Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 15
Effect of Anagliptin versus Sitagliptin on Renal Function: Subanalyzes from the REASON Trial
Authors Teragawa H , Morimoto T , Fujii Y , Ueda T, Sakuma M, Shimabukuro M, Arasaki O, Node K, Nomiyama T, Ueda S
Received 21 November 2021
Accepted for publication 17 February 2022
Published 3 March 2022 Volume 2022:15 Pages 685—694
DOI https://doi.org/10.2147/DMSO.S350518
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ming-Hui Zou
Hiroki Teragawa,1 Takeshi Morimoto,2 Yuichi Fujii,1 Tomohiro Ueda,1 Mio Sakuma,2 Michio Shimabukuro,3 Osamu Arasaki,4 Koichi Node,5 Takashi Nomiyama,6 Shinichiro Ueda7
1Department of Cardiovascular Medicine, JR Hiroshima Hospital, Hiroshima, Japan; 2Department of Clinical Epidemiology, Hyogo College of Medicine, Nishinomiya, Japan; 3Deparment of Diabetes, Endocrinology and Metabolism, Fukushima Medical University, Fukushima, Japan; 4Department of Cardiology, Yuuai Medical Center, Tomigusuku, Okinawa, Japan; 5Department of Cardiovascular Medicine, Saga University, Saga, Japan; 6Department of Diabetes, Metabolism and Endocrinology, International University of Health and Welfare Ichikawa Hospital, Ichikawa, Japan; 7Department of Clinical Pharmacology and Therapeutics, University of the Ryukyus, Nishihara, Okinawa, Japan
Correspondence: Hiroki Teragawa, Department of Cardiovascular Medicine, JR Hiroshima Hospital, 3-1-36 Futabanosato, Higashi-ku, Hiroshima, 732-0052, Japan, Tel +81-82-262-1171, Fax +81-82-262-1449, Email [email protected]
Purpose: The effects of two types of dipeptidyl peptidase-4 (DPP-4) inhibitors on renal function remain unclear. Thus, we investigated the effect of anagliptin (ANA) and sitagliptin (SITA) on renal function in patients with type 2 diabetes who participated in the randomized evaluation of ANA versus SITA on low-density lipoprotein-cholesterol (LDL-C) in diabetes (REASON) trial.
Patients and methods: We measured the estimated glomerular filtration rate (eGFR) and urinary albumin–creatinine ratio (UACR) before and after the REASON trial. ANA 200 mg/day was administered to 177 patients for 52 weeks, while SITA 50 mg/day was given to 176 patients. We investigated the relationship between differences in renal function and differences in hemoglobin A1c (HbA1c) levels, LDL-C levels, and blood pressure (BP).
Results: No significant differences were found in baseline eGFR and UACR between the two groups. The eGFR levels were significantly decreased in both groups; however, the UACR level was unchanged in the ANA group but elevated in the SITA group, although the difference did not reach significance between the two groups. The difference in eGFR was affected by the differences in HbA1c level and BP, and the difference in the UACR was affected by the differences in LDL-C level and BP, which were reduced only in the ANA group.
Conclusion: These findings imply that the effects of DPP-4 inhibitors on renal function, especially on UACR, may be different between the types of DPP-4 inhibitors.
Keywords: glomerular filtration rate, dipeptidyl peptidase 4, dipeptidyl peptidase 4 inhibitors, albuminuria
Introduction
Diabetes mellitus (DM) is currently the leading cause of chronic kidney disease and end-stage kidney disease worldwide.1 For this reason, there has been much interest in nephroprotective therapy, including lifestyle modifications and types of drugs in patients with DM.1,2 Needless to say, sufficient glycemic control without hypoglycemia and strict control of blood pressure (BP) using renin–angiotensin–aldosterone system (RAAS) blockers constitute the mainstream nephroprotective therapy in patients with DM.1
In addition to the effects of glycemic control, some antidiabetic drugs such as glucagon-like peptide-1 (GLP-1) receptor agonist (GLP-1-RA)3 and sodium-glucose co-transporter 2 (SGLT2) inhibitors4,5 have demonstrated a nephroprotective effect. DPP-4 inhibitors increase the activity of GLP-1, the most physiologically significant incretin, and hence have various effects, including glucose-dependent insulin secretion stimulation, glucagon secretion inhibition, stomach emptying inhibition, and appetite modulation.6 Some researchers have focused on the nephroprotective effects of DPP-4 inhibitors7–13 as, although DPP-4 inhibitors have not been recommended as first-line medications in patients with DM with atherosclerotic cardiovascular diseases or very high risk of cardiovascular diseases,1 they have been widely used in the clinical setting because of their ability to improve hemoglobin A1c (HbA1c) levels with fewer severe complications. We recently conducted a randomized evaluation of anagliptin (ANA) versus sitagliptin (SITA) on low-density lipoprotein-cholesterol (LDL-C) in diabetes (REASON) trial. This trial included patients with type 2 DM, dyslipidemia, and existing atherosclerotic vascular lesions, demonstrating that the reduction in LDL-C level in the ANA group was superior to that in the SITA group, with a significant estimated treatment difference at −4.5 mg/dL.14 As one of the subanalyzes of the REASON trial, we previously examined the effects of these two DPP-4 inhibitors on inflammatory markers and reported that they did not affect inflammatory markers.15 Since the anti-inflammatory effect is involved in the inhibition of atherosclerosis progression,16 it appears important to study the effect on inflammatory markers. However, as mentioned above, considering that DM is the most common cause of the progression of chronic kidney disease (CKD),1 the effect of antidiabetic drugs on renal function must be examined. Therefore, in this study, we investigated the effects of two DPP-4 inhibitors, ANA and SITA, on renal function as another subanalysis of the REASON trial.
Methods
Trial Design and Participants
The design and participants of the REASON trial were previously detailed.17 The REASON trial investigated the efficacy of ANA or SITA in patients with type 2 DM, dyslipidemia, and existing atherosclerotic vascular lesions in a multicenter, randomized, open-label, active-controlled, parallel-group trial. Adults (aged 20 years) with type 2 DM who were treated with diet and exercise alone or in combination with hypoglycemic agents, had existing atherosclerotic vascular lesions, were treated with statins for dyslipidemia for 8 weeks, and documented with an LDL-C level (100 mg/dL in at least one measurement after statin use) were eligible participants. The major exclusion criteria were as follows: type 1 diabetes with a triglyceride level of 400 mg/dL in a previous fasting blood sample, pregnancy, potential pregnancy, or lactation, severe infections, surgery, serious trauma, serum creatinine level of 2.4 mg/dL for men or 2.0 mg/dL for women, and use of GLP-1-RA.
This study followed the Declaration of Helsinki and the Japanese Ethical Guidelines for Medical and Health Research Involving Human Subjects. Before randomization, the institutional review boards of the University of the Ryukyus (No. 731) and each participating center received written informed consent from all patients or their legally authorized representatives. This study was registered in Clinicaltrials.gov (NCT02330406).
Randomization and Intervention
The randomization and intervention protocols were also discussed in the earlier work.17 In this study, we used the standard dosage of the drug recommended in Japan. In a nutshell, the ANA group received 100 mg of ANA twice daily orally for 52 weeks. The dose could be increased to 200 mg orally twice daily if the effects were insufficient. SITA 50 mg orally once daily was given to the SITA group for 52 weeks. The dose could be increased to 100 mg/day if the effects were insufficient. If the patients were using antidiabetic medicines at the start of the experiment, save DPP-4 inhibitors, the study drug was given concurrently, and the antidiabetic treatments were not replaced. The participants’ and treating physicians’ treatment assignments were not kept a secret.
No hypoglycemic agents or antidyslipidemic drugs were introduced or doses altered during the trial period; a change in the insulin dose was not considered a change in the hypoglycemic agent. The need for additional therapy was established by the lead physician; however, adjustments in other medications that could affect the outcome were not allowed. Participants and their physicians were intensively supervised by clinical research coordinators at each visit to ensure adherence to the study medication and dose. As per protocol, individuals were removed if there was a crossover.
Measurements
Blood tests were performed at the core laboratory (SRL Inc., Tokyo, Japan). The estimated glomerular filtration rate (eGFR, mL/min/1.73 m2)18 and urinary albumin–creatinine ratio (UACR, mg/gCr) using spot urine collection were checked at baseline and final follow-up. In the present subanalyzes, the differences in these renal markers were expressed as markers at the final follow-up minus those at baseline. To evaluate the relationship between the differences in eGFR and UACR and clinical parameters, the latter included factors with differences in HbA1c level, LDL-C level, and systolic and diastolic BP; baseline eGFR and UACR; whether the patient was using a RAAS inhibitor; and whether the patient did not previously use a DPP-4 inhibitor or SGLT2 inhibitor.
Statistical Analysis
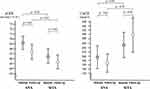
The intention-to-treat principle was applied to all analyses. Categorical variables were expressed as frequencies with percentages, whereas continuous variables were expressed as means with standard deviations or medians with interquartile ranges. In Figure 1 only, the graph bar was displayed using standard error because the range became large when standard deviation was used. We estimated the changes in outcomes between baseline and 52-week follow-up and their 95% confidence intervals, and their differences were compared with the paired t-test. The differences in changes between the ANA and SITA groups were compared with the two-sample t-test. We conducted the same analyses according to the clinically selected subgroups, including the eGFR at baseline and use of a RAAS inhibitor, previous DPP-4 inhibitor, and SGLT2 inhibitor for eGFR and UACR at baseline and use of a RAAS inhibitor, previous DPP-4 inhibitor, and SGLT2 inhibitor for UACR. Moreover, we assessed the correlations among changes in eGFR, UACR, HbA1c level, LCL-C level, and systolic and diastolic BP. The study statisticians (Morimoto T) performed all statistical analyses at the data center (Institute for Clinical Effectiveness) using JMP 13.1 (SAS Institute Inc., Cary, NC) and SAS 9.4 (SAS Institute Inc.) based on the SAP. All P-values were two-sided, and a P-value of less than 0.05 was considered significant.
Results
Patient Characteristics
Of the 353 participants, 177 and 176 patients were assigned to the ANA group and SITA group, respectively. Table 1 presents the patient characteristics. No difference was found in the use of antihypertensive drugs, including RAAS inhibitors, calcium channel blockers, diuretics, and beta-receptors; use of antidiabetic drugs, such as a previous DPP-4 inhibitor or SGLT2 inhibitor; body weight; body mass index; systolic and diastolic BP; eGFR; and UACR at baseline between the two groups.
 |
Table 1 Patient Characteristics |
Changes in eGFR and UACR
No change in systolic BP was noted in both groups at the final follow-up. Diastolic BP was significantly reduced in only the ANA group (P = 0.02, Table 2), whereas no significant difference was found between the two arms (P = 0.09). In both groups, the eGFRs at the final follow-up were lower than those at baseline, with the difference in the eGFR of −1.66 ± 9.83 mL/min/1.73 m2 (P = 0.03) in the ANA group and −1.39 ± 8.02 mL/min/1.73 m2 (P = 0.02) in the SITA group; however, the values in both groups were comparable (P = 0.79, Figure 1, Table 2). The UACRs at the final follow-up were not different in the two groups, but the differences in UACRs from baseline to the final follow-up were 7.7 ± 185.7 mg/gCr in the ANA group (P = 0.61) and 57.2 ± 345.3 mg/gCr in the SITA group (P = 0.04), although this difference was not significant (P = 0.12, Figure 1, Table 2).
 |
Table 2 Clinical Parameters at Baseline and Final Follow-Up and Difference |
Relationship Between the Differences in eGFR and UACR and Clinical Parameters
First, differences in eGFR were positively related to the differences in A1c level and systolic and diastolic BP, and the difference in UACR was positively related to the differences in LDL-C level and systolic and diastolic BP (Table 3). We evaluated the relationship between the differences in eGFR and clinical factors, such as eGFR at baseline, use of a RAAS inhibitor, and previous use of a DPP-4 inhibitor and SGLT2 inhibitor, and we found no significant factor responsible for the difference in eGFR (Figure 2). We also examined the relationship between the difference in UACR and clinical factors, such as UACR at baseline, use of a RAAS inhibitor, and previous use of a DPP-4 inhibitor or SGLT2 inhibitor, and found no significant factors, except for the UACR at baseline, which was responsible for the difference in UACR (Figure 3). Regarding the relationship between the difference in UACR and UACR at baseline, although no significant difference was noted in each of the three subgroups (UACR < 30, 30 ≤ UACR < 300, and UACR ≥ 300), there was a tendency for a wider difference in UACR in the ANA and SITA groups, when the baseline UACR was ≥ 300 mg/gCr (Figure 3).
 |
Table 3 Relationship Between the Differences in Renal Function and Clinical Parameters |
Discussion
Herein, we investigated the effects of ANA versus SITA on renal function in patients with DM with a high cardiovascular burden by subanalyzes of the REASON trial. In this study, eGFR was significantly reduced in both groups and the change in UACR was somewhat different in the two groups: it did not change in the ANA group but increased in the SITA group, although this difference did not reach significance. Furthermore, the difference in eGFR was affected by the differences in HbA1c level and systolic and diastolic BP; however, differences in eGFR according to the use of various DPP-4 inhibitors with ANA or SITA were not affected by either the baseline eGFR or use of a RAAS inhibitor or previous use of a DPP-4 inhibitor or SGLT2 inhibitor. Conversely, the difference in UACR was affected by the difference in LDL-C level and systolic and diastolic BPs. Furthermore, the difference in UACR was not affected by the use of a RAAS inhibitor or previous use of a DPP-4 inhibitor or SGLT2 inhibitor. However, we found a different tendency between the difference in UACR and baseline UACR.
According to several experimental studies, DPP-4 expression and enzymatic activity were discovered in the glomerulus only in pathological renal circumstances, not in healthy kidneys.19–21 Furthermore, when compared with nonalbuminuric diabetic patients or healthy individuals, patients with type 2 DM and albuminuria had considerably higher urine DPP-4 activity.21,22 Using these experimental observations, we hypothesized that DPP-4 plays a pathogenic function in diabetic nephropathy, implying that DPP-4 inhibitors could have a nephroprotective effect on this patient population.
Clinical studies that investigated the effects of DPP-4 inhibitors on eGFR have presented various results. Concerning eGFR, some studies9,12,23 have reported that using a DPP-4 inhibitor reduced eGFR, whereas others have shown that eGFR did not change after administration of DPP-4 inhibitor7,10,24,25 or that the influence of DPP-4 inhibitors on eGFR varied according to the baseline eGFR.11 One important factor is that a decline in eGFR is sometimes followed by an improvement in preceding glomerular hyperfiltration.26 In the present subanalyzes, changes in eGFR were positively correlated with changes in HbA1c and BP. On the contrary, diastolic BP decreased significantly only in the ANA group. A study also reported that HbA1c did not change significantly in both groups in the REASON trial.14 Nonetheless, the present study revealed that eGFR was significantly lower in both groups. Considering all these findings, the decrease in eGFR in this study group may be due to a class effect of DPP-4 inhibitors or natural history of eGFR within 52 weeks. We believed that this difference in eGFR may not be clinically significant.
As regards the effects of DPP-4 inhibitors on UACR, clinical studies8–10,12,13,24,25,27 have reported similarly beneficial results, namely, the lowering effect of UACR after DPP-4 inhibitor administration. These effects may be dependent, to some extent, on the type of DPP-4 inhibitor, duration of administration, accompanying diseases, and degree of baseline renal function. The present subanalyzes revealed that the difference in UACR was somewhat different in the two groups and associated with the difference in LDL-C level and BP. The main finding of the REASON trial indicated that the reduction in LDL-C level in the ANA group was superior to that in the SITA group,14 and the present study showed that the decline in diastolic BP was observed only in the ANA group. As studies have reported that lipid-lowering or antihypertensive therapy can improve renal function or prevent worsening of renal function,28,29 ANA-induced lowering effects on LDL-C level and diastolic BP might account for the unchanged UACR, which was observed only in the ANA group. Moreover, the ANA-induced effect on UACR might be greater in patients with albuminuria (UACR ≥300 mg/gCr); thus, future studies should be conducted to confirm the latter finding.
The present study has several limitations. First, as presented above, renal parameter data at follow-up, especially in UACR, were not completely obtained from the studied patients; thus, insufficient data may contribute to the results of the present subanalyzes. Second, the majority of the patients (82%) had previously used a DPP-4 inhibitor; thus, only a small group of the patients had never used a DPP-4 inhibitor (ie, DPP-4 inhibitor naive) for whom it was expected that starting a new DPP-4 inhibitor would have any effect on renal function. Third, the number of patients investigated in the REASON trial was not initially assessed in these subanalyzes. Fourth, to evaluate the natural history of eGFR and UACR, a control group without DPP-4 inhibitor should have been included during the study period. Thus, given the lack of a control group in this study, evaluating the natural history of these indices was not possible. Fifth, the study included patients with severe CKD (serum creatinine level ≥ 2.4 mg/dL for men or ≥ 2.0 mg/dL for women).17 The effects of ANA and SITA on renal function in these patients with severe CKD were not evaluated. Sixth, no difference was noted in the antihypertensive drugs at the time of entry between the two groups. However, since there was no information on changes in antihypertensive drugs during the observation period, the possibility that changes in antihypertensive drugs in each group affected BP trends could not be denied. Finally, in the REASON trial, spot urine collection was used to assess the UACR. Timed urine collection would have provided a more accurate assessment of albuminuria. However, in many clinical studies, spot urine collection is used for the assessment of UACR because of the complexity of timed urine collection.
Conclusions
In the present subanalyzes of the REASON trial, we found that the two DPP-4 inhibitors, ANA and SITA, had a small but significant reduction effect on eGFR in both groups, but the effect on UACR may be somewhat different. The former finding may be due to the class effect of DPP-4 inhibitors or the natural history of patients with DM having a high atherosclerotic burden. The latter finding should be re-evaluated in the future in patients with severe albuminuria.
Abbreviations
ANA, anagliptin; BP, blood pressure; CKD, chronic kidney disease; DM, diabetes mellitus; DPP-4, dipeptidyl peptidase-4; eGFR, estimated glomerular filtration rate; GLP-1-RA, glucagon-like peptide-1 receptor agonist; HbA1c, hemoglobin A1c; LDL-C, low-density lipoprotein cholesterol; RAAS, renin-angiotensin-aldosterone system; REASON trial, Randomized Evaluation of Anagliptin and sitagliptin in reducing low-density lipoprotein cholesterol in diabetes trial; SGLT2, sodium-glucose co-transporter 2; SITA, sitagliptin; UACR, urinary albumin-creatinine ratio.
Data Sharing Statement
The data sets analysed during the current study are available from the corresponding author on reasonable request ([email protected]).
Ethical Approval
This study was conducted in accordance with the Declaration of Helsinki and the Ethical Guidelines for Medical and Health Research Involving Human Subjects in Japan. The institutional review boards of the University of the Ryukyus (No. 731) and each participating center gave their approval for this study. This trial was registered on ClinicalTrials.gov (NCT02330406).
Informed Consent
All patients or their legally authorized representatives provided written informed consent prior to randomization.
Acknowledgments
Data management was handled by Ms. Makiko Ohtroii, Ms. Ai Sunagawa, Ms. Sachiko Kitamura, Ms. Kaori Yamamoto, Ms. Hirono Saito, and Ms. Saeko Nagano, while project management was handled by Ms. Takako Okumura.
Funding
Kowa Company, Ltd. contributed to the REASON trial. Kowa had a representative participate in the research idea and evaluation of the final draft of the REASON trial’s main report. The academic authors, on the other hand, were exclusively responsible for the study design, operation, data collection, statistical analysis, and paper drafting.
Disclosure
Dr H.T. reports lecturer fees from Daichi Sankyo, Mitsubish Tanabe, Bayer, Kowa, and Nihon Medi-Physics. Dr. T.M. has lecturer fees from AbbVie, AstraZeneca, Daiichi Sankyo, Japan Lifeline, Kowa, Toray, Tsumura, Kyorin, Mitsubishi Tanabe, Pfizer, and Bayer, as well as manuscript payments from Pfizer and advisory board roles with Asahi Kasei, Boston Scientific, Bristol-Myers Squibb, and Novartis. Dr. Y.F. and T.U. declare that they have no competing interests. Dr. M.S. is a member of Enomoto Pharmaceutical’s advisory board. Dr. M.S. has received research grants from AstraZeneca, Ono, and Sanwa Kagaku Kenkyusho, as well as nonpurpose research grants from Astellas, AstraZeneca, Bayer, Boehringer Ingelheim, Chugai, Eli Lilly, Kowa, Mitsubishi Tanabe, MSD, Novo Nordisk, Ono, Taisho Toyama, and Takeda; lecturer fees from Astella. Dr O.A. reports lecturer fees from Abbott, Astellas, Boehringer Ingelheim, Medtronic, and St. Jude Medical. Dr. K.N. reports research grants from Abbott, Actelion, Air Water, Asahi Kasei, Astellas, Bayer, Terumo, Boehringer Ingelheim Japan, Mochida Pharmaceutical, Fuji Yakuhin, Medtronic, Daiichi Sankyo, Eli Lilly Japan, Takeda Pharmaceutical, GlaxoSmithKline, Mebix, Mitsubishi Tanabe, MSD, Novartis, Novo Nordia, Ono Pharmaceutical, and Teijin; nonpurpose research grants from Abbott, Astellas, AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb. Dr. T.N. has received research grants from Eli Lilly, Mitsubishi Tanabe, MSD, Novartis, Novo Nordisk, Ono, Sanofi, Sanwa Kagaku Kenkyusho, Sumitomo Dainippon, Taisho Toyama, Takeda, and Terumo, as well as lecturer fees from Arkray, Astellas, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Johnson & Johnson Dr. S.U. has received research funding from Bristol-Myers Squibb and Kowa, as well as non-purpose research grants from Bristol-Myers Squibb, Chugai, MSD, Pfizer, and Takeda. He also has lecturer fees from Boehringer Ingelheim and MSD. The authors report no other conflicts of interest in this work.
References
1. Cosentino F, Grant PJ, Aboyans V, et al. 2019 ESC guidelines on diabetes, pre-diabetes, and. cardiovascular diseases developed in collaboration with the EASD. Eur Heart J. 2020;41(2):255–323. doi:10.1093/eurheartj/ehz486
2. Buse JB, Wexler DJ, Tsapas A, et al. 2019 update to: management of hyperglycemia in type 2 diabetes, 2018. a consensus report by the American Diabetes Association (ADA) and the European Association for the study of diabetes (EASD). Diabetes Care. 2020;43(2):487–493. doi:10.2337/dci19-0066
3. Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375(4):311–322. doi:10.1056/NEJMoa1603827
4. Zelniker TA, Wiviott SD, Raz I, et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet. 2019;393(10166):31–39. doi:10.1016/S0140-6736(18)32590-X
5. Perkovic V, Jardine MJ, Neal B, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380(24):2295–2306. doi:10.1056/NEJMoa1811744
6. Drucker DJ. The biology of incretin hormones. Cell Metab. 2006;3(3):153–165. doi:10.1016/j.cmet.2006.01.004
7. White WB, Cannon CP, Heller SR, et al. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med. 2013;369(14):1327–1335. doi:10.1056/NEJMoa1305889
8. Goldshtein I, Karasik A, Melzer-Cohen C, et al. Urinary albumin excretion with sitagliptin compared to sulfonylurea as add on to metformin in type 2 diabetes patients with albuminuria: a real-world evidence study. J Diabetes Complicat. 2016;30(7):1354–1359. doi:10.1016/j.jdiacomp.2016.05.012
9. Cornel JH, Bakris GL, Stevens SR, et al. Effect of sitagliptin on kidney function and respective cardiovascular outcomes in type 2 diabetes: outcomes from TECOS. Diabetes Care. 2016;39(12):2304–2310. doi:10.2337/dc16-1415
10. Mosenzon O, Leibowitz G, Bhatt DL, et al. Effect of saxagliptin on renal outcomes in the SAVOR-TIMI 53 trial. Diabetes Care. 2017;40(1):69–76. doi:10.2337/dc16-0621
11. Esaki H, Tachi T, Goto C, et al. Renoprotective effect of dipeptidyl peptidase-4 inhibitors in patients with type 2 diabetes mellitus. Front Pharmacol. 2017;8:835. doi:10.3389/fphar.2017.00835
12. Bae JH, Kim S, Park EG, et al. Effects of dipeptidyl peptidase-4 inhibitors on renal outcomes in patients with type 2 diabetes: a systematic review and meta-analysis. Endocrinol Metab. 2019;34(1):80–92. doi:10.3803/EnM.2019.34.1.80
13. Williams DM, Nawaz A, Evans M. Renal outcomes in type 2 diabetes: a review of cardiovascular and renal outcome trials. Diabetes Ther. 2020;11(2):369–386. doi:10.1007/s13300-019-00747-3
14. Morimoto T, Sakuma I, Sakuma M, et al. Randomized evaluation of anagliptin vs sitagliptin on low-density lipoproteiN cholesterol in diabetes (REASON) trial: a 52-week, open-label, randomized clinical trial. Sci Rep. 2019;9(1):8537. doi:10.1038/s41598-019-44885-x
15. Teragawa H, Morimoto T, Fujii Y, et al. Effect of anagliptin versus sitagliptin on inflammatory markers: sub-analysis from the REASON trial. Diabetes Metab Syndr Obes. 2020;13:4993–5001. doi:10.2147/DMSO.S282968
16. Ross R. Atherosclerosis–an inflammatory disease. N Engl J Med. 1999;340(2):115–126. doi:10.1056/NEJM199901143400207
17. Ueda S, Shimabukuro M, Arasaki O, et al. Effect of anagliptin and sitagliptin on low-density lipoprotein cholesterol in type 2 diabetic patients with dyslipidemia and cardiovascular risk: rationale and study design of the REASON trial. Cardiovasc Drugs Ther. 2018;32(1):73–80. doi:10.1007/s10557-018-6776-z
18. Matsuo S, Imai E, Horio M, et al. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53(6):982–992. doi:10.1053/j.ajkd.2008.12.034
19. Stiller D, Bahn H, August C. Demonstration of glomerular DPP IV activity in kidney diseases. Acta Histochem. 1991;91(1):105–109. doi:10.1016/S0065-1281(11)80302-8
20. Sharkovska Y, Reichetzeder C, Alter M, et al. Blood pressure and glucose independent renoprotective effects of dipeptidyl peptidase-4 inhibition in a mouse model of type-2 diabetic nephropathy. J Hypertens. 2014;32(11):2211–2223. doi:10.1097/HJH.0000000000000328
21. Kanasaki K. The role of renal dipeptidyl peptidase-4 in kidney disease: renal effects of dipeptidyl peptidase-4 inhibitors with a focus on linagliptin. Clin Sci. 2018;132(4):489–507. doi:10.1042/cs20180031
22. Sun AL, Deng JT, Guan GJ, et al. Dipeptidyl peptidase-IV is a potential molecular biomarker in diabetic kidney disease. Diab Vasc Dis Res. 2012;9(4):301–308. doi:10.1177/1479164111434318
23. Nakamura K, Oe H, Kihara H, et al. DPP-4 inhibitor and alpha-glucosidase inhibitor equally improve endothelial function in patients with type 2 diabetes: EDGE study. Cardiovasc Diabetol. 2014;13:110. doi:10.1186/s12933-014-0110-2
24. Kitada M, Tsuda SI, Konishi K, et al. Anagliptin ameliorates albuminuria and urinary liver-type fatty acid-binding protein excretion in patients with type 2 diabetes with nephropathy in a glucose-lowering-independent manner. BMJ Open Diabetes Res Care. 2017;5(1):e000391. doi:10.1136/bmjdrc-2017-000391
25. Rosenstock J, Perkovic V, Johansen OE, et al. Effect of linagliptin vs placebo on major cardiovascular events in adults with type 2 diabetes and high cardiovascular and renal risk: the CARMELINA randomized clinical trial. JAMA. 2019;321(1):69–79. doi:10.1001/jama.2018.18269
26. Melsom T, Mathisen UD, Ingebretsen OC, et al. Impaired fasting glucose is associated with renal hyperfiltration in the general population. Diabetes Care. 2011;34(7):1546–1551. doi:10.2337/dc11-0235
27. Kim YG, Byun J, Yoon D, et al. Renal protective effect of DPP-4 inhibitors in type 2 diabetes mellitus patients: a cohort study. J Diabetes Res. 2016;2016:1423191. doi:10.1155/2016/1423191
28. Sandhu S, Wiebe N, Fried LF, et al. Statins for improving renal outcomes: a meta-analysis. J Am Soc Nephrol. 2006;17(7):2006–2016. doi:10.1681/ASN.2006010012
29. Hanratty R, Chonchol M, Havranek EP, et al. Relationship between blood pressure and incident chronic kidney disease in hypertensive patients. Clin J Am Soc Nephrol. 2011;6(11):2605–2611. doi:10.2215/CJN.02240311
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.