Back to Journals » Clinical Ophthalmology » Volume 16
Effect of Autologous Platelet-Rich Plasma Drops in the Treatment of Ocular Surface Disease
Authors Nadelmann JB , Bunya VY, Ying GS, Hua P, Massaro-Giordano M
Received 6 October 2022
Accepted for publication 23 November 2022
Published 15 December 2022 Volume 2022:16 Pages 4207—4213
DOI https://doi.org/10.2147/OPTH.S391536
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Jennifer B Nadelmann,1,2 Vatinee Y Bunya,1 Gui-Shuang Ying,3 Peiying Hua,3 Mina Massaro-Giordano1
1Department of Ophthalmology, Scheie Eye Institute, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, USA; 2Department of Ophthalmology, Weill Cornell Medicine, New York, NY, USA; 3Center for Preventive Ophthalmology and Biostatistics, University of Pennsylvania, Philadelphia, PA, USA
Correspondence: Mina Massaro-Giordano, Department of Ophthalmology, Scheie Eye Institute, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, USA, Tel +1-215-662-8100, Email [email protected]
Purpose: Platelet rich plasma (PRP) is an autologous preparation that concentrates platelets in a small volume of plasma. The purpose of this study was to determine if PRP eye drops improved the symptoms and signs of ocular surface disease.
Patients and Methods: A retrospective case series was conducted of patients who were prescribed PRP eye drops. Subjects were excluded if they did not have follow-up, underwent intraocular surgery prior to follow-up, received nerve growth factor treatments, or did not have a baseline examination with photography. Symptoms were assessed using the Ocular Surface Disease Index (OSDI). Patients also underwent a slit lamp exam, ocular surface staining with fluorescein and lissamine green, and Schirmer testing.
Results: The charts of 47 patients treated with PRP drops for ocular surface disease were reviewed. Sixty-four eyes of 32 patients were included in the study who had photographs of lissamine green staining taken at baseline and at follow-up. Thirteen patients (28%) had ocular graft-versus-host disease, 16 patients (34%) had Sjögren’s syndrome, and 4 patients (8.5%) had rheumatoid arthritis. There was a statistically significant decrease in OSDI score from baseline to follow-up (39.5 vs 30.8 points, p = 0.02). Among the 64 eyes included, 9 (14%) had an improvement in conjunctival lissamine green staining, while 6 (9%) had an increase in staining at follow-up. Among the 20 eyes with Schirmer testing, there was a borderline significant increase in score from baseline to follow-up (5.9 vs 9.7, p = 0.06). Among the 44 eyes that had corneal fluorescein staining (CFS) reported, 8 (18.2%) had decreased staining and 2 (4.5%) had increased staining at follow-up.
Conclusion: Treatment with PRP drops was associated with a significant improvement in symptoms in patients with ocular surface disease. Future larger prospective studies are needed to further evaluate the efficacy of PRP drops for treating ocular surface disease.
Keywords: ocular surface disease, cornea, lissamine green, Ocular Surface Disease Index, platelet-rich plasma
Introduction
Human blood-derived topical treatments are increasingly being used in clinical practice for treating chronic severe ocular surface disease. Blood-derived products are advantageous in that they mimic certain components of natural tears, providing a mixture of growth factors and cytokines to the ocular surface.1 In addition, blood-derived therapies have also been studied for the treatment of pigment epithelial detachments, corneal ulcers, recurrent erosion syndrome, chemical injury, limbal stem cell deficiency and superior limbic keratoconjunctivitis.2
There are various blood-derived preparations that can be used to treat ocular surface disease. The first is autologous serum drops. Some studies have shown autologous serum to be effective in treating severe dry eye disease compared to preservative free artificial tears with varying results.3–5 However, the efficacy of autologous serum may be limited if circulating antibodies and pro-inflammatory cytokines are present as this may limit wound healing.6 Platelet preparations have also been used to treat ocular surface disease, including platelet-rich plasma (PRP), plasma rich in growth factors (PRGF) and platelet lysate.7 The advantage of platelet preparations is that they contain increased concentrations of growth factors, anti-inflammatory cytokines, and other platelet derivatives that have a role in wound healing and tissue regeneration.8 However, while they show promising results, blood-derived products can be costly and inconvenient for some patients.2 In addition, there is a lack of consensus regarding the use of blood products in the field of ophthalmology since many studies are based on heterogeneous blood products. In addition, there is also a need for more randomized clinical trials and stringent diagnostic tools to evaluate the impact of these blood products on ocular surface disease.9,10 An additional limitation to their use is that there is variability in regard to regulatory oversight and methods of preparation, and there are no standardized international protocols.1 Of note, allogenic blood products are now becoming an important alternative source as their production is not based on the health status of the patient.9,11
Platelet rich plasma is made by isolating and subsequently concentrating platelets in plasma. PRP can be prepared using a single or two-step centrifugation process.12 PRP is prepared from whole blood that is collected in the presence of a 3.2% sodium citrate anticoagulant solution, which is then centrifuged to isolate a platelet-enriched supernatant plasma. The PRP is divided into 3–4 mL aliquots and can be stored at 4°C for one week or at −20°C for an extended period of time.13 The growth factors present in PRP are thought to activate macrophages, induce cell repair and angiogenesis.8 Platelets store several biologically active agents such as growth factors inside granules, including platelet-derived growth factor (PDGF), epidermal growth factor (EGF), fibroblast growth factor (FGF), transforming growth factor (TGF), nerve growth factor (NGF) and insulin-like growth factor (IGF).12 PDGF, which is present in PRP, is thought to play a major role in wound healing, increasing the number of repaired cells, stimulating angiogenesis and supporting the development of new blood vessels and activated macrophages.1 To date, there is not a study that indicates an optimal concentration of growth factors; in addition, growth factors do not directly correlate with a patient’s platelet count.14 Blood products also include important metabolites.15 In addition, these products, unlike serum drops, do not contain leukocytes that may increase levels of pro-inflammatory cytokines.16
There are a small number of studies that suggest that PRP eye drops promote wound healing in ocular surface disease. However, there is a need for additional clinical studies to elucidate the role and efficacy of PRP eye drops in treating dry eye disease. The purpose of this study is to determine whether the addition of PRP eye drops for patients with severe ocular surface disease refractory to conservative measures provides subjective and objective clinical improvements.
Materials and Methods
Study Population
A retrospective case series was conducted of patients who were prescribed autologous PRP eye drops for the treatment of ocular surface disease. Patients were treated with 20% PRP eye drops four times daily for an average of three months. Subjects were excluded if they did not have a follow-up visit, underwent intraocular surgery prior to their follow-up visit, received nerve growth factor treatments or did not have a baseline examination with photography.
Grading of Images of Conjunctival Staining with Lissamine Green
Lissamine green was administered using GreenGlo strips (Figure 1, GreenGloTM, HUB Pharmaceuticals, LLC, Scottsdale, AZ), which each contain 1.5 mg of lissamine green. Slit lamp photographs of lissamine green staining of the bulbar conjunctiva were graded using the Oxford grading scale based on standard reference images, assigning an ordinal scale number to indicate disease severity. The nasal and temporal conjunctiva of each eye was evaluated on a scale of 0 to 3.
 |
Figure 1 Photograph of lissamine green staining at baseline visit. |
Assessment of Dry Eye Disease
Symptoms were assessed using the Ocular Surface Disease Index (OSDI). Patients also underwent a slit lamp exam, which included ocular surface staining with fluorescein and lissamine green.
Statistical Methods
We performed descriptive analyses using mean, standard deviation (SD), median, interquartile and range for continuous measures, and using count and percentage for categorical measures. A statistical comparison between baseline and follow-up visits for person-level OSDI score was performed using a paired t-test, and a comparison of eye-level scores of signs (conjunctival lissamine green staining score, Schirmer test score) was performed using the generalized linear model. Inter-eye correlation was accounted for by using generalized estimating equations. All statistical comparisons were performed in SAS v9.4 (SAS Institute Inc., Cary, NC) and two-sided p < 0.05 was considered statistically significant.
Results
The charts of 47 patients with a history of ocular surface disease were reviewed who had been prescribed PRP drops and were included in this analysis (Table 1). Sixty-four eyes of 32 patients had evaluable photographs of lissamine green staining taken at the baseline and at follow-up. The mean (SD) age at the baseline visit was 60 (13) years (range: 33 to 87) and 39/47 patients (83%) were females. Thirty-one of the 47 patients had a history of a systemic disease including 13 patients (28%) with ocular graft-versus-host disease, 16 patients (34%) with Sjögren’s syndrome, and 4 patients (8.5%) with rheumatoid arthritis. Two of these patients had a diagnosis of both Sjögren’s syndrome and rheumatoid arthritis. The median length of follow-up was 182 days (interquartile: 126–245 days), and 162.6 days for 32 patients who had follow-up photographs taken.
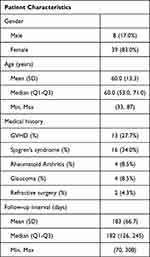 |
Table 1 Descriptive Statistics for Baseline Patient Characteristics (n = 47) |
There was a statistically significant decrease in OSDI score (ie, improvement in symptoms) from baseline to follow-up (39.5 vs 30.8 points, p = 0.02, Table 2). Among the 64 eyes included, 9 (14%) eyes had an improvement in conjunctival lissamine green staining (improving by ≥1 point), 49 (77%) eyes had stable staining and 6 (9%) eyes had increased staining (by ≥1 point) at follow-up. Among the 20 eyes with Schirmer testing, there was a borderline significant increase in score (ie, improvement in sign) from baseline to follow-up visit (5.9 vs 9.7, p = 0.06). There were no statistically significant associations between change in OSDI score or Schirmer’s score and baseline characteristics. Among the 44 eyes that had corneal fluorescein staining (CFS) reported at baseline and follow-up examinations, 8 (18.2%) had decreased staining and 2 (4.5%) had increased staining on follow-up. There was not sufficient data available regarding tear film breakup time to perform an analysis.
 |
Table 2 Symptoms and Signs at Baseline and Follow-Up |
Discussion
Recently, there has been a large expansion in treatment modalities available to treat the different causes of dry eye disease. The treatment of dry eye should follow a stepwise approach based upon the patient’s symptoms and the underlying pathophysiology of their condition.7 For patients with symptoms that are refractory to conservative measures and lubricants, there are several prescription medications that can be utilized. In addition, blood products are increasingly being used that have resulted in an improvement in the symptoms and signs of dry eye disease.
In our study, we found that patients treated with PRP had a statistically significant improvement in dry eye symptoms as measured by the OSDI. The mean difference in the OSDI score was 8.6 points, which is clinically significant. Based on Miller et al article, the minimal clinically important difference (MCID) ranges from 7.0 to 9.9 for all OSDI categories. In patients with mild or moderate symptoms, the MCID for OSDI is 4.5 to 7.3 whereas for those with severe symptoms, the MCID is 7.3 to 13.4.17 In addition, most eyes that were treated had stable or improved lissamine green conjunctival staining at follow-up. Among the 44 eyes that had fluorescein staining reported at baseline and follow-up examinations, 18.2% had decreased staining and 4.5% had increased staining on follow-up.
Previous studies have shown promising results for PRP drops in treating the signs and symptoms of moderate-to-severe DED. For example, Alio et al showed PRP was associated with a significant improvement in dry eye symptoms and signs in 368 patients with moderate-to-severe dry eye at 6 weeks of follow-up.18 There was an improvement of dry eye symptoms in 87.5% of cases and a decrease in CFS in 76.1% of patients. They also observed that 28.8% of patients improved by at least one line of best corrected visual acuity (BCVA). In addition, there was a statistically significant decrease in OSDI scores and in CFS (Oxford scale) after treatment.18 Another study was conducted in 156 eyes of 80 patients with chronic ocular surface syndrome (OSS) following laser in situ keratomileusis (LASIK) who were treated with autologous platelet-rich plasma E-PRP six times daily for six weeks. In this study, Alio et al observed a significant improvement in dry eye symptoms in 85% of cases. The study also found an improvement of CFS in 89.6% of patients, complete corneal healing in all three (1.9%) patients who presented with severe punctate keratitis at baseline, and improvement of conjunctival hyperemia in 93.3% of patients who had ocular surface inflammation. There was also a significant improvement in logMAR CDVA.19 Finally, García-Conca et al compared 44 patients treated with PRP to 39 patients treated with sodium hyaluronate artificial tears who had hyposecretory dry eyes at 15 and 30 days of treatment. They observed a statistically significant reduction in symptoms. In addition, they observed visual improvement, as well as improvements in hyperemia, ocular surface staining, Schirmer test scores, and osmolarity in the PRP group compared to the artificial tear group. They also found statistically significant improvements in the PRP group in visual acuity, hyperemia, osmolarity and ocular surface staining when compared to baseline values.20
While all of these results were promising, future larger prospective studies are needed to further evaluate the efficacy of PRP drops for treating ocular surface disease. Of note, a double-blind, randomized, parallel non-inferiority trial will compare the efficacy of 100% autologous platelet-rich plasma (APRP) to 100% autologous serum (AS) drops in patients with moderate-to-severe DED. The trial is currently in the recruitment stage.21
While dry eye disease affects millions of patients, the advances that have been made in the treatment of DED are helping to decrease the burden of disease on patients’ lives. Blood derivatives including PRP eye drops may have a strong therapeutic role in healing advanced ocular surface disease. Of note, PRP eye drops should be used as an adjunct to other conservative treatments in patients with severe ocular surface disease. PRP has the advantage of containing a large quantity of growth factors that promote wound healing at the ocular surface. We found that treatment with PRP drops was associated with a significant improvement in symptoms in patients with ocular surface disease and showed improvement in certain clinical signs. While the limitations of this study include study size and length of follow-up, we believe that this study is clinically relevant to patients with severe ocular surface disease refractory to conservative treatment measures. Future larger prospective and comparative studies with more advanced diagnostic techniques and standardization of protocols are needed to further evaluate the efficacy of PRP drops for treating ocular surface disease.
Conclusions
We found that treatment with PRP drops was associated with a significant improvement in dry eye symptoms in patients with ocular surface disease. The majority of eyes treated had stable or decreased ocular surface staining at follow-up. Future larger prospective and comparative studies are needed to further evaluate the efficacy of PRP drops for treating ocular surface disease.
Ethics Approval and Informed Consent
The report does not contain any personal identifying information. Written consent to publish this case was not obtained given that this was a retrospective study. An IRB exemption was granted by the University of Pennsylvania Institutional Review Board, IRB Protocol #827060 titled “Dry Eye Treatment Study” and was approved on the grounds of involving no more than minimal risk to patients. All research maintained patient confidentiality and was performed in accordance with the principles stated in the Declaration of Helsinki.
Acknowledgments
The abstract of this paper was presented at the Association for Research in Vision and Ophthalmology 2021 Annual Meeting as a poster presentation with interim findings. The poster’s abstract was published in the June 2021 Abstract Issue of IOVS: https://iovs.arvojournals.org/article.aspx?articleid=2774804&resultClick=1
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing of the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted and agree to be accountable for all aspects of the work.
Funding
Vision Core Grant (P30 EY001583) and National Eye Institute (R01 EY026972). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. Additional funding was provided by Research to Prevent Blindness and the Elizabeth King Trust.
Disclosure
Mina Massaro-Giordano reports being on advisory boards for Dompe and Lynthera; non-financial support from Lynthera, being an investor in PRN neutraceuticals (owns minor stock) and being a consultant for Lynthera and Claris, outside the submitted work. She is also part of advisory board for Kala, Oyster Point, Claris, and Anida. The authors report no other potential conflicts of interest for this work.
References
1. Giannaccare G, Versura P, Buzzi M, Primavera L, Pellegrini M, Campos EC. Blood derived eye drops for the treatment of cornea and ocular surface diseases. Transfus Apher Sci. 2017;56(4):595–604. doi:10.1016/j.transci.2017.07.023
2. Soni NG, Jeng BH. Blood-derived topical therapy for ocular surface diseases. Br J Ophthalmol. 2016;100(1):22–27. PMID: 26178904. doi:10.1136/bjophthalmol-2015-306842
3. Hussain M, Shtein RM, Sugar A, et al. Long-term use of autologous serum 50% eye drops for the treatment of dry eye disease. Cornea. 2014;33(12):1245–1251. PMID: 25299423. doi:10.1097/ICO.0000000000000271
4. Urzua CA, Vasquez DH, Huidobro A, Hernandez H, Alfaro J. Randomized double-blind clinical trial of autologous serum versus artificial tears in dry eye syndrome. Curr Eye Res. 2012;37(8):684–688. PMID: 22670856. doi:10.3109/02713683.2012.674609
5. Yılmaz U, Küçük E, Ç K, Gökler E. Comparison of autologous serum versus preservative free artificial tear in patients with dry eyes due to systemic isotretinoin therapy. Curr Eye Res. 2017;42(6):827–831. PMID: 28139163. doi:10.1080/02713683.2016.1255758
6. Sanchez-Avila RM, Merayo-Lloves J, Muruzabal F, Orive G, Anitua E. Plasma rich in growth factors for the treatment of dry eye from patients with graft versus host diseases. Eur J Ophthalmol. 2020;30(1):94–103. doi:10.1177/1120672118818943
7. Jones L, Downie LE, Korb D, et al. TFOS DEWS II management and therapy report. Ocular Surf. 2017;15(3):575–628.
8. Alio JL, Colecha JR, Pastor S, Rodriguez A, Artola A. Symptomatic dry eye treatment with autologous platelet-rich plasma. Ophthal Res. 2007;39(3):124–129. doi:10.1159/000100933
9. Bernabei F, Roda M, Buzzi M, Pellegrini M, Giannaccare G, Versura P. Blood-based treatments for severe dry eye disease: the need of a consensus. J Clin Med. 2019;8(9):1478. doi:10.3390/jcm8091478
10. Di Cello L, Pellegrini M, Vagge A, et al. Advances in the noninvasive diagnosis of dry eye disease. Applied Sci. 2021;11(21):10384. doi:10.3390/app112110384
11. Merolle L, Iotti B, Berni P, et al. Platelet-rich plasma lysate for treatment of eye surface diseases. J Vis Exp. 2022;(186). doi:10.3791/63772
12. You J, Hodge C, Hoque M, Petsoglou C, Sutton G. Human platelets and derived products in treating ocular surface diseases - a systematic review. Clin Ophthalmol. 2020;14:3195–3210. doi:10.2147/OPTH.S265701
13. Drew VJ, Tseng C-L, Seghatchian J, Burnouf T. Reflections on dry eye syndrome treatment: therapeutic role of blood products. Front Med. 2018;5:33. doi:10.3389/fmed.2018.00033
14. Pulcini S, Merolle L, Marraccini C, et al. Apheresis platelet rich-plasma for regenerative medicine: an in vitro study on osteogenic potential. Int J Mol Sci. 2021;22(16):8764. doi:10.3390/ijms22168764
15. Quartieri E, Marraccini C, Merolle L, et al. Metabolomics comparison of cord and peripheral blood-derived serum eye drops for the treatment of dry eye disease. Transfus Apher Sci. 2021;60(4):103155. doi:10.1016/j.transci.2021.103155
16. O’Neil EC, Henderson M, Massaro-Giordano M, Bunya VY. Advances in dry eye disease treatment. Curr Opin Ophthalmol. 2019;30(3):166–178. doi:10.1097/ICU.0000000000000569
17. Miller KL. Minimal clinically important difference for the ocular surface disease index. Arch Ophthalmol. 2010;128(1):94. doi:10.1001/archophthalmol.2009.356
18. Alio JL, Rodriguez AE, Ferreira-Oliveira R, Wróbel-Dudzińska D, Abdelghany AA. Treatment of dry eye disease with autologous platelet-rich plasma: a prospective, interventional, non-randomized study. Ophthalmol Ther. 2017;6(2):285–293. doi:10.1007/s40123-017-0100-z
19. Alio JL, Rodriguez AE, Abdelghany AA, Oliveira RF. Autologous platelet-rich plasma eye drops for the treatment of post-LASIK chronic ocular surface syndrome. J Ophthalmol. 2017;2017:2457620. doi:10.1155/2017/2457620
20. García-Conca V, Abad-Collado M, Hueso-Abancens JR, et al. Efficacy and safety of treatment of hyposecretory dry eye with platelet-rich plasma. Acta Ophthalmol. 2019;97(2):e170–e178. PMID: 30450721. doi:10.1111/aos.13907
21. Jongkhajornpong P, Numthavaj P, Anothaisintawee T, et al. Comparison of treatment efficacy between 100% platelet-rich plasma and 100% serum eye drops in moderate-to-severe dry eye disease: a randomised controlled trial protocol. BMJ Open. 2021;11(6):e048479. PMID: 34193498; PMCID: PMC8246355. doi:10.1136/bmjopen-2020-048479
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
