (IBM Corp., Armonk, NY, USA). Measurement data are expressed as mean ± standard deviation (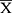 ±s). The t-test was used for comparisons between groups. Enumeration data are expressed as rate (n, %), and the χ2 test or Fisher’s exact test was used for comparisons between groups. Statistical significance was set at P<0.05.
±s). The t-test was used for comparisons between groups. Enumeration data are expressed as rate (n, %), and the χ2 test or Fisher’s exact test was used for comparisons between groups. Statistical significance was set at P<0.05.
Results
Baseline Comparison Between Patient Groups Before the Study
During implementation of the pharmaceutical care interventions, no patients were lost to follow-up or discontinued their participation in the study group, except one patient in the control group who withdrew their participation owing to financial reasons. Basic information on the patients in both groups is presented in Table 2. We analyzed data of age, sex, educational level, CHA2DS2-VASC score, comorbidities, and creatinine clearance rate for patients in both groups; the observed difference was not statistically significant (P>0.05 in all cases). Therefore, the basic information regarding all enrolled patients was comparable.
 |
Table 2 Baseline Comparison Between Study Group and Control Group |
Comparison of Study Results Between Patient Groups
Comparison of Satisfaction and Cognition Between Patient Groups Before Intervention
There was no significant difference in the degree of satisfaction and cognition between the study group and control group before the implementation of pharmaceutical care (P>0.05), as shown in Table 3.
 |
Table 3 Comparison of Satisfaction and Cognition Between Two Groups Before Intervention ( |
Comparison of Satisfaction and Cognition Between Patient Groups After Intervention
We compared satisfaction among the two groups after the different interventions. We observed statistically significant differences in service attitude, professional level, detail of response, and total satisfaction (P<0.05). There was no significant difference in the degree of problem resolution and response time (P> 0.05). The comparison of cognition between the two groups after implementation of pharmaceutical care showed statistically significant differences with respect to disease, dosage and administration of drugs, effectiveness and safety of drugs, and total cognition (P<0.05). There was no significant difference regarding indications and medication monitoring indexes (P>0.05), as shown in Table 4.
 |
Table 4 Comparison of Satisfaction and Cognition Between Two Groups After Intervention ( |
Self-Comparison of Satisfaction and Cognition in Patients Before and After Intervention
The satisfaction and cognition of the two groups were assessing in a self-comparison before and after the implementation of the different pharmaceutical care interventions, and the differences were statistically significant (P<0.05), as shown in Tables 5 and 6.
 |
Table 5 Comparison of Satisfaction of Two Groups Before and After Intervention ( |
 |
Table 6 Comparison of Cognition of Two Groups Before and After Intervention ( |
Discussion
Effects of an Evidence-Based Pharmaceutical Care Model on Patient Satisfaction and Cognition
Before the implementation of pharmaceutical care interventions in the study group and control group, there was no significant difference between groups regarding the degree of satisfaction and cognition. After implementation of the different care interventions, satisfaction and cognition were significantly improved among participants in the two groups, and the difference was statistically significant (P<0.05), indicating the need for implementation of evidence-based pharmaceutical care.
After implementation of the different pharmaceutical care models, a comparison between the participant groups showed that patients who received evidence-based pharmaceutical care had more obvious satisfaction in terms of the service attitude, professional level, and detail of response. In terms of response time, although more time was needed to collect, evaluate, and summarize the evidence for patients in the study group, these patients were willing to spend more time with a clinical pharmacist. Because pharmacists spent a relatively long contact time with patients, they could better understand the needs and preferences of their patients, answer treatment-related questions in more detail and with greater authority, and provide more appropriate medication guidance. Furthermore, there was no significant difference for the degree of problem solving, which may be related to the fact that rivaroxaban had not been used for a long time in the study population; the questions raised about rivaroxaban overlapped with evidence-based information in the instructions and guidelines. Moreover, rivaroxaban is a new drug and therefore, evidence about this drug is relatively lacking.
Both groups were also similar in terms of cognition about drug indications and medication monitoring indicators. This may be related to the fact that the indications for rivaroxaban in China are relatively clear and easy to remember after repeated explanation. At the same time, rivaroxaban does not require routine monitoring and has little interaction with other drugs or foods, and a regular review is only needed according to renal function. In this study, patients were monitored with less frequency, and insignificant differences in treatment cognition were observed. However, following EBP care, cognition in other dimensions showed obvious improvement, indicating that through EBP, full and detailed pharmacological care can help to improve patients’ understanding of their disease and medications, thereby improving overall medication compliance and clinical efficacy and reducing adverse events.
Importance of Evidence-Based Pharmaceutical Care
Under a background of the urgent pursuit of health-related knowledge and the contradictory relationship between supply and demand for medical care, the relationship between doctors and patients is becoming increasingly strained. Patients are desperate to understand their illness but doctors are too busy to provide a detailed explanation to each patient. Additionally, with the development of modern technologies, patients can acquire non-professional medical knowledge via the Internet. However, specious suggestions and conclusions from online platforms may further aggravate the already strained relationship between doctors and patients. The provision of high-quality pharmaceutical care can alleviate the aforementioned problem, to some extent. As early as 2006, the World Health Organization and the International Federation of Pharmacy jointly compiled a pharmacist’s manual entitled “Developing Pharmacy Practice-A Focus on Patient Care,”24 which clearly proposes the use of evidence-based medicine concepts and methods in pharmacy practice.
The ultimate purpose of evidence-based pharmaceutical care is rational drug use, which requires that patients receive drugs that are suitable for their clinical needs within the appropriate time, in doses that meet their individual needs, and at the lowest cost to them and to the community. When making a drug treatment decision, understanding the needs of patients and seeking reliable evidence are complicated by the increasing number of electronic information resources, as well as the increasing number and types of drugs on the market. Patients have different conditions and different expectations of treatment. Not all data have the same value in terms of evidence.19 Obtained data require extensive scientific evaluation and interpretation, and different sources of evidence provide different levels of evidence in decision making.25,26
In this study, we innovatively applied EBP care among patients using the principle of PICO-S (Participants’ research object, Intervention, Comparison, Outcome, Study design). PICO-S emphasizes that patients should actively participate in the treatment process to establish a good relationship between pharmacists and patients. Clinical pharmacists should fully understand the needs and preferences of patients, as well as the characteristics of their disease, and they should then search the relevant evidence, evaluate the level of evidence, and conduct systematic analysis (eg, meta-analysis) of the evidence. Finally, clinical pharmacists should use appropriate language to make recommendations for individualized treatment decisions, which adds additional value with respect to clinical outcomes.27,28
The ESC guidelines for atrial fibrillation also emphasize that patients should be actively involved in treatment decisions. A patient study on the influence of direct oral anticoagulants (DOACs) on stroke prevention in atrial fibrillation showed that greater patient involvement in decision making can help to prevent and address negative effects on their daily life to improve compliance and overall satisfaction with treatment and may improve prognosis and increase uptake of DOACs.29 In another study of pharmacists’ experiences in clinical pharmaceutical care during the COVID-19 pandemic,30 many pharmacists working in hospitals had to adjust their working mode and were reassigned to the intensive care unit. These pharmacists participated in the provision and evaluation of evidence in their new position, which offered a new reference for doctors in treating patients. Owing to the severity of the pandemic, many patients could not enter a hospital to receive treatment from specialists but could only seek care in the community. Pharmacists in the community could fully understand the needs and fears of patients through communication with their patients, providing patients with evidence-based care after evaluation and participating in the compilation and dissemination of educational materials. Verbal advice could also be provided to patients, members of the public, and health professionals. During the pandemic, pharmacists have become an important member of the treatment team as a bridge between doctors and patients, which has been affirmed by doctors, and have improved patients’ satisfaction with pharmacists and their understanding of their disease. Similarly, the evidence-based pharmaceutical care model has received positive reports in terms of monitoring adverse drug reactions and the rational use of antibiotics.31,32 This can significantly reduce the occurrence of adverse reactions and improve the rate of reasonable antibiotics use.
The implementation of evidence-based pharmaceutical care has significantly improved patient satisfaction and cognition. In clinical practice and in other previous studies, it has been shown that encouraging patients to participate in treatment decision making and evaluating the available evidence are crucial. Only by thoroughly understanding patients’ needs and preferences as well as their existing difficulties and past drug experiences can drug treatment be optimized in patients (literature).14–16 Clinical pharmacists scientifically evaluate the evidence to arrive at a conclusion through systematic analysis (such as meta-analysis), then recommend the most appropriate treatment to patients using language that the patient can understand. Patients can receive individualized treatment, which can help to improve patient compliance and enhance the effect of treatment. Of course, it takes time for the pharmacist and patient to develop a trusting relationship.
In conclusion, although thorough systematic analysis and comprehensive evaluation of the available evidence in EBP care of patients requires a slightly longer time than usual care, patients’ desire for high-quality medical care that meets their health needs can be satisfied and patients’ knowledge levels can be increased. EBP care can also promote the development of high-quality professional skills among pharmacists.
Strengths and Limitations
Compared with previous studies, this study included more homogeneous populations and a clear control group. It used statistical methods to compare the satisfaction and cognition of these patients under different pharmaceutical care modes on the premise that there was no statistical difference at baseline. This study shows obvious statistical differences in satisfaction and cognition, which can better reflect the characteristics and advantages of evidence-based pharmacy. It also illustrates the necessity of implementing evidence-based pharmacy. But it also has some shortcomings. The sample size in this study was not sufficiently large; also, the follow-up period could have been extended and the frequency of follow-up could have been increased (preferably to once a month or more). Particularly in the case of outpatients, detailed follow-up should be conducted before and after treatment, which can assist in identifying additional problems. In addition to evidence regarding basic diseases, drug science, and humanistic care, most of the evidence-based pharmacy services in this study were according to patients’ questions. This has led to incidents that may affect the results. In future work, an initiative to help guide patients in identifying problems and that uses EBP to solve these problems should be adopted. Due to the single blind method used in this study, there may be subjective bias of the researcher, but this is the nature of the experiment dictates that the two groups need to be treated differently.
Implications for Future Research and Clinical Practice
Relevant studies have pointed out that pharmacists are now extensively involved in the treatment and management of chronic diseases, including the distribution of educational material, review of drug therapy, as well as the provision of pharmaceutical advice to patients. Pharmacist-led care has an impact on the clinical progression of chronic diseases; however, determining which method of care can best serve patients deserves further discussion.33 We believe that evidence-based pharmaceutical care in patients with NVAF who are treated with rivaroxaban can be comprehensive and accurate in mitigating issues associated with the use of this drug. The care model in this study can enable patients to have a more comprehensive understanding of the advantages and disadvantages of the drug and improve patient satisfaction and cognition. Thus, quality of medical treatment can be assured, the doctor–patient relationship can be improved, safety and effectiveness of drug use can be enhanced, and the burden on clinicians can be reduced. Whether there are significant differences in patient outcomes, readmission rates, test indicators, and various economic indicators requires further follow-up study. Educational institutions of pharmacy in many countries have established courses related to evidence-based practice or that require pharmaceutical students to master evidence-based practice skills.34 As members of the pharmaceutical profession, pharmacists should keep pace with the times, constantly improving their professional skills, such as by applying the abovementioned approaches to other diseases and drugs and by constantly improving pharmaceutical care based on scientific methods.
Conclusions
The present novel evidence-based pharmaceutical care model is conducive to improving satisfaction and cognition among patients with NVAF who are being treated with rivaroxaban. Clinical pharmacists should respect patients’ wishes by understanding their preferences and should encourage them to participate in the decision-making process. In this model and according to patients’ clinical indicators, clinical pharmacists search the relevant literature, evaluate the level of evidence, conduct systematic analysis (such as meta-analysis), and use the most appropriate language to provide patients with individualized recommendations for treatment decisions, which can lead to greater safety and efficacy of subsequent drug use.
Acknowledgment
The authors are grateful to all study participants.
Funding
This research was funded by Suzhou Science and Technology Development Plan (People’s Livelihood Technology-Basic Research on Medical and Health Applications), no. SYSD2018224.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Hindricks G, Potpara T, Dagres N, et al. ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association of Cardio-Thoracic Surgery (EACTS). Eur Heart J. 2020;42(5):373–498.
2. Benjamin EJ, Muntner P, Alonso A, et al.; American Heart Association Council on Epidemiology and Prevention Statistics. Committee and stroke statistics subcommittee. Heart disease and stroke statistics −2019 update: a report from the American Heart Association. Circulation. 2019;139(10):e56–e528.
3. Chugh SS, Havmoeller R, Narayanan K, et al. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation. 2014;129(8):837–847. doi:10.1161/CIRCULATIONAHA.113.005119
4. Colilla S, Crow A, Petkun W, et al. Estimates of current and future incidence and prevalence of atrial fibrillation in the US adult population. Am J Cardiol. 2013;112(8):1142–1147. doi:10.1016/j.amjcard.2013.05.063
5. Krijthe BP, Kunst A, Benjamin EJ, et al. Projections on the number of individuals with atrial fibrillation in the European Union, from 2000 to 2060. Eur Heart J. 2013;34(35):2746–2751. doi:10.1093/eurheartj/eht280
6. Zhou Z, Hu D. An epidemiological study on the prevalence of atrial fibrillation in the Chinese population of mainland China. J Epidemiol. 2008;18(5):209–216. doi:10.2188/jea.JE2008021
7. Kishore A, Vail A, Majid A, et al. Detection of atrial fibrillation after ischemic stroke or transient ischemic attack: a systematic review and meta-analysis. Stroke. 2014;45(2):520–526. doi:10.1161/STROKEAHA.113.003433
8. Henriksson KM, Farahmand B, Åsberg S, et al. Comparison of cardiovascular risk factors and survival in patients with ischemic or hemorrhagic stroke. Int J Stroke. 2012;7(4):276–281. doi:10.1111/j.1747-4949.2011.00706.x
9. Grond M, Jauss M, Hamann G, et al. Improved detection of silent atrial fibrillation using 72-hour Holter ECG in patients with ischemic stroke: a Prospective Multicenter Cohort Study. Stroke. 2013;44(12):3357–3364. doi:10.1161/STROKEAHA.113.001884
10. Fatemi O, Yuriditsky E, Tsioufis C, et al. Impact of intensive glycemic control on the incidence of atrial fibrillation and associated cardiovascular outcomes in patients with type 2 diabetes mellitus(from the action to control cardiovascular Risk in Diabetes Study). Am J Cardiol. 2014;114(8):1217–1222. doi:10.1016/j.amjcard.2014.07.045
11. Lin KJ, Cho SI, Tiwari N, et al. Impact of metabolic syndrome on the risk of atrial fibrillation recurrence after catheter ablation: systematic review and meta-analysis. J Interv Card Electrophysiol. 2014;39(3):211–223. doi:10.1007/s10840-013-9863-x
12. Chang SH, Wu LS, Chiou MJ, et al. Association of metformin with lower atrial fibrillation risk among patients with type 2 diabetes mellitus: a population-based dynamic cohort and in vitro studies. Cardiovasc Diabetol. 2014;13(1):123. doi:10.1186/s12933-014-0123-x
13. Nguyen TN, Hilmer SN, Cumming RG. Review of epidemiology and management of atrial fibrillation in developing countries. Int J Cardiol. 2013;167(6):2412–2420. doi:10.1016/j.ijcard.2013.01.184
14. Etminan M, Wright JM, Carleton BC. Evidence based pharmacotherapy: review of basic concepts and applications in clinical practice. Ann Pharmacother. 1998;32(11):1193–1200. doi:10.1345/aph.17333
15. Siyan Z. Evidence-based medicine and evidence-based pharmacy practice. J Clin Pharmacother. 2008;6(3):47.
16. Theresa LC, Tamara D, Ross TT. Systematic reviews of pharmacy practice research: methodologic issues in searching evaluating, interpreting, and disseminating results. Ann Pharmacother. 2009;43(1):118–122.
17. John CA, Lip Gregory YH, De CR, et al. 2012 focused update of the ESC guidelines for the management of atrial fibrillation: an update of the 2010 ESC guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. Eur Heart J. 2012;33(21):1385–413.
18. Chen Z, Zhang M. A review of the 2014 AHA/ACC/HRS guidelines for the management of atrial fibrillation. J Clin Cardiol. 2014;30(11):929–931.
19. Toklu HZ. Promoting evidence-based practice in pharmacies. Integr Pharm Res Pract. 2015;4:127–131. PMID: 29354526; PMCID: PMC5741015. doi:10.2147/IPRP.S70406
20. Cook DA, Beckman TJ. Current concepts in validity and reliability for psychometric instruments: theory and application. Am J Med. 2006;119(2):
21. Yu M. Development of a transition process scale for high-risk infant’s caregiver. Iran J Public Health. 2016;45(2):158–169.
22. Shi J, Mo X, Sun Z. Application of content validity index in scale formulation. J Cent South Univ. 2012;37(2):152–155.
23. Minglong WU. Practice of Questionnaire Statistical Analysis: SPSS Operation and Application. Chongqing: Chongqing University Press; 2010:476–477.
24. Wiedenmayer K, Summers R, Mackie C, Gous A, Everard M, Tromp D. Developing pharmacy practice: a focus on patient care. World Health Organization; International Pharmaceutical Federation; 2006.
25. Albrecht S. Evidence-based medicine in pharmacy practice. US Pharmacist. 2009;34(10):HS14–HS18.
26. Schindler B, Gointher J, Suter K. Qualitätsbewertung klinischer Studien [Evidence-based pharmacy – assessment of study quality]. Med Monatsschr Pharm. 2014;37(11):413–418. German.
27. Wiedenmayer K, Summers RS, Mackie CA, Gous AG, Everard M, Tromp D. Developing Pharmacy Practice – Focus Patient Care. Netherlands: World Health Organization and International Pharmaceutical Federation; 2006.
28. Toklu HZ, Hussain A. The changing face of pharmacy practice and the need for a new model of pharmacy education. J Young Pharm. 2013;5(2):38–40. doi:10.1016/j.jyp.2012.09.001
29. Medlinskiene K, Richardson S, Fylan B, et al. Patient perspectives on factors affecting direct oral anticoagulant use for stroke prevention in atrial fibrillation. Patient Prefer Adherence. 2021;15:953–966. doi:10.2147/PPA.S302016
30. Paudyal V, Cadogan C, Fialová D, et al. Provision of clinical pharmacy services during the COVID-19 pandemic: experiences of pharmacists from 16 European countries. Res Social Adm Pharm. 2020;17(8):1507–17.
31. Han AL. Study on the application value of evidence-based pharmacy to drug adverse reactions. Electron J Clin Med Lit. 2018;5(16):152.
32. Yao JJ. Effect of evidence-based pharmacy on rational use of antibiotics in clinic. Medicine. 2016;3:145.
33. Jia X, Zhou S, Luo D, et al. Effect of pharmacist-led cares on medication adherence and inhalation technique in adult patients with asthma or COPD: a systematic review and meta-analysis. J Clin Pharm Ther. 2020;45(5):904–917. doi:10.1111/jcpt.13126
34. Zhang LL, Yi L, Die HU, et al. Systematic review of evidence-based pharmacy definitions and literature. J CN EBM. 2011;11(1):7–13.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.