Back to Journals » International Journal of Women's Health » Volume 15
Effect of COVID-19 on Menstruation and Lower Reproductive Tract Health
Authors Li J, Bai J, Xiang X, Guo Y, Yu H
Received 2 August 2023
Accepted for publication 13 December 2023
Published 23 December 2023 Volume 2023:15 Pages 1999—2013
DOI https://doi.org/10.2147/IJWH.S433516
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Marleen van Gelder
Jiaosheng Li,1 Jiaojiao Bai,2 Xuanxuan Xiang,3 Yifan Guo,3 Haotian Yu4
1Department of Gynecology and Obstetrics, Central People’s Hospital of Zhanjiang, Zhanjiang, People’s Republic of China; 2Department of Gynecology and Obstetrics, Hebei North University, Zhangjiakou, People’s Republic of China; 3Department of Gynecology and Obstetrics, Hainan Hospital of Chinese PLA General Hospital, Sanya, People’s Republic of China; 4Department of Gynecology and Obstetrics, The Eighth Medical Center of Chinese PLA General Hospital, Beijing, People’s Republic of China
Correspondence: Yifan Guo; Haotian Yu, Email [email protected]; [email protected]
Background: To evaluate the dynamically impact of the coronavirus disease 2019 (COVID-19) on the female reproductive system.
Methods: An online survey was shared to women of reproductive age who had been infected with COVID-19 and recovered in China between January and March 2023.
Results: In total, 610 women of childbearing age completed the menstrual component of the survey and 82.6% (n=504) women self-purchased medications without hospitalization. 254 women were menstruating during COVID-19 infection. 66.9% of them reported changes in menstruation, including cycle length (64.7%), menstrual flow (72.4%), and duration (53%), compared to pre–COVID-19. COVID-19–related chest tightness (OR: 9.5; 95% CI: 1.9– 46.3), COVID-19–related stress (OR: 18.4; 95% CI: 1.4– 249.7), and COVID-19–related low mood (OR: 6.2; 95% CI: 1.4– 28.2) were associated with these menstrual changes. However, over 73% of women who menstruated during and after COVID-19 regained their pre-infection menstrual cycle (73%), duration (79.6%), and flow (75.2%) during their first menstruation after COVID-19 recovery. Compared to pre-infection, 19.7% (n=124) women reported changes in lower reproductive tract during COVID-19, including volume and color of vaginal discharge, vulvar pruritus, and vaginitis. These changes were significantly increased in those with a history of pelvic inflammatory disease (OR: 12.1; 95% CI: 3.1– 48.2), ovarian cysts (OR: 4.9; 95% CI: 1.2– 19.4), and vaginitis (OR: 5.5; 95% CI: 2.1– 14.4) prior to COVID-19. However, 52.4% reported that their lower reproductive tract health had returned to its pre-infection within the first month after recovery from COVID-19, while 73.5% reported a return to the pre-infection within 2 months.
Conclusion: Changes in menstruation and lower reproductive tract associated with COVID-19 are transient. Menstruation and lower reproductive tract health will gradually return to pre-COVID-19 status within 2 months of recovery, which can help alleviate excessive concerns about the effects of COVID-19 on the reproductive system.
Keywords: COVID-19, menstrual abnormalities, lower reproductive tract health, female reproductive system
A Letter to the Editor has been published for this article.
Introduction
Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has been a global public health problem since 2019. As of December 26, 2022, COVID-19 has caused more than 734 million cumulative cases, and 6.7 million deaths globally.1 On December 27, 2022, the Chinese government issued a new policy to downgrade the management level of COVID-19 from Class A to Class B infectious disease, effective January 8, 2023.2 More than 12 million women will be exposed to and infected with COVID-19.1,3
The incubation duration for COVID-19 varied from 1 to 14 days; common symptoms include fatigue, fever, and cough.4 SARS-CoV-2 enters and infects human cells via binding its spike (S) protein to an obligate receptor, angiotensin-converting enzyme 2 (ACE2),5,6 regulating ACE2 expression and causing serious physical damage to the cardiovascular, digestive and nervous systems.7–9 The abundant expression of ACE2 in the ovary, uterus, and vagina may enable the interference of COVID-19 with female reproductive functions.10
However, systematic studies of the impact of COVID-19 on the female reproductive system are lacking. There are numerous reports on the effects of COVID-19 and its related stress on the female reproductive system and menstruation,11–16 reporting that women with COVID-19 experience changes in menstrual volume and cycle. A common limitation of these reports was the lack of longitudinal data that could reflect the rise and fall of menstrual changes following COVID-19, because these studies only provided specific insight into the menstrual changes experienced by women at the onset of COVID-19 infection or subsequent lockdown. Therefore, clinical investigations are required to verify the effects of COVID-19 on the female reproductive system as transient or permanent.
In this study, we evaluated how COVID-19 dynamically affects the female reproductive system, such as menstruation (menstrual cycle, menstrual flow, and period duration) and lower reproductive tract health (volume and color of vaginal discharge, vulvar pruritus, and vaginitis) during COVID-19 infection and after recovery from COVID-19, and further identified associated risk factors.
Furthermore, we hypothesized that women infected with COVID-19 would experience predominant abnormal changes in menstruation and lower genital tract health compared with pre-infection, and that these changes would gradually disappear as they recovered. Our findings will help address the concerns regarding the effect of COVID-19 infection on the reproductive system.
Methods
Participant
A cross-sectional study design was used to recruit an age-diverse population of women across China. An online questionnaire was distributed via social media (WeChat) and the clinic where the researchers work from January 15, 2023 to March 25, 2023. In order to obtain data on women of childbearing age in different age groups, first, we selected young mothers who accompanied their children to pediatrics outpatient clinic (predominantly between 27 and 40 years old), and women who attended general medical-surgical and gynecological outpatient clinics (predominantly middle-aged and older women). We also forwarded the online questionnaire to young women on the Internet using social media such as WeChat to obtain data from women aged 18–30. We reviewed and pre-tested the survey questions for relevance and context among a subset of women in the target population.
Participants were eligible to participate in the study if they have established regular menstruation, tested positive for COVID-19, and were not tested for COVID-19 but had been exposed to COVID-19-positive patients and are symptomatic.
The exclusion criteria of the study included pregnant women, lactating women who had not yet menstruated, and women who had undergone hysterectomy (or removal of both ovaries). Further exclusion of menopausal women is needed to study menstrual changes during COVID-19 infection and after recovery from COVID-19.
Survey Development
This questionnaire comprised five sections. The first section consisted of demographic-related questions, such as age, height, weight, marital status, number of children, smoking status, COVID-19 vaccine status, systemic, and gynecologic underlying disease prior to COVID-19.The second section captured details about COVID-19 symptoms, duration of symptoms, treatment options for COVID-19, mood changes following COVID-19 infection, timing of menstruation when COVID-19 symptoms first appeared, and menstrual patterns 6 months prior to COVID-19 infection. Compared to pre-COVID-19, the third section requested detail on changes in menstruation during COVID-19 infection, and the fourth section contained questions concerning the changes in the first menstruation after recovery from COVID-19. Menstrual parameters were self-reported by women based on menstrual cycle length, period duration, menstrual flow. Women who selected “no change” or “didn’t notice or forgot” for all three menstrual parameters were classified as “no change” and all other changes were classified as “change.” Dysmenorrhea and non-menstrual bleeding were also asked in the third and fourth sections. The final section focused on the predominant abnormal observations of the lower genital tract during and after recovery from COVID-19 compared to pre-COVID-19. These included changes in the volume and color of vaginal discharge, vulvar pruritus, and vaginitis. Participants were categorized as “No change” when “No change” or “Didn’t notice or forgot” was selected for all four parameters, while all other changes were categorized as “Change”.
Data processing is shown in Figure 1.
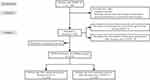 |
Figure 1 Schematic of study methods. COVID-19, coronavirus disease 2019. |
Ethical Consideration
This study was granted approval by the ethics committee of PLA General Hospital (309202302331311) and complied with the Declaration of Helsinki. Participants completed the questionnaires voluntarily and were informed that all data would be collected anonymously, with only the researchers having access to the data.
Statistical Analysis
Data were analyzed using IBM SPSS version 26 and GraphPad Prism version 9. Data are expressed as mean ± standard deviation (SD) for continuous variables and as frequencies and percentages for categorical variables. Chi-square test was used to analyze significant differences between categorical variables under the same questionnaire section, and the McNemar test was used to evaluate differences in menstrual patterns during COVID-19 infection and after recovery from COVID-19. Multifactorial binary logistic regression models were used to assess independent risk factors of changes in menstruation and predominant abnormal observations of the lower genital tract during COVID-19. Correlation between the independent variables was tested for collinearity. Differences were considered statistically significant when p<0.05.
Results
Participant Demographics
The survey was conducted from January 15, 2023 to March 25, 2023. A total of 628 responses with COVID-19 infection were included for analysis of the predominant abnormal changes in the lower genital tract. After the exclusion of menopausal women, 610 participants were included in the analysis of menstrual changes during COVID-19 and the first month after recovery from COVID-19.
As shown in Table 1, the mean age of 610 participants was 32.21±7.2 years (range,17–55 years) and body mass index (BMI) was 20.8±3.5 kg/m2 (range, 13–37 kg/m2). Among the women, 58.5 (n=397), 38.7 (n=236), and 53.3% (n=325) were married, single, and reported having no children, respectively. In addition, 64.1% (n=391) of the women received three doses of COVID-19 vaccine. Most women were healthy, without systemic underlying diseases (n=531, 87%) nor gynecological diseases (n=382, 62.6%). Most women reported discomfort during COVID-19 infection. As shown in Table 2, common symptoms were fever (n=543, 89%), cough (n=466, 76.4%), and fatigue (n=390, 63.9%). Only 0.7% (n=4) women with COVID-19 required hospitalization, and 82.6% (n=504) women self-purchased medications for symptomatic treatment including ibuprofen and cough medicines. More than half of the women (51.5%) infected with COVID-19 reported a negative mood; sleep changes [lighter sleep and insomnia (n=279, 45.8%)], anxiety (n=133, 21.8%), and low mood (n=125, 20.5%) were the most prevalent mental health symptoms. Symptoms of COVID-19 occurred mainly in women during ovulation (n=119, 19.5%),7 days before menstruation (n=99, 16.2%), and within 48 h of menstruation (n=92, 15.1%).
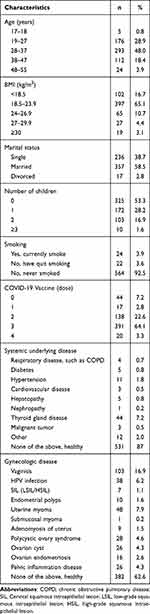 |
Table 1 Demographic Characteristics of Child-Bearing Age Women with COVID-19 |
 |
Table 2 Cross Tabulation of COVID-19 Infection Symptoms, Duration of Symptoms, Treatment, Mood Changes Following COVID-19 Infection and Time of Menstruation When COVID-19 Symptoms First Appeared |
Changes in Menstruation During COVID-19 Infection
A total of 254 women menstruated during the infection, and 66.9% (n=170) reported a change in menstruation (p<0.001), compared with pre-COVID-19 periods. Women who had irregular menstruation (n=36, 57.1%) and those with regular menstruation 6 months prior to infection (n=134, 70.2%) reported a change in menstruation during the COVID-19 infection (Figure 2).
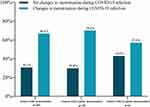 |
Figure 2 The proportion of changes in menstruation during COVID-19 infection. |
Amongst the 254 women who had menstruation during the infection, 64.7% reported an overall change in their cycle length: 40% observed an earlier menstrual cycle; 72.4% reported a change in menstrual flow: 33.6% reported a heavier flow, and 38.8% reported a lighter flow; 53% reported a change in menstruation duration: 29.1% reported a longer period and 23.9% reported a shorter period, compared with that pre–COVID-19 (Figure 3A). No significant change in dysmenorrhea (72.9%) and 76.8% did not experience non-menstrual bleeding during infection (Figure 3B).
Menstrual changes in women who had regular menstruation in the 6 months prior to COVID-19 infection were similar to the changes described above.
Risk Factors Influencing Menstrual Changes During COVID-19 Infection
A binary logistic regression model was used to estimate the risk factors for changes in menstruation during COVID-19 infection. We observed a significantly increased risk of menstrual changes among women who had COVID-19 symptoms occurring more than 1 month after their last menstruation (OR: 9.9; 95% CI: 2.4–41.1), chest tightness during COVID-19 (OR: 9.5; 95% CI: 1.9–46.3), COVID-19–related low mood (OR: 6.2; 95% CI: 1.4–28.2), and COVID-19–related stress (OR: 18.4; 95% CI: 1.4–249.7). (Figure 3C). The collinearity analysis showed no multiple covariates among the risk factors.
Post-Recovery Menstrual Cycle Parameters Return to Those Seen During Menstruation Pre-COVID19 Infection
After recovery from COVID-19, 358 women had or should have menstruation as expected, and 63.7% (n=228) of them reported that their first menstrual period had returned to pre-COVID-19 infection.
A total of 137 women menstruated during the COVID-19 infection and after recovery. Significant differences in menstrual changes were observed during the COVID-19 infection and the first menstruation after recovery (p < 0.001). During the first menstrual period after recovery from COVID-19, more than 73% of women underwent menstruation both during and after COVID-19 returned to their pre-infection menstrual cycle (73%), duration (79.6%), and flow (75.2%). Furthermore, 85.4% of the women did not experience non-menstrual bleeding (Table 3).
 |
Table 3 Comparison of Participant’s Responses About the Effect of COVID-19 on Menstruation During COVID-19 Infection and After Recovery from COVID-19 |
As shown in the Figure 4, regardless of the regularity of menstruation 6 months prior to COVID-19 infection, menstruation gradually returned to the pre-COVID-19 period as COVID-19 was treated.
 |
Figure 4 Trends in menstrual changes during COVID-19 infection and after recovery from COVID-19. |
Abnormality Observed in Lower Genital Tract in Relation to COVID-19 is Transient
Compared to pre-infection, 19.7% (n=124) had abnormal observations of the lower reproductive tract during the COVID-19 infection, including abnormal volume of vaginal discharge (n=83, 66.9%), change in color of vaginal discharge (n=36, 29%), appearance or worsening of vulvar pruritus, and vaginitis (n=49, 39.5% and n=30, 24.2%). Meanwhile, 46.8% (n=58) and 20.2% (n=25) reported increased and decreased vaginal discharge compared to pre-infection, respectively (Figure 5A).
However, among these women (n=124) who experienced changes in the lower reproductive tract during COVID-19, 52.4% (n=65) reported that these changes had disappeared after COVID-19, 27.4% (n=34) reported that these changes persisted, and 20.2% (n=25) had no concerns about these changes. Of 34 women who continued to experience changes after COVID-19 recovery, 73.5% reported that their lower reproductive tract health had returned to pre-infection within two months after recovery from COVID-19, respectively (Table 4).
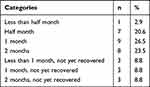 |
Table 4 Time Required for Lower Reproductive Tract to Return to Pre-Infection in Women with Abnormal Lower Reproductive Tract Observations After COVID-19 Negativity (n=34) |
Only 2.2% (n=9) of the 408 women who did not report abnormal observations of the lower reproductive tract during COVID-19 reported changes after recovery from COVID-19 infection. However, half of them (n=5, 55.6%) reported that their lower reproductive tract health returned to normal in approximately 1 month.
A binary logistic regression model was used to assess the risk factors contributing to the predominant abnormal observation of the lower genital tract during COVID-19. We observed significantly increased odds of changes in the lower reproductive tract during COVID-19 infection among women with COVID-19 symptoms occurring more than 1 month after the last menstrual period (OR: 4.5; 95% CI: 1.1–18.6) or 7 days before the next period (OR: 2.4; 95% CI: 1.0–5.6), light-headedness and headaches during COVID-19 (OR: 1.9; 95% CI 1.1–3.5), history of pelvic inflammatory disease (OR: 12.1; 95% CI: 3.0–48.2), history of ovarian cysts (OR: 4.9; 95% CI: 1.2–19.4), and history of vaginitis (OR: 5.5; 95% CI: 2.1–14.4) prior to COVID-19 (Figure 5B). The collinearity analysis showed no multiple covariates among the above risk factors.
Discussion
In this study, we recruited 610 Chinese women of reproductive age who had recovered from COVID-19 to assess the dynamic effects of COVID-19 on menstruation and lower reproductive tract health, and determine the persistence of these changes after a COVID-19 infection and duration for the female reproductive system to return to the pre–COVID-19 state in most women. A large proportion of the women (n=228, 63.7%) reported changes in menstrual flow, period duration, and cycle length due to COVID-19 infection. We found that COVID-19 symptoms occurring more than 1 month after the last menstrual period, chest tightness, and COVID-19–related low mood and stress were important risk factors for changes in menstruation during COVID-19 infection. However, these changes were transient. Regardless of the regularity of menstruation in the 6 months prior to infection, after recovery from COVID-19 infection, women’s menstruation gradually returns to their pre-infection menstruation. Meanwhile, 19.7% (n=124) reported some abnormal observations of the lower reproductive tract during COVID-19 infection; however, 52.4% and 73.5% reported that these changes had disappeared, and their lower reproductive tract health had returned to that observed pre-infection within one and two months after recovery from COVID-19.
There are numerous reports that COVID-19 and its related stress may drive menstrual changes exist. However, available data are limited in determining the persistence of menstrual changes after a COVID-19 infection and duration for menstruation to return to the pre–COVID-19 state in most women. Further research is required to draw definitive conclusions.
Over half of the respondents (n=228, 63.7%) reported significant changes in menstrual flow, period duration, and cycle length during the COVID-19 period. Similarly, Omer et al found a reduction in menstrual duration and daily pad use among women during the pandemic compared to pre-COVID-19.17 However, we find that these changes are temporary. As patients recover from COVID-19, their menstruation will gradually return to a pre-infection state. In addition, 63.7% of women reported that their first menstrual period had returned to the pre-infection period. During the first menstrual period after recovery from COVID-19, more than 73% of women returned to their pre-infection menstrual cycle (73%), duration (79.6%), and flow (75.2%); further, 85.4% did not experience non-menstrual bleeding. In a 2-month post-discharge follow-up of 56 hospitalized COVID-19 patients who experienced menstrual changes, Li et al found that 84% returned to normal menstrual flow and 99% returned to normal cycles within 1–2 months after discharge, indicating that COVID-19-induced menstrual changes were temporary.18 This was consistent with findings from our study. However, unlike our study, their sample did not reflect the majority of women infected with COVID-19 who did not require hospitalization. In our study sample, only 0.7% (n=4) of the women with COVID-19 required hospitalization, and 82.6% (n=504) were treated symptomatically with self-purchased medications such as ibuprofen and cough suppressants, suggesting a wider usage of these medications among women.
Multiple studies on sex hormone changes in COVID-19 patients showed that no significant differences were observed in any of the sex hormone and AMH concentrations between pre– and post–COVID-19 infection, COVID-19 patients and age-matched controls, or mild and severe patients.18,19 These results suggested that the ovarian endocrine system is not severely affected by COVID-19 infection in most women and that menstrual changes in relation to COVID-19 infection are temporary, with no long-term consequences reported.19
However, we found that menstruation was significantly altered during the COVID-19 infection. Binary logistic regression analyses showed a significantly increased risk of changes in menstruation during COVID-19 among women with COVID-19 symptoms, occurring more than 1 month after the last menstrual period, chest tightness during COVID-19, and COVID-19–related low mood and stress.
The mechanisms of COVID-19 effects on the reproductive system are still being explored; however, three hypotheses dominate the literature: (a) SARS-CoV-2 relies on its obligate receptor, ACE2, which is widely expressed in the endometrium and ovaries,20 to enter cells21; hence, follicular development and ovulation are dysregulated, affecting regular changes in the endometrial tissue and leading to menstrual changes;22 (b.) immune response-mediated stress may be a major cause of transient changes in menstruation.23 During COVID-19 infection, immune alterations, such as T cell alterations and innate immune responses, may lead to cytokine storm syndrome, which could alter the leukocyte environment of the endometrium and decrease the LH pulse amplitude or frequency by upregulating the hypothalamic-pituitary-adrenal (HPA) axis and suppressing the HPG axis.10,24 Consequently, menstrual changes may occur and these effects disappear when the cytokine storm subsides and the products of the HPA axis return to physiological concentrations23; (c.) SARS-CoV-2 may directly affect the hypothalamic-pituitary-ovarian (HPO) axis, leading to temporary amenorrhea, menorrhagia, and changes in menstrual cycles.25
COVID-19-related psychological factors are associated with menstrual cycle irregularities. In this study, over half of the women (51.5%) infected with COVID-19 reported negative moods, such as changes in sleep (lighter sleep, insomnia), anxiety, low mood, and irritability. We found that women with increased stress and low mood during COVID-19 were approximately 18 and 6 times more likely to have menstrual changes, respectively, than those without negative moods. Our findings are consistent with those of previous studies. Several studies have revealed that high stress related to COVID-19 is associated with menstrual changes14,16,26 and female with high-stress levels during COVID-19 are likely to have longer periods and more menstrual bleeding than those with moderate stress levels.26 Stress can activate the HPO axis,27 inhibit the secretion of gonadotropin-releasing hormone (GnRH), and lead to a decrease in FSH, LH, follicular development, and estrogen secretion,28 resulting in menstrual changes.
However, little is known about the effect of COVID-19 on lower genital tract health in women. Therefore, we assessed the predominant abnormalities of the lower reproductive tract in women during COVID-19 and after recovery from COVID-19, including changes in the volume and color of vaginal discharge, vulvar pruritus, and vaginitis.
Compared to changes that occurred during menstruation pre-infection, 19.7% (n=124) of women reported changes in the lower genital tract during COVID-19 infection, including abnormal volume of vaginal discharge, change in color of vaginal discharge, appearance or worsening of vulvar pruritus, and vaginitis. However, 52.4% and 73.5% of the patients reported that these changes had disappeared, and their lower reproductive tract health had returned to the conditions observed pre-infection within one or two months after recovery from COVID-19, respectively. To date, only two studies have reported the effects of COVID-19 on the female lower genital tract. Aolymat et al indicated that the incidence of abnormal vaginal discharge, genital itching, and lower abdominal pain was significantly increased during COVID-19 infection compared to pre-infection.29 In the study by Xiao et al30 using a TRACE-seq-based metatranscriptomic analysis, there were significant differences in the vaginal microbiome between the COVID-19 group and the healthy control groups. Compared to healthy controls, patients with COVID-19 had higher intragroup heterogeneity of vaginal microbiota structure, more frequent vaginal dysbacteriosis, and more frequent detection of bacterial vaginosis (BV) pathogens, such as Mycoplasma spp., Gardnerella spp., Ureaplasma spp., Bacteroides spp., and Atopobium spp. They further suggested that the adverse microbiome changes in COVID-19 patients may be due to immunosuppression or the administration of multiple antimicrobials when in the ICU or both. However, all participants were elderly women with a mean age of 32.76±7.976 years (628 participants, including postmenopausal women and women of reproductive age; data not displayed in this article), which may improve the generalizability of our study findings to women of multiple ages.
To the best of our knowledge, the reason why the abnormality observed in the lower genital tract in relation to COVID-19 is transient remains unelucidated. We found that these changes in the female lower genital tract are associated with a significant increase in history of pelvic inflammatory disease, ovarian cysts, and vaginitis prior to COVID-19. For females of reproductive age, the vaginal microbiome seems to be mainly influenced by the impact of estrogen on vaginal epithelial cells, the dominance of lactobacilli, and suitable pH.31 Low estrogen, resulting in atrophy and thinner vaginal squamous epithelium, reduced vaginal secretions, and depletion of the dominant Lactobacillus are the common causes of abnormal vaginal microbiology and vaginal discharge.32 Xiao et al suggested that activation of the NF-κB pathway during COVID-19 infection and the cytokine storm it triggers, particularly the IL-1/IL-36 pathway and its downstream NF-κB pathway, are critical in the response of the female reproductive system to infection.30
Our study had some limitations. First, recall bias is inevitable. Studies have reported that the validity of self-assessment of female fertility history ranges from 92.9–100%.33 Therefore, self-assessment can be used to assess menstrual patterns and can be further validated by obtaining objective data, such as sex hormone concentrations. Another potential limitation is sampling error because the online nature of the survey may have excluded individuals who are not internet savvy. To minimize this error, we took advantage of the high penetration of smartphones in China and generated a QR code for the survey; hence, any woman with a smartphone could scan the QR code and complete the survey.
The strengths of this study include the novel nature of the data, which longitudinally documents that COVID-19-induced changes in menstruation and abnormalities observed in the lower reproductive tract are temporary and self-limiting, potentially helping to alleviate concerns regarding the effects of COVID-19 on the reproductive system. Another strength is the large population of women surveyed in this study. In addition, we recommend that COVID-19 women with abnormal menstruation should be observed at home after ruling out pregnancy. This will provide clinicians with an additional referral option and help avoid wasting medical resources.
In this study, we found significant changes in menstruation and the lower reproductive tract health resulting from COVID-19 infection compared with those pre-infection. In addition, this study showed that these changes are transient and self-limiting and that menstruation and lower reproductive tract health will gradually return to their pre-COVID-19 status after recovery from COVID-19. In our study, the follow-up period of was 2 months after recovery from COVID-19. Future studies that include larger sample sizes and conduct longer follow-up periods are needed to validate this finding, especially for the small subset of women who have not fully resumed menstruation within 2 months of COVID-19 recovery. Although the study briefly mentions prevalent mental health symptoms, such as sleep changes, anxiety, and low mood, it does not provide detailed assessments of mental health. Further information on the severity and impact of these symptoms would have strengthened the findings. In addition, because the SARS-CoV-2 virus has mutated and people are still threatened by COVID-19, more studies on the long-term impact of COVID-19 on the female reproductive system are needed in the future.
Abbreviations
COVID-19, Coronavirus disease 2019; ACE2, Angiotensin-converting enzyme 2; SD, Standard deviation; COPD, Chronic obstructive pulmonary disease; SIL, Cervical squamous intraepithelial lesion; LSIL, Low-grade squamous intraepithelial lesion; HSIL, High-grade squamous intraepithelial lesion.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics Approval
This study was approved by the ethics committee of PLA General Hospital (309202302331311) and complied with the Declaration of Helsinki.
Consent to Participate
Informed consent was obtained from every participant in this study.
Consent for Publish
All authors gave consent for publication.
Acknowledgments
Data were collected by the Hainan Hospital of Chinese PLA General Hospital.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Disclosure
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript.
References
1. WHO Coronavirus (COVID-19) Dashboard | WHO Coronavirus (COVID-19) Dashboard With Vaccination Data; 2022. Available from: https://covid19.who.int/.
2. China strengthens COVID-19 response for key groups, locations; 2023. Available from: http://english.www.gov.cn/news/topnews/202212/27/content_WS63aae802c6d0a757729e4d37.html.
3. Statistical communiqué of the people’s republic of China on the 2022 national economic and social development; 2023. Available from: http://www.stats.gov.cn/english/PressRelease/202302/t202302271918979.html.
4. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi:10.1016/S0140-6736(20)30183-5
5. Zhou P, Yang XL, Wang XG, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. doi:10.1038/s41586-020-2012-7
6. Lu R, Zhao X, Li J, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395(10224):565–574. doi:10.1016/S0140-6736(20)30251-8
7. Li YC, Bai WZ, Hashikawa T. The neuroinvasive potential of SARS‐CoV2 may play a role in the respiratory failure of COVID‐19 patients. J Med Virol. 2020;92(6):552–555. doi:10.1002/jmv.25728
8. Zheng YY, Ma YT, Zhang JY, Xie X. COVID-19 and the cardiovascular system. Nat Rev Cardiol. 2020;17(5):259–260. doi:10.1038/s41569-020-0360-5
9. Zhuang MW, Cheng Y, Zhang J, et al. Increasing host cellular receptor-angiotensin-converting enzyme 2 expression by coronavirus may facilitate 2019-nCoV (or SARS-CoV-2) infection. J Med Virol. 2020;92(11):2693–2701. doi:10.1002/jmv.26139
10. Jing Y, Run-Qian L, Hao-Ran W, et al. Potential influence of COVID-19/ACE2 on the female reproductive system. Mol Hum Reprod. 2020;26(6):367–373. doi:10.1093/molehr/gaaa030
11. Khan SM, Shilen A, Heslin KM, et al. SARS-CoV-2 infection and subsequent changes in the menstrual cycle among participants in the Arizona CoVHORT study. Am J Obstet Gynecol. 2022;226(2):270–273. doi:10.1016/j.ajog.2021.09.016
12. Lebar V, Laganà AS, Chiantera V, Kunič T, Lukanović D. The effect of COVID-19 on the menstrual cycle: a systematic review. J Clin Med. 2022;11(13):3800. doi:10.3390/jcm11133800
13. Ding T, Wang T, Zhang J, et al. Analysis of ovarian injury associated with COVID-19 disease in reproductive-aged women in Wuhan, China: an observational study. Front Med Lausanne. 2021;8:635255. doi:10.3389/fmed.2021.635255
14. Anto-Ocrah M, Valachovic T, Chen M, et al. Coronavirus disease 2019 (COVID-19)-related stress and menstrual changes. Obstetrics Gynecol. 2023;141(1):176. doi:10.1097/AOG.0000000000005010
15. Taşkaldıran I, Vuraloğlu E, Bozkuş Y, et al. Menstrual changes after COVID-19 infection and COVID-19 vaccination. Int J Clin Pract. 2022;2022:1–5. doi:10.1155/2022/3199758
16. Phelan N, Behan LA, Owens L. The Impact of the COVID-19 pandemic on women’s reproductive health. Front Endocrinol (Lausanne). 2021;12:642755. doi:10.3389/fendo.2021.642755
17. Demir O, Sal H, Comba C. Triangle of COVID, anxiety and menstrual cycle. J Obstet Gynaecol. 2021;41(8):1257–1261. doi:10.1080/01443615.2021.1907562
18. Li K, Chen G, Hou H, et al. Analysis of sex hormones and menstruation in COVID-19 women of child-bearing age. Reprod Biomed Online. 2021;42(1):260–267. doi:10.1016/j.rbmo.2020.09.020
19. Madendag IC, Madendag Y, Ozdemir AT. COVID-19 disease does not cause ovarian injury in women of reproductive age- an observational before-and-after COVID-19 study. Reprod BioMed Online. 2022;45(1):153–158. doi:10.1016/j.rbmo.2022.03.002
20. Chadchan SB, Popli P, Maurya VK, Kommagani R. The SARS-CoV-2 receptor, angiotensin-converting enzyme 2, is required for human endometrial stromal cell decidualization. Biol Reprod. 2021;104(2):336–343. doi:10.1093/biolre/ioaa211
21. Jackson CB, Farzan M, Chen B, Choe H. Mechanisms of SARS-CoV-2 entry into cells. Nat Rev Mol Cell Biol. 2022;23(1):3–20. doi:10.1038/s41580-021-00418-x
22. Alvergne A, Vlajic WM, Hogqvist TV. Do sexually transmitted infections exacerbate negative premenstrual symptoms? Insights from digital health. Evol Med Public Health. 2018;2018(1):138–150. doi:10.1093/emph/eoy018
23. Saadedine M, Sabeh ME, Borahay MA, Daoud G. The influence of COVID-19 infection-associated immune response on the female reproductive system. Biol Reprod. 2023;108(2):1–11. doi:10.1093/biolre/ioac187
24. Jara LJ, Lopez-Zamora B, Ordonez-Gonzalez I, et al. The immune-neuroendocrine system in COVID-19, advanced age and rheumatic diseases. Autoimmun Rev. 2021;20(11):102946. doi:10.1016/j.autrev.2021.102946
25. Sharp GC, Fraser A, Sawyer G, et al. The COVID-19 pandemic and the menstrual cycle: research gaps and opportunities. Int J Epidemiol. 2022;51(3):691–700. doi:10.1093/ije/dyab239
26. Nguyen BT, Darney B. Re: ”Impact of stress on menstrual cyclicity during the COVID-19 pandemic: a survey study” by Ozimek et al. J Womens Health (Larchmt). 2022;31(2):299–300. doi:10.1089/jwh.2021.0587
27. Xiang YT, Yang Y, Li W, et al. Timely mental health care for the 2019 novel coronavirus outbreak is urgently needed. Lancet Psychiatry. 2020;7(3):228–229. doi:10.1016/S2215-0366(20)30046-8
28. Huhmann K. Menses Requires Energy: a Review of How Disordered Eating, Excessive Exercise, and High Stress Lead to Menstrual Irregularities. Clin Ther. 2020;42(3):401–407. doi:10.1016/j.clinthera.2020.01.016
29. Aolymat I, Khasawneh AI, Al-Tamimi M. COVID-19-associated mental health impact on menstrual function aspects: dysmenorrhea and premenstrual syndrome, and genitourinary tract health: a cross sectional study among Jordanian medical students. Int J Environ Res Public Health. 2022;19(3):1439. doi:10.3390/ijerph19031439
30. Xiao M, Lu B, Ding R, et al. Metatranscriptomic analysis of host response and vaginal microbiome of patients with severe COVID-19. Sci China Life Sci. 2022;65(7):1473–1476. doi:10.1007/s11427-021-2091-0
31. Paavonen J, Brunham RC. Bacterial vaginosis and desquamative inflammatory vaginitis. N Engl J Med. 2018;379(23):2246–2254. doi:10.1056/NEJMra1808418
32. Gliniewicz K, Schneider GM, Ridenhour BJ, et al. Comparison of the vaginal microbiomes of premenopausal and postmenopausal women. Front Microbiol. 2019;10:193. doi:10.3389/fmicb.2019.00193
33. Sampson GA, Prescott P. The assessment of the symptoms of premenstrual syndrome and their response to therapy. Br J Psychiatry. 1981;138(5):399–405. doi:10.1192/bjp.138.5.399
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


