Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 15
Effect of Azelaic Acid on Psoriasis Progression Investigated Based on Phosphatidylinositol 3-Kinase (PI3K)/Protein Kinase B (AKT) Signaling Pathway
Authors Li L, Lu H, Zhang Y, Li Q, Shi S, Liu Y
Received 13 September 2022
Accepted for publication 10 November 2022
Published 23 November 2022 Volume 2022:15 Pages 2523—2534
DOI https://doi.org/10.2147/CCID.S389760
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Licui Li,1,2 Huixiu Lu,1,3 Yanli Zhang,4 Qian Li,4 Shaomin Shi,4 Yaling Liu1,4
1Department of Dermatology and Venereology, Hebei Medical University, Shijiazhuang, People’s Republic of China; 2Department of Dermatology, Shijiazhuang Gaocheng People’s Hospital, Shijiazhuang, People’s Republic of China; 3Department of Dermatology, Shijiazhuang People’s Hospital, Shijiazhuang, People’s Republic of China; 4Department of Dermatology, The Third Hospital of Hebei Medical University, Shijiazhuang, People’s Republic of China
Correspondence: Yaling Liu, Department of Dermatology and Venereology, Hebei Medical University, Shijiazhuang, People’s Republic of China, Email [email protected]
Objective: To probe into the effect of azelaic acid on psoriasis based on the phosphatidylinositol 3-kinase (PI3K)/protein kinase B (AKT) signaling pathway.
Methods: Psoriasis gene expression data were downloaded from the GEO database for differential expression analysis to identify differentially expressed genes (DEGs). KEGG and GSEA analyses were performed to identify important signaling pathways that may be involved in psoriasis progression for subsequent validation. Thirty-six C57BL/6 mice aged 8 weeks old were randomly assigned into the blank control group (n = 9), negative control group (n = 9), psoriasis model group (n = 9), and azelaic acid treat group (n = 9). Mice models of psoriasis were prepared with imiquimod (IMQ) in the latter two groups, and azelaic acid ointment was applied in azelaic acid treat group. Then, hematoxylin-eosin (HE) staining was carried out to detect the effect of azelaic acid on the pathological damage of mice models of psoriasis in each group. HaCaT cells cultured in vitro were divided into blank control group, negative control group (addition of azelaic acid), IL-17 group (20 ng/mL) and IL-17+azelaic acid group, with 3 replicates for each group. Immunofluorescence assay and Western blotting were used to detect the protein expression of PI3K/AKT signaling pathway related molecules.
Results: KEGG analysis showed that DEGs were significantly enriched in PI3K-AKT signaling pathway. GSEA analysis showed that PI3K and MTOR signaling pathways were up-regulated in psoriasis, while AUTOPHAGY signaling pathway was down-regulated. HE staining showed that azelaic acid could significantly inhibit the local skin injury in mice caused by IMQ-induced psoriasis. Moreover, azelaic acid can inhibit the expression of PI3K/AKT signaling pathway related proteins phosphorylated (p)-PI3K, p-AKT, p-mammalian target of rapamycin (mTOR), vascular endothelial growth factor (VEGF), cyclooxygenase-2 (COX-2), angiogenin-1 and hypoxia-inducible factor-1α (HIF-1α). These results imply that azelaic acid may inhibit the activation of PI3K/AKT signaling pathway and angiogenesis, thereby improving the symptoms of psoriasis.
Conclusion: Azelaic acid may inhibit the activation of PI3K/AKT signaling pathway and angiogenesis, thereby improving the symptoms of psoriasis.
Keywords: psoriasis, azelaic acid, PI3K/AKT signaling pathway, angiogenesis
Introduction
Psoriasis is a chronic inflammatory dermatosis, which has strong genetic susceptibility and autoimmune pathogenic characteristics.1 Currently, it is believed that psoriasis has an association with genetic, environmental, immune and other factors. It is mediated by the innate and adaptive immune systems, involves multiple links and pathways, and notably affects the health and quality of life of patients.2,3
Phosphatidylinositol‑4,5‑bisphosphate 3‑kinase (PI3K)/protein kinase B (AKT) signaling pathway is a key pathway for cell survival and a typical autophagy-dependent transduction pathway, which is involved in cell growth, proliferation, vascular proliferation and other processes.4 PI3K in cells can be activated by corresponding cytokines, further activating downstream AKT and related proteins and triggering corresponding cellular effects. In psoriasis, this pathway can regulate the upstream and downstream related targets together to induce pathological proliferation of epidermal cells.5 In the upstream, tumor suppressor phosphatase and tensin homolog (PTEN) expression is down-regulated, and in the downstream, mammalian target of rapamycin (mTOR) is abnormally expressed. Inhibiting the PI3K/AKT signaling pathway can repress endothelial growth factors, thereby relieving the symptoms of psoriasis.6
Azelaic acid gel is a common anti-inflammatory drug. It is mostly used in the treatment of acne, chloasma and cutaneous lesions caused by ultraviolet rays in clinic.7–9 It can effectively improve the damaged skin barrier function of patients, and has good anti-inflammatory, anti-ultraviolet and anti-microbial damage effects. A previous study found that 15% azelaic acid gel is effective in reducing the purity, scaling and hyperkeratosis of psoriasis plaques. This treatment is also effective in reducing the percent of psoriatic skin involvement.10 However, the specific mechanism of azelaic acid in the treatment of psoriasis has not been reported. Exploring the potential mechanism of action of azelaic acid on psoriasis is beneficial to the treatment and management of patients. Therefore, in this study, the regulatory effects of azelaic acid on skin inflammation in mice with psoriasis through the PI3K/AKT signaling pathway were investigated by establishing mice models of psoriasis with imiquimod (IMQ), offering a theoretical basis for the treatment of psoriasis in clinical practice.
Materials and Methods
Identification of Differentially Expressed Genes in Psoriasis Public Datasets
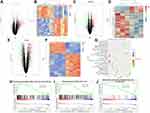
From the Gene Expression Omnibus (GEO) database (https://www.ncbi.nlm.nih.gov/gds/) search psoriasis-related datasets. Psoriasis-related gene expression datasets GSE141804, GSE160932 and GSE164400 were found and downloaded. Differential gene expression analysis was performed after quantile normalization of RNA-seq data using the R language limma software package. P-value <0.05 and |log fold change| (|logFC|) >0.2 were the screening criteria for DEGs. The differentially expressed gene (DEG) volcano plots of the GSE141804, GSE160932 and GSE164400 datasets were constructed using the R language ggplot2 package. In addition, cluster analysis heatmaps of DEGs were constructed using the R language pheatmap package.
Functional Enrichment Analysis
The intersection of DEGs in GSE141804, GSE160932 and GSE164400 datasets were taken. In order to explore the biological functions of common DEGs, Kyoto Encyclopedia of Genes and Genomes (KEGG) functional enrichment analysis was performed for common DEGs. False discovery rate (FDR) <0.05 was considered statistically significant. The R language ggplot2 package was used to draw the KEGG pathway enrichment map. In addition, Gene Set Enrichment Analysis (GSEA) tool (http://www.gsea-msigdb.org/) was used for GSEA of all genes. P < 0.05 was considered statistically significant.
Experimental Animals and Cell
Thirty-six male 8-week-old C57BL/6 mice, specific pathogen free (SPF) grade, weight (25 ± 2) g, were purchased from Beijing Weitong Lihua Laboratory Animal Technology Co. LTD. The experiment was carried out in animal Experiment Center of Hebei Ex&Invivo Biotechnology Co., Ltd. This experiment was carried out in strict accordance with the “3R” principle of laboratory animal welfare, and was approved by the Committee for The Management and Use of Experimental Animals (SY2022-03) of Hebei Ex&Invivo Biotechnology Co., Ltd. Guidelines for the ethical review of laboratory animal welfare11 were followed for the welfare and treatment of the laboratory animals. HaCaT cell lines were purchased from the National Experimental Cell Remote Sharing Service Platform (Beijing Headquarters).
Main Reagents and Instruments
Azelaic acid ® (1 mL: 150 mg, Cosentyx, Novartis, Switzerland); Imiquimod (IMQ) cream (181201, Sichuan Mingxin Pharmaceutical Co., LTD.); Rabbit anti-p-AKT (AB38449), anti-p-mTOR (AB109268), anti-VEGF (AB184784), anti-COX-2 (AB179800), anti-angiogenin-1 (AB254222), anti-HIF-1α (AB179483) were purchased from Abcam Company, UK. Rabbit anti-p-PI3K (17366S) was purchased from Cell Signaling Technology inc., USA. Mouse anti-β-actin (66009-1-IG) was purchased from Proteintech Company, USA. Microplate reader (SYmefield, USA); ECL Gel Imaging System (Bio-RAD, USA), etc.
Experimental Group of Mice
After 1 week of adaptive feeding, the mice were randomly divided into 4 groups (blank control group, negative control group, psoriasis model group and azelaic acid treat group) with 9 mice in each group. The specific treatment for grouping is as follows:
Blank control group: only skin preparation was performed to expose a square hairless area of about 4 cm × 4 cm on the back of the mice.
Negative control group: The skin was prepared to expose a square hairless area of about 4 cm×4 cm on the back of the mice. Then, on the 1st, 6th and 13th day, azelaic acid® was applied to the square hairless area, twice a day.
Psoriasis model group: The skin was prepared to expose a square hairless area of about 4 cm×4 cm on the back of the mice. Then, 0.06~0.07 g imiquimod (IMQ) cream was evenly applied to the hairless area of the mice back to a thickness of about 1~2 mm. Twice a day for 7 days. After 7 days, scales, erythema and desquamation appeared in the area of skin modeling, indicating that the modeling was successful. At the same time, on the 1st, 6th, and 13th day of modeling, blank gel matrix (without azelaic acid) was applied to the psoriatic modeling site, twice a day to a thickness of about 1–2mm.
Azelaic acid treat group: The azelaic acid treat group was modeled in the same way as the psoriasis model group. Then, on the 1st, 6th and 13th day of modeling, azelaic acid® was applied to the psoriatic modeling site, twice a day to a thickness of about 1–2mm.
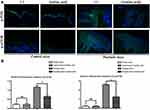
Immunofluorescence Detection
Mice skin tissue was fixed with 4% neutral formaldehyde solution, dehydrated with gradient ethanol, transparent with xylene, embedded in paraffin, sliced, and then routinely dewaxed to water. After high pressure repair with PH6.0 citric acid repair solution for 3 min, holding for 30 min, washed with tris buffered saline (TBS) for 3 times, incubated with H2O2 for 30 min to remove peroxidase in the tissue, washed with TBS for 3 times. The non-specific expression of antibody was blocked by 3%BSA for 30 min. Then, the primary antibody p-PI3K, p-mTOR (1:200) was dropped overnight at 4°C. The goat anti-rabbit secondary antibody was dropped after washing the next day, and incubated at room temperature without light for 1 h. Subsequently, 4’,6-diamidino2-phenylindole (DAPI) was stained for 3 min, and the tablets were sealed with anti-fluorescence quenching sealing tablets. The images were observed under fluorescence microscope. IPP6.0 software was used to analyze the fluorescence intensity.
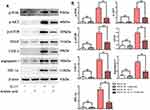
Cell Culture and Grouping
Human Keratinocyte cell lines (HaCaT cell lines) were cultured in dulbecco’s modified eagle medium (DMEM) containing 10% fetal bovine serum at 5% CO2, 37°C, and saturated humidity. Then, the medium was changed every 2 to 3 days. When the cell density was moderate, they were divided into 4 groups: blank control group, negative control group (adding azelaic acid), interleukin (IL)-17 group (20 ng/mL) and IL-17+azelaic acid group. Cells were harvested for Western blot detection 24 h after grouping treatment.
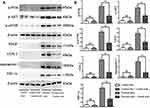
Western Blot Detection
Total protein of HaCaT cells was extracted and quantified by bicinchoninic acid (BCA) method. The samples were added according to the protein content of 20 μg per well. Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) was conducted at an initial voltage of 80V. After bromophenol blue reached the separation gel, the voltage was increased to 120V and electrophoresis was continued until bromophenol blue reached the bottom of the gel. The separated protein was transferred to polyvinylidene fluoride (PVDG) membrane and blocked with 50 g/L nonfat dry milk. Then, p-PI3K, p-AKT, p-MTOR, vascular endothelial growth factor (VEGF), cyclooxygenase-2 (COX-2), angiogenin-1 and hypoxia-inducible factor-1α (HIF-1α) (1:1000) antibodies were added, overnight at 4°C. After washing the membrane the next day, horseradish peroxidase labeled secondary antibody was added, and then the membrane was washed after incubation at room temperature for 1 h. The enhanced chemiluminescence (ECL) developing system fixed and developed color. The gray value of the strip was scanned and analyzed, and the value was represented by the ratio of the gray value of the target strip to the gray value of the internal reference β-actin (1:5000).
Statistical Analysis
All data were statistically analyzed by SPSS 20.0 professional software. All values were expressed as mean ± standard deviation (X ± S). P < 0.05 was considered statistically significant.
Results
Identification of DEGs
Psoriasis-related datasets GSE141804, GSE160932 and GSE164400 were searched from GEO database. Differential gene expression analysis was performed using the R language limma software package. According to the screening criteria P-value <0.05 and |logFC| >0.2, DEGs in the GSE141804, GSE160932 and GSE164400 datasets were identified. In GSE141804 dataset, 215 DEGs were identified, among which 122 were up-regulated and 93 were down-regulated. The volcano and heatmap are shown in Figure 1A and B. In GSE160932 dataset, 135 DEGs were identified, among which 36 were up-regulated and 99 were down-regulated. The volcano and heatmap are shown in Figure 1C and D. In GSE164400 dataset, 292 DEGs were identified, among which 195 were up-regulated and 97 were down-regulated. The volcano and heatmap are shown in Figure 1E and F.
Enrichment Analysis
KEGG enrichment analysis was performed for common DEGs in GSE141804, GSE160932 and GSE164400. FDR < 0.05 was considered statistically significant. KEGG analysis revealed that PI3K-AKT signaling pathway was significantly enriched (Figure 1G). In addition, GSEA tool was used for GSEA of all genes. P < 0.05 was considered statistically significant. GSEA analysis showed that PI3K and MTOR signaling pathways were up-regulated in psoriasis, while AUTOPHAGY signaling pathway was down-regulated (Figure 1H–J). These results suggest that PI3K pathway plays an important role in the progression of psoriasis.
Azelaic Acid Alleviated the Degree of Skin Injury in Mice Models of Psoriasis
The structure of the skin tissue of the mice in each group was observed by hematoxylin-eosin (HE) staining. In blank control group, the epidermal structure of skin tissue was intact and clear, with obvious stratum corneum, thin stratified squamous epithelium, and relatively orderly and tightly arranged cells. The dermis exhibited staggered collagen fibers and red and uniform cytoplasm, and the connective tissues and fat layer displayed clearly visible structure. The subcutaneous muscularis had neatly arranged muscle fibers, with a uniform size, morphologically normal nuclei and nucleoli mostly distributed around. No obvious pathological changes were observed (Figure 2A). In negative control group, the skin tissue had an intact and clear structure, and cells were arranged orderly and tightly (Figure 2B). After modeling with IMQ, the stratified squamous epithelium was thickened, the cells were arranged disorderly, and the outer epidermis was covered with a layer of crust. Necrosis of collagen fibers was found in the superficial layer of the dermis. In the necrotic area, a small number of deeply stained single round lymphocytes infiltrated, and many oblong fibroblasts and long spindle fibroblasts proliferated. Bleeding was found in the dermis, with infiltration of many red blood cells between fibrous tissues (Figure 2C). Following treatment with azelaic acid, the thickening of the stratified squamous epithelium, disordered of cell arrangement, partial collagen fiber necrosis and lymphocyte infiltration in the superficial layer of the dermis, and bleeding in the dermis were all improved (Figure 2D). This suggests that azelaic acid significantly inhibits local skin injury in mice caused by IMQ-induced psoriasis.
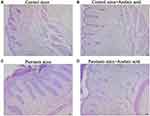 |
Figure 2 Microscopic observation of target skin tissue of mice in blank control (A), negative control (B), psoriasis model (C) and azelaic acid treat groups (D). Observe under 100x microscope. |
Immunofluorescence Detection of p-PI3K and p-mTOR
As PI3K/AKT signaling pathway related proteins, p-PI3K and p-mTOR were analyzed by immunofluorescence staining. The results showed that compared with the blank control group, there was no significant change in the negative control group, while the fluorescence intensity of the psoriasis model group was significantly stronger. Moreover, compared with the psoriatic model group, the fluorescence intensity of azelaic acid treat group was significantly decreased (Figure 3). This result implies that azelaic acid may inhibit the activation of the PI3K/AKT signaling pathway and improve the symptoms of psoriasis.
Western Blot Detection of the Protein Expression Levels of p-PI3K, p-AKT, p-mTOR, VEGF, COX-2, angiogenin-1 and HIF-1α in Each Group
In order to further prove that azelaic acid may treat psoriasis by inhibiting the PI3K pathway, the expression of PI3K/AKT signaling pathway related proteins was detected by Western blot. Compared with the blank control group, the relative protein expression of p-PI3K, p-AKT, p-mTOR, VEGF, COX-2, angiogenin-1 and HIF-1α in the negative control group was not significantly different. The relative protein expressions of p-PI3K, p-AKT, p-mTOR, VEGF, COX-2, angiogenin-1 and HIF-1α in the psoriasis model group were significantly higher than those in the blank control group (Figure 4). This suggests that the PI3K/AKT signaling pathway is activated in IMQ-induced psoriasis with concomitant proliferation of angiogenic factors. Notably, compared with the psoriasis model group, the relative protein expressions of p-PI3K, p-AKT, p-mTOR, VEGF, COX-2, angiogenin-1 and HIF-1α were significantly decreased in the azelaic acid treat group (Figure 4). This suggests that azelaic acid can inhibit the activation of the PI3K/AKT signaling pathway and inhibit angiogenic factor proliferation.
We also validated the potential effects of azelaic acid at the cellular level (Figure 5). Compared with the blank control group, the relative protein expression of p-PI3K, p-AKT, p-mTOR, VEGF, COX-2, angiogenin-1 and HIF-1α in the negative control group (adding azelaic acid) was not significantly different. The relative protein expressions of p-PI3K, p-AKT, p-mTOR, VEGF, COX-2, angiogenin-1 and HIF-1α in the IL-17 group were significantly higher than those in the blank control group. Compared with the IL-17 group, the relative protein expressions of p-PI3K, p-AKT, p-mTOR, VEGF, COX-2, angiogenin-1 and HIF-1α were significantly decreased in the IL-17+ azelaic acid group. These results are consistent with those in the mice model and further suggest that azelaic acid can inhibit the activation of the PI3K/AKT signaling pathway and inhibit the proliferation of angiogenic factors, thereby ameliorating the symptoms of psoriasis.
Discussion
As a chronic inflammatory disease mediated by immunity, psoriasis is caused by multiple factors affecting skin tissues and results in dryness and scaly lesions in any part of the body.12 At present, it is considered that the core of the pathogenesis of psoriasis is that cytokines such as tumor necrosis factor-alpha (TNF-α) and IL-12 produced by dendritic cells under stimulation, as well as IL-17 and IL-22 produced by Th17 cells stimulated by IL-23, act on epidermal keratinocytes together with TNF-α through the janus kinase (JAK)/signal transducer and activator of transcription (STAT) pathway, giving rise to various manifestations of psoriasis.13 The central mechanism of the disease is the IL-23/Th17 axis, whose executive cytokines IL-22 and IL-17A/F lead to keratinocyte proliferation and production of proinflammatory cytokines, chemokines, and antimicrobial peptides (AMPs), and the formation of a positive feedback loop, which maintains the inflammatory process.14 In recent years, studies have found that the PI3K/AKT signaling pathway plays an important mediating role in the progression of psoriasis.15–17 In this study, KEGG analysis revealed that PI3K-AKT signaling pathway was significantly enriched. GSEA analysis showed that PI3K and MTOR signaling pathways were up-regulated in psoriasis. Therefore, we explored the potential role of azelaic acid in psoriasis based on PI3K-AKT signaling pathway. The results showed that azelaic acid could significantly inhibit the local skin injury caused by IMQ in mice and inhibit the expression of PI3K/AKT signaling pathway related proteins p-PI3K, p-AKT, mTOR, VEGF, COX-2, angiopoietin-1 and HIF-1α. These results suggest that azelaic acid may play a role in psoriasis treatment by regulating PI3K/AKT signaling pathway.
Cell proliferation can be triggered by the PI3K/AKT signaling pathway through the regulation of upstream and downstream targets, and it can also be induced by highly expressed IL-22 and IL-17 in skin lesions through activation of mTOR.18 Furthermore, IL-17 is a key inflammatory cytokine in the pathogenesis of psoriasis.19 A study showed that the protein level of p-AKT and the activity of AKT in skin lesions of patients with psoriasis are significantly higher than those in normal skin. Increased AKT activity can result in excessive proliferation of keratinocytes and proliferation of papillary dermal blood vessels.20 In the mice model of IMQ-induced psoriasis, PI3K inhibition resulted in reduced epidermal thickness, number of infiltrating immune cells, and levels of psoriasis-related cytokines.21,22 In this study, immunohistochemistry and Western blot showed that azelelic acid could reduce the expression of p-PI3K, p-AKT, p-mTOR in psoriasis mice, suggesting that azelaic acid may play an important role in mediating the PI3K/AKT signaling pathway in the progression of psoriasis.
COX-2, a key enzyme in fatty acid metabolism, is released at the site of tissue injury and can stimulate pain and inflammation. The prostaglandin E2 (PGE2) derived from it can induce angiogenesis and cell proliferation.23 Furthermore, COX-2 is also closely related to the PI3K/AKT signaling pathway and is a downstream signaling effector of the PI3K/AKT signaling pathway.24–26 COX-2 activity is enhanced in psoriasis and psoriatic arthritis.27 HIF-1α, a downstream signaling protein of PI3K/AKT, has a close relation to VEGF activity and angiogenesis.28,29 In the case of hypoxia, HIF-1α transfers to the nucleus and participates in the modulation of transcription of VEGF.30 The expression of HIF-1α is increased in psoriatic lesions and serum, and may play a key role in the pathogenesis of psoriasis by promoting keratinocyte proliferation, inducing excessive angiogenesis and abnormal activation of the immune system.31 Angiogenesis is a complex regulatory process, which is affected by many factors, and VEGF and angiogenin-1 are important angiogenic factors involved in angiogenesis. Following binding of VEGF to vascular endothelial growth factor receptor 2 (VEGFR2), the autophosphorylation of VEGFR2 is activated at the tyrosine site, thus mediating angiogenesis and other biological effects.32 Vascular hyperplasia is one of the characteristics of psoriasis. VEGF is a potent mediator of angiogenesis. VEGF-induced blood vessels often show increased permeability, resulting in hemorrhages and reduced vessel functionality. Previous studies have found that decreased VEGF secretion in HaCaT inhibits angiogenesis.33,34 Eunah Suhng et al found that LL-37 and IL-33 enhanced VEGF mRNA expression and VEGF release in HaCaT cells. Thus, IL-33 and LL-37 may mediate the angiogenesis of rosacea skin through VEGF.35 In many cases, VEGF induced by various factors has a potent angiogenic effect. Insulin-like growth factor II (IGF-II) is a potent inducer of VEGF synthesis by immortalized keratinocyte line HaCaT. VEGF induced by IGF-II may contribute to the angiogenesis of psoriasis.36 Through the activation of the PI3K/AKT, angiopoietin promotes the synthesis and release of nitric oxide, a vasodilator factor, involved in the vascular physiology.37 VEGF and angiogenin-1 are also important upstream molecules of PI3K/AKT signaling pathway.38,39 Moreover, activation of the PI3K/AKT signaling pathway can also regulate the expression of VEGF and angiopoietin.28 In this study, azelaic acid could not only regulate the expression of p-PI3K, p-AKT, p-mTOR, but also the expression of VEGF, COX-2, angiogenin-1 and HIF-1α. Therefore, we hypothesized that azelaic acid may regulate psoriasis angiogenesis through PI3K/AKT signaling pathway.
Azelaic acid, a compound molecule with multiple activities, is widely used in medicine. A growing body of research has shown that azelaic acid has anti-inflammatory, anti-infective and anti-bacterial effects.40–43 Azelaic acid can inhibit or kill anaerobes and aerobic bacteria in the skin and resist cell proliferation and cytotoxic effects mainly by destroying mitochondrial respiratory pathways and cellular DNA synthesis.7 Moreover, azelaic acid can also regulate the expression of AKT related to the PI3K/AKT signaling pathway in acute myeloid leukemia, thereby regulating the progression of the disease. In this study, we found that azelaic acid could azelaic acid significantly inhibits local skin injury in mice caused by IMQ-induced psoriasis. In addition, azelaic acid also inhibited the expression of p-PI3K, p-AKT, p-mTOR, VEGF, COX-2, angiogenin-1 and HIF-1α in cutaneous lesion tissues of psoriasis mice model and in HaCaT cell lines. To our knowledge, this study may be the first study to show that azelaic acid may play a role in psoriasis by regulating the PI3K/AKT signaling pathway, which lays a theoretical foundation for the treatment of psoriasis. In conclusion, azelaic acid also has anti-inflammatory, anti-infective, antibacterial effects and can prevent angiogenesis in psoriasis. The mechanism may be to play a regulatory role by inhibiting the PI3K/AKT signaling pathway, which provides theoretical support for the clinical application of azelaic acid in the treatment of psoriasis.
However, this study also has a certain degree of limitations. It is necessary to continue to conduct additional research to explore the specific mechanism of azelaic acid in the treatment of psoriasis. Firstly, the effect of azelaic acid on psoriasis angiogenesis should be further explored by cultured vascular endothelial cells. Secondly, it is necessary to detect the differences of protein and gene between psoriatic mice treated with azelaic acid and untreated mice. Thirdly, psoriasis area and severity index (PASI) should also be performed on psoriatic mice. In brief, further animal experiments and clinical trials are needed in future studies to improve the reliability of the conclusions.
Data Sharing Statement
All data generated or analyzed during this study are included in this published article.
Ethics Approval
This experiment was carried out in strict accordance with the “3R” principle of laboratory animal welfare, and was approved by the Committee for The Management and Use of Experimental Animals (SY2022-03) of Hebei Ex&Invivo Biotechnology Co., Ltd. HaCaT cell lines were purchased from the National Experimental Cell Remote Sharing Service Platform (Beijing Headquarters).
Consent for Publication
All of the authors have agreed to the publication of the article.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
There is no funding to report.
Disclosure
All authors declare that they have no competing interests in this work.
References
1. Korman NJ. Management of psoriasis as a systemic disease: what is the evidence? Br J Dermatol. 2020;182(4):840–848. doi:10.1111/bjd.18245
2. Sato Y, Ogawa E, Okuyama R. Role of innate immune cells in psoriasis. Int J Mol Sci. 2020;21(18):6604. doi:10.3390/ijms21186604
3. Palijan T, Kovačević D, Vlastelica M, Dadić-Hero E, Sarilar M. Quality of life of persons suffering from schizophrenia, psoriasis and physical disabilities. Psychiatr Danub. 2017;29(1):60–65. doi:10.24869/psyd.2017.60
4. Xie Y, Shi X, Sheng K, et al. PI3K/Akt signaling transduction pathway, erythropoiesis and glycolysis in hypoxia (Review). Mol Med Rep. 2019;19(2):783–791. doi:10.3892/mmr.2018.9713
5. Zhang M, Zhang X. The role of PI3K/AKT/FOXO signaling in psoriasis. Arch Dermatol Res. 2019;311(2):83–91. doi:10.1007/s00403-018-1879-8
6. Lv H, Liu X, Chen W, et al. Yangxue jiedu fang ameliorates psoriasis by regulating vascular regression via Survivin/PI3K/Akt pathway. J Immunol Res. 2021;2021:4678087. doi:10.1155/2021/4678087
7. Searle T, Ali FR, Al-Niaimi F. The versatility of azelaic acid in dermatology. J Dermatol Treat. 2022;33(2):722–732. doi:10.1080/09546634.2020.1800579
8. Dayal S, Sahu P, Dua R. Combination of glycolic acid peel and topical 20% azelaic acid cream in melasma patients: efficacy and improvement in quality of life. J Cosmet Dermatol. 2017;16(1):35–42. doi:10.1111/jocd.12260
9. Pazoki-Toroudi H, Nilforoushzadeh MA, Ajami M, et al. Combination of azelaic acid 5% and clindamycin 2% for the treatment of acne vulgaris. Cutan Ocul Toxicol. 2011;30(4):286–291. doi:10.3109/15569527.2011.581257
10. Iraji F, Faghihi G, Siadat AH, et al. Efficacy of 15% azelaic acid in psoriasis vulgaris: a randomized, controlled clinical trial. J Drugs Dermatol. 2010;9(8):964–968.
11. MacArthur Clark JA, Sun D. Guidelines for the ethical review of laboratory animal welfare People’s Republic of China National Standard GB/T 35892–2018 [Issued 6 February 2018 Effective from 1 September 2018]. Anim models exp med. 2020;3(1):103–113. doi:10.1002/ame2.12111
12. de Alcantara CC, Reiche EMV, Simão ANC. Cytokines in psoriasis. Adv Clin Chem. 2021;100:171–204.
13. Tokuyama M, Mabuchi T. New treatment addressing the pathogenesis of psoriasis. Int J Mol Sci. 2020;21(20):7488. doi:10.3390/ijms21207488
14. Vičić M, Kaštelan M, Brajac I, Sotošek V, Massari LP. Current concepts of psoriasis immunopathogenesis. Int J Mol Sci. 2021;22(21):11574. doi:10.3390/ijms222111574
15. Koycheva IK, Mihaylova LV, Todorova MN. Leucosceptoside a from Devil’s Claw modulates psoriasis-like inflammation via suppression of the PI3K/AKT signaling pathway in keratinocytes. Molecules. 2021;26(22):7014. doi:10.3390/molecules26227014
16. Wang H, Ran LW, Hui K, Wang XY, Zheng Y. Expressions of survivin, PI3K and AKT in keratinocytes in skin lesions and their pathogenic role in psoriasis vulgaris. Nan fang yi ke da xue xue bao. 2017;37(11):1512–1516.
17. Gao J, Guo J, Nong Y, et al. 18β-Glycyrrhetinic acid induces human HaCaT keratinocytes apoptosis through ROS-mediated PI3K-Akt signaling pathway and ameliorates IMQ-induced psoriasis-like skin lesions in mice. BMC Pharmacol Toxicol. 2020;21(1):41. doi:10.1186/s40360-020-00419-0
18. Chamcheu JC, Esnault S, Adhami VM. Fisetin, a 3,7,3’,4’-Tetrahydroxyflavone inhibits the PI3K/Akt/mTOR and MAPK pathways and ameliorates psoriasis pathology in 2D and 3D organotypic human inflammatory skin models. Cells. 2019;8(9):1089. doi:10.3390/cells8091089
19. Blauvelt A, Chiricozzi A. The immunologic role of IL-17 in psoriasis and psoriatic arthritis pathogenesis. Clin Rev Allergy Immunol. 2018;55(3):379–390. doi:10.1007/s12016-018-8702-3
20. Mercurio L, Albanesi C, Madonna S. Recent updates on the involvement of PI3K/AKT/mTOR molecular cascade in the pathogenesis of hyperproliferative skin disorders. Front med. 2021;8:665647. doi:10.3389/fmed.2021.665647
21. Chamcheu JC, Adhami VM, Esnault S, et al. Dual inhibition of PI3K/Akt and mTOR by the dietary antioxidant, delphinidin, ameliorates psoriatic features in vitro and in an imiquimod-induced psoriasis-like disease in mice. Antioxid Redox Signal. 2017;26(2):49–69. doi:10.1089/ars.2016.6769
22. Roller A, Perino A, Dapavo P, et al. Blockade of phosphatidylinositol 3-kinase PI3Kδ or PI3Kγ reduces IL-17 and ameliorates imiquimod-induced psoriasis-like dermatitis. J Immunol. 2012;189(9):4612–4620. doi:10.4049/jimmunol.1103173
23. Desai SJ, Prickril B, Rasooly A. Mechanisms of phytonutrient modulation of Cyclooxygenase-2 (COX-2) and inflammation related to cancer. Nutr Cancer. 2018;70(3):350–375. doi:10.1080/01635581.2018.1446091
24. Zhang X, Qu P, Zhao H, Zhao T, Cao N. COX‑2 promotes epithelial‑mesenchymal transition and migration in osteosarcoma MG‑63 cells via PI3K/AKT/NF‑κB signaling. Mol Med Rep. 2019;20(4):3811–3819. doi:10.3892/mmr.2019.10598
25. Sharma P, Caldwell TS, Rivera MN, Gullapalli RR. Cadmium exposure activates Akt/ERK signaling and pro-inflammatory COX-2 expression in human gallbladder epithelial cells via a ROS dependent mechanism. Toxicol in Vitro. 2020;67:104912. doi:10.1016/j.tiv.2020.104912
26. Dinicola S, Fabrizi G, Masiello MG, et al. Inositol induces mesenchymal-epithelial reversion in breast cancer cells through cytoskeleton rearrangement. Exp Cell Res. 2016;345(1):37–50. doi:10.1016/j.yexcr.2016.05.007
27. Wójcik P, Biernacki M, Wroński A, Łuczaj W. Altered lipid metabolism in blood mononuclear cells of psoriatic patients indicates differential changes in psoriasis vulgaris and psoriatic arthritis. Int J Mol Sci. 2019;20(17):4249. doi:10.3390/ijms20174249
28. Karar J, Maity A. PI3K/AKT/mTOR pathway in angiogenesis. Front Mol Neurosci. 2011;4:51. doi:10.3389/fnmol.2011.00051
29. Yang C, Liu X, Zhao K, et al. miRNA-21 promotes osteogenesis via the PTEN/PI3K/Akt/HIF-1α pathway and enhances bone regeneration in critical size defects. Stem Cell Res Ther. 2019;10(1):65. doi:10.1186/s13287-019-1168-2
30. Ahluwalia A, Tarnawski AS. Critical role of hypoxia sensor--HIF-1α in VEGF gene activation. Implications for angiogenesis and tissue injury healing. Curr Med Chem. 2012;19(1):90–97. doi:10.2174/092986712803413944
31. Zhu WJ, Li P, Wang L, Xu YC. Hypoxia-inducible factor-1: a potential pharmacological target to manage psoriasis. Int Immunopharmacol. 2020;86:106689. doi:10.1016/j.intimp.2020.106689
32. Pulkkinen HH, Kiema M, Lappalainen JP, et al. BMP6/TAZ-Hippo signaling modulates angiogenesis and endothelial cell response to VEGF. Angiogenesis. 2021;24(1):129–144. doi:10.1007/s10456-020-09748-4
33. Zhi S, Li J, Kong X, Xie X, Zhang Q, Fang G. Insulin-like growth factor 2 mRNA binding protein 2 regulates proliferation, migration, and angiogenesis of keratinocytes by modulating heparanase stability. Bioengineered. 2021;12(2):11267–11276. doi:10.1080/21655979.2021.2002495
34. Zhang C, Xu Q, Tan X, et al. Astilbin decreases proliferation and improves differentiation in HaCaT keratinocytes. Biomed Pharmacother. 2017;93:713–720. doi:10.1016/j.biopha.2017.05.127
35. Suhng E, Kim BH, Choi YW, Choi HY, Cho H, Byun JY. Increased expression of IL-33 in rosacea skin and UVB-irradiated and LL-37-treated HaCaT cells. Exp Dermatol. 2018;27(9):1023–1029. doi:10.1111/exd.13702
36. Kwon YW, Kwon KS, Moon HE, et al. Insulin-like growth factor-II regulates the expression of vascular endothelial growth factor by the human keratinocyte cell line HaCaT. J Invest Dermatol. 2004;123(1):152–158. doi:10.1111/j.0022-202X.2004.22735.x
37. Cucci LM, Satriano C, Marzo T, La Mendola D. Angiogenin and copper crossing in wound healing. Int J Mol Sci. 2021;22(19):10704. doi:10.3390/ijms221910704
38. Tian X, Zhang N, Yan C, et al. CREG promotes vasculogenesis by activation of VEGF/PI3K/Akt pathway. Front Biosci. 2014;19(8):1215–1226. doi:10.2741/4277
39. Liu XB, Jiang J, Gui C, Hu XY, Xiang MX, Wang JA. Angiopoietin-1 protects mesenchymal stem cells against serum deprivation and hypoxia-induced apoptosis through the PI3K/Akt pathway. Acta Pharmacol Sin. 2008;29(7):815–822. doi:10.1111/j.1745-7254.2008.00811.x
40. Searle T, Ali FR, Al-Niaimi F. Perioral dermatitis: diagnosis, proposed etiologies, and management. J Cosmet Dermatol. 2021;20(12):3839–3848.
41. Dall’Oglio F, Tedeschi A, Lacarrubba F, et al. A novel azelaic acid formulation for the topical treatment of inflammatory rosacea: a multicentre, prospective clinical trial. J Cosmet Dermatol. 2021;20 Suppl 1(Suppl1):28–31. doi:10.1111/jocd.14098
42. Liu H, Yu H, Xia J, et al. Topical azelaic acid, salicylic acid, nicotinamide, sulphur, zinc and fruit acid (alpha-hydroxy acid) for acne. Cochrane Database Syst Rev. 2020;5(5):Cd011368. doi:10.1002/14651858.CD011368.pub2
43. Coda AB, Hata T, Miller J, et al. Cathelicidin, kallikrein 5, and serine protease activity is inhibited during treatment of rosacea with azelaic acid 15% gel. J Am Acad Dermatol. 2013;69(4):570–577. doi:10.1016/j.jaad.2013.05.019
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.