Back to Journals » Chronic Wound Care Management and Research » Volume 7
Effect of Aloe vera Gel, Calendula officinalis Ointment and Simple Prophylactic Sacral Dressings for Pressure Injury Development
Authors Baghdadi M, Rafiei H, Rashvand F, Oveisi S
Received 2 April 2020
Accepted for publication 25 May 2020
Published 11 June 2020 Volume 2020:7 Pages 19—26
DOI https://doi.org/10.2147/CWCMR.S256537
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Marco Romanelli
Mahmood Baghdadi,1 Hossein Rafiei,2 Farnoosh Rashvand,2 Sonia Oveisi3
1Student Research Committee, Qazvin University of Medical Sciences, Qazvin, Iran; 2Social Determinants of Health Research Center, Research Institute for Prevention of Non‐Communicable Diseases, Qazvin University of Medical Sciences, Qazvin, Iran; 3Metabolic Diseases Research Center, Research Institute for Prevention of Non-Communicable Diseases, Qazvin University of Medical Sciences, Qazvin, Iran
Correspondence: Farnoosh Rashvand Email [email protected]
Background and Purpose: Prophylactic dressings play a significant role in the prevention of pressure injury. However, no herbal products have been used in this regard, so far. Therefore, the present study was designed with the aim of comparing the effect of Aloe vera gel, Calendula officinalis ointment and simple sacral prophylactic dressings on pressure injury development in patients admitted to intensive care unit.
Materials and Methods: The sample of this clinical trial study consisted of 90 critically ill patients. Randomization was performed by head nurse in intensive care unit. Patients in group “A” received Aloe vera gel prophylactic dressing twice a day; patients in group “B” received Calendula officinalis ointment prophylactic dressing twice a day; and patients in group “C” received simple prophylactic dressing twice a day. The development of pressure injury was compared between these three groups after the intervention.
Results: Demographic variables were similar in all three groups (p> 0.05). The results showed that 3 patients (10%) who received the simple dressing, 2 patients (6.6%) who received the Calendula officinalis ointment dressing were wounded, while the development of pressure injury in patients who received the Aloe vera gel dressing was zero. The results of chi-square test showed that the frequency distribution of the pressure injury development in the two groups receiving Aloe vera gel and simple dressing was different. This difference was statistically significant (p < 0.05). However, there was no significant difference between the Aloe vera gel and Calendula officinalis ointment groups as well as the Calendula officinalis ointment group and the simple dressing.
Conclusion: The results of the present study demonstrated that using Aloe vera gel and Calendula officinalis ointment prophylactic dressing twice a day can be effective in prevention of pressure injury in patients admitted to intensive care unit.
Keywords: Aloe vera gel, Calendula officinalis ointment, critically ill patient, medicinal plants, prophylactic dressings
Introduction
Patients admitted to the intensive care units (ICUs) are at high risk of pressure injury development. These patients are immobilized on the bed because of various reasons.1,2 Other factors such as nutritional deficiencies, loss of consciousness, severity of illness, taking certain medications, urinary incontinence and/or bowel incontinence, sweating and excessive moisture in the skin, edema, and inappropriate use of medical devices also increase the risk of pressure injury development.1–4 Studies on these types of ulcers in Iran indicate the high prevalence and incidence. The results of three studies conducted in 2014, 2015 and 2016 can be mentioned as an example, in which the prevalence of pressure injury development in the ICUs in Iran was reported to be 27, 45, 32%, respectively.5–7 Studies in other countries also indicate high prevalence of these wounds in the ICUs. For instance, a study in China in 2016 showed that the prevalence of pressure injury development in the ICUs is 31%.8
The development of pressure injury in hospitalized patients is considered as a negative point of healthcare providers and systems. Therefore, the prevention of pressure injury is highly important, especially for the nursing team.8,9 For this, many guidelines and references have provided a variety of suggestions, including; identifying the patients at risk, patients change position, using support surfaces, and physical examination of the patients’ skin in terms of any symptoms of pressure injury in the early stages.2 In addition to the previous approaches, the debate about the use of pressure injury prophylactic dressings has attracted the attention of many researchers, and several studies have been done in this regard. In a study in 2016, Byrne et al studied the effects of prophylactic sacral dressing for pressure injury development in critically ill patients. The results of Byrne et al study showed that using prophylactic sacral dressing for pressure injury development in these patients significantly reduced the development of pressure injury.2 In another study in the United State, Walsh et al concluded that the use of prophylactic dressing is effective in reducing the development of pressure injury in patients hospitalized admitted to the ICU.10 In a randomized controlled clinical trial Santamaria et al examined the use of multi‐layered soft silicone foam dressings for prevention of sacral and heel pressure injury in 440 trauma and critically ill patients that were randomly assigned into two groups. The results of their study revealed that the use of multi‐layered soft silicone foam dressings reduced the rate of pressure injury in critically ill patients in intervention group compared to control group (3.1% versus 13.1%).11 In another study, Bishopp et al examined the effect of using prophylactic hydrocolloid dressings on nasal bridge for prevention medical-device-related pressure injury. Results of Bishopp et al’s study revealed that using prophylactic hydrocolloid dressings decrease rate of medical-device-related pressure injury related to non-invasive ventilation mask.12 In another study in this regard in 2014, Kohta et al reported that using polyurethane film dressings decreased the risk of pressure injury development in surgery patients.13
Although there are a variety of prophylactic dressing types for pressure injury in previous studies, they can be generally divided into three categories: Film dressings, Hydrocolloid dressings and Foam dressings.14 The materials used in these three categories are usually industrial materials and associated with relatively high cost for the patient and healthcare systems. Cheaper and more affordable alternatives, such as plant products, can solve this problem. But there is no study conducted on the preventive role of plant products in pressure injury. However, there are several studies on the therapeutic effects of plant products on the improvement of inflammation and skin wounds.15–19 Therefore, this hypothesis was proposed to researchers whether the plant product prophylactic dressing decreases the development of pressure injury. After searching from available databases, two materials of Aloe vera and Calendula officinalis were selected for this purpose. Aloe vera also named yellow Aloe is related to Liliaceae family and usually found in hot and arid areas such as some parts of Asia, Europe, Africa and America.20–22 This plant has several functions such as wound healing, anti-inflammatory action, moisturizing and anti-aging effect, antiseptic effect and laxative effect.21 Calendula officinalis also named pot marigold used for several skin health condition.23,24 Calendula officinalis extract rich of monoesters, triterpenoids, triterpene alcohols, triterpene oligoglycosides, and flavonoids.25 Thus, the present study was designed with the aim of comparing the effect of Aloe vera gel, Calendula officinalis ointment and simple prophylactic dressings on pressure injury development in patients admitted to ICU.
Materials and Methods
Study Environment and Samples
The present study, which is a clinical trial, conducted in the ICU of Alborz Hospital in Karaj, Iran, during the years 2017 and 2018. The ICU of this hospital has 20 active beds and the hospitalized patients are composed of patients from different groups including surgical, internal and neurological wards. Our hospital has wound care team that guides nurses for pressure injuries prevention and treatment. We also use standard guideline for pressure injuries prevention in ICU.
The present study included all the patients who were classified in the moderate to high-risk category (score 14 and below) for pressure injury development based on the Braden Scale.
Sample Size
According to a study conducted by Eghdampour et al in 2013, the mean and average standard deviation scores in the two groups’ placebo and intervention (Aloe vera) were estimated to be 1.10 ± 0.73 and 0.59 ± 0.59, respectively.26 Therefore, considering type I error, α=0.05 and the type II error, β=0.20 (power=80%), the sample size was calculated using the following formula. Therefore, considering a drop-out rate of 10%, the total sample size required for each group is 30 participants and totally 90.
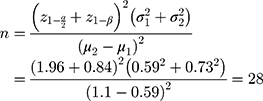
Inclusion Criteria
No sign of pressure injury at the time of ICU admission, participants with a moderate and high risk of pressure injury development based on a score they achieved in the Braden Scale (score 14 and below) and aged over 18 years old (Braden Scale is only used for people with over 18 years old).
Exclusion Criteria
Absolute movement limitation requested by the physicians that inhibit the patient from changing position. Drug allergy to the Aloe vera in group “A” and drug allergy to the Calendula officinalis ointment in group “B” based on the patient’s or their relative report and the development of pressure injury in the first 12 hours after admission.
Data Collection Instrument
Demographic Checklist
This checklist included age, sex, cause of hospitalization, drug addiction, other diseases, type of respiration in the ICU (endotracheal tube, tracheostomy and spontaneous respiration)
Braden Scale for Predicting Pressure Injury Risk
Braden Scale is an instrument using to predict pressure injury risk. It was first developed by Braden and Bergstrom. This scale assesses six criteria: Sensory perception, Moisture, Activity, Mobility, Nutrition, Friction and Shear. Each category is rated on a scale of 1 to 4 (excluding the “friction and shear” category which is rated on a scale of 1 to 3). Score 1 indicates a worse condition, while a score of 4 a better one. A total score is calculated by combining these categories. Higher scores denote lower risk of developing a pressure injury. The validity and reliability of the Persian version of this instrument have been determined to the desired in previous studies.3
Pressure Injury Grading Tool
The instrument was developed in 2014 by the American Pressure Ulcer Advisory Panel and the European Pressure Ulcer Advisory Panel. Based on this, pressure injury is divided into six stages (Stage 1: Non-blanchable erythema of intact skin, stage 2: Partial-thickness skin loss with exposed dermis, stage 3: Full-thickness skin loss, stage 4: Full-thickness skin and tissue loss, Unstageable Pressure Injury: Obscured full-thickness skin and tissue loss and Deep Tissue Pressure Injury: Persistent non-blanchable deep red, maroon or purple discoloration) in severity.27
Interventions
All the patients aged over 18 years old were selected in the first 12 hours after admission to the ICU of Alborz Hospital in Karaj by the researchers. If patients did not have any pressure injury at the time of admission, Braden scale was used to assess them. Those patients, who were at a moderate and high risk of pressure injury development based on a score they achieved in the Braden Scale (score 14 and below), recruited to the study. It needs to be said that the Braden scale was carried out only once by the researcher and it was at the time of the initial evaluation of the patient.
Patients were randomly assigned to three groups. A ball was chosen by one of the head nurses of the ICU who was blinded to the study groups. Patients in the group “A” received Aloe vera gel prophylactic dressing, patient in group “B” received Calendula officinalis ointment prophylactic dressing and patient in group “C” (control group) received simple prophylactic dressing. Considering that the most common area of pressure injury is sacrum area and the average duration of pressure injury development in critically ill patients were the first seven days,28 the skin of patients in three groups was examined by the clinical nurse who were trained in wound care for a maximum of seven days and twice a day in terms of any symptoms of pressure injury. She was blinded to the study groups. Usual care such as change position, alternating pressure air mattress replacements, static mattress replacements, turning regimes and daily/per shift skin inspection were similar in all patients in three groups.
Patients in group “A” received Aloe vera gel prophylactic dressing. In this group, the skin of the sacrum area was firstly washed and cleaned with a normal saline solution. Then, 5 cc of Aloe vera gel was rubbed in the sacrum area, and a 10×10 cm simple dressing gauge was placed and kept on the skin with mattress glue. The dressing was changed two times in 24-hour. The Aloe vera gel, which was used in the patients in group “A”, was 100% concentration and produced by the Seagull Company located in Karaj, Iran. Dressing in this group performed by one of the researchers (MB).
Patients in group “B” received Calendula officinalis prophylactic dressing. In this group, the skin of the sacrum area was firstly washed and cleaned with a normal saline solution. Then, 5 cc of Calendula officinalis was rubbed in the sacrum area, and a 10×10 cm simple dressing gauge was placed and kept on the skin with mattress glue. The dressing was changed two times in 24-hour. The Calendula officinalis, which was used in the patients in group “A”, was 2% concentration and produced by the Dineh Company located in Qazvin, Iran. Dressing in this group performed by one of the researchers (MB).
Patients in group “C” (control group) received simple dressing. In this group, the skin of the sacrum area was firstly washed and cleaned with a normal saline solution. Then a 10×10 cm simple dressing gauge was placed without any Calendula officinalis ointment or the Aloe vera gel and kept on the skin with mattress glue. The dressing was changed two times in 24-hour. Dressing in this group performed by one of the researchers (MB).
The sacral dressing was different in different group but all the patients received the routine nursing care related to pressure injury prevention such as change position, alternating pressure air mattress replacements, static mattress replacements, turning regimes and daily/per shift skin inspection were similar in all patients in three groups.
Ethical Considerations
Official permission and ethical approval for the study were granted by the research and technology department of the Qazvin University of Medical Sciences. Study also registered in Iranian Registry of Clinical Trials (ethics code: IRCT20170620034653N2). Study conducted in accordance with the Declaration of Helsinki. Participants or their legal guardians were approached and informed about the study methods and objectives and they were requested to complete the informed consent (of 90 patients, 7 patients have enough consciousness to read and sign informed consent form). The information kept confidential after the collection, and was used only for the study objectives.
Statistical Analysis
Data were distributed normally based on the results of Kolmogorov–Smirnov test. Frequency, percentages, mean and standard deviation were used to describe the data. Chi-square test (Fisher’s exact test) was also used to compare the groups based on the development of pressure injury. P-values less than 0.05 were considered significant in all tests.
Results
A total number of 90 patients participated in the present study. The demographic variables studied included age, sex, respiratory conditions, length of ICU stay, level of consciousness, surgical history and the hemodynamic variables such as blood pressure and temperature, which all were homogeneous and same among the patients in three groups. Table 1 shows the demographics variables in detail.
 |
Table 1 Participants’ Demographics Characteristics |
The results showed that 3 patients (10%) who received the simple dressing, 2 patients (6.6%) who received the Calendula officinalis ointment dressing developed pressure injury, while the development of pressure injury in patients who received the Aloe vera gel dressing was zero. The results of chi-square test showed that the difference in the frequency distribution of the pressure injury development in the two groups receiving Aloe vera gel and simple dressing was statistically significant (p= 0.04) (Table 2). The results of chi-square test showed that the frequency distribution of the pressure injury development in the two groups receiving Calendula officinalis ointment and simple dressing was the same and there was no significant difference between them (p=0.05) (Table 3). The results of chi-square test showed that the frequency distribution of the pressure injury development in the two groups receiving Calendula officinalis ointment and Aloe vera gel dressing was the same and there was no significant difference between them (p >0.05) (Table 4).
 |
Table 2 Pressure Injury Development in Patients in Aloe vera and Simple Dressing |
 |
Table 3 Pressure Injury Development in Patients in Calendula officinalis and Simple Dressing |
 |
Table 4 Pressure Injury Development in Patients in Aloe vera and Calendula officinalis |
Discussion
Prevention of pressure injury is one of the priorities of the healthcare team members for hospitalized patients, especially for those who are admitted to the ICUs. One of the effective strategies for pressure injury prevention in patients who are at high risk is to use prophylactic dressings. In this study, the effect of two plant products, which were Calendula officinalis ointment and Aloe vera gel, on the prevention of pressure injury was evaluated. The results showed that both of them had better efficacy in the prevention of pressure injury compared to the simple dressing; however, the difference was significant only when using the Aloe vera gel.
The use of this plant is mostly associated with wound healing, and there are very limited studies related to the preventive effects of this product, especially in the prevention of pressure injury. The searches showed only one study that examined the effect of topical Aloe vera gel on pressure injury prevention in one group of orthopedic patients. In this study that conducted in 2018 in Iran, Hekmatpou et al reported that using topical Aloe vera gel have significant effects on pressure injury prevention.29 Searches also showed two studies that examined the use of Aloe vera gel in the treatment of pressure injury. In the first study, which was a quasi-experimental study, Egyptian researchers evaluated the effect of 1% Aloe vera cream on the treatment of stage 2 pressure injury in patients hospitalized in the ICU. In this study, 60 patients were divided into two groups. Patients in the intervention group received 1% Aloe vera cream. The results of Egyptian researchers’ study showed that the use of Aloe vera cream significantly improved the stage 2 pressure injury in patients hospitalized in the ICU.30 Another study, which was a case-report, conducted in Japan, in which the effects of Aloe vera gel powder with high molecular (by washing with running water using the patented freeze-drying under micro wave and far infra-red irradiations) were studied in 6 patients. The results of this study also showed that Aloe vera gel powder had a positive effect on the treatment of pressure injury.31 As previously mentioned, this study is the first one to evaluate the preventive effects of Aloe vera gel on the development of pressure injury. The preventive effects of Aloe vera gel in this study can be attributed to some of the properties of this plant. Mucopolysaccharides, amino acids and zinc in the Aloe vera gel can help to maintain skin integrity, maintain moisture and refresh skin, and reduce skin sensitivity and erythema. Aloe vera gel also boosts the immune system and cytokines synthesis, which itself can play a role in the prevention effects of this gel.21,32 However, further studies with more details are recommended to evaluate the effects of Aloe vera gel in the prevention of pressure injury.
Calendula officinalis has been used to treat a various types of wounds. In the treatment of pressure injury, searches showed two studies that evaluated the efficacy of this product. In a study in Iran, the effect of Calendula officinalis ointment was evaluated in the treatment of pressure injury in 20 patients. The results of this study showed that the treatment of pressure injury was significantly improved by using Calendula officinalis ointment dressing.33 The other study was conducted in Brazil by Buzzi et al. In this cohort study, 41 patients diagnosed the pressure injury, were treated by using Calendula officinalis product over 30 weeks. Patients were assessed in terms of pressure injury development, infection, tissue type and amount of exudate. The results of Buzzi et al study revealed that the use of Calendula officinalis product significantly promoted the healing process of pressure injury.34 However, no study was found about the use of Calendula officinalis ointment prophylactic dressing in the prevention of pressure injury. In fact, the present study is the first one in this field. It seems that the preventive effects of Calendula officinalis in the present study are mostly related to the anti-inflammatory properties of this plant. The extract of this plant contains carotenes, flavonoids, sterols, alkaloids, terpenes, resins and calcium, which have anti-inflammatory properties.35 However, further studies are needed to evaluate the effect of Calendula officinalis in the prevention of pressure injury in more detail.
This study has some limitations. The first limitation is that the time of the patients’ evaluation was considered 7 days and it is recommended that future studies consider more time. Also, we only enrolled critically ill patient that were at moderate and high risk of pressure injury according to Braden scale and this limited the generalizability of the findings to those patients who are at low risk of pressure injury development. Other limitation is our patients’ age in inclusion criteria. We only selected patients aged 18 and older because Braden scale could only be used for this group of people. So, the result can not be generalized to pediatric and neonate in critical care units. Missing calendula test group in the sample size calculation is also another limitation of our study.
Conclusion
The results of the present study demonstrated that the use of Aloe vera gel and Calendula officinalis ointment prophylactic dressing twice a day can be effective in the prevention of pressure injury in patients admitted to ICU. Further study is needed for confirmation of this effect. The findings of this study can also provide a significant improvement in the prevention of pressure injury related to the use of prophylactic dressings, because the materials used in these dressings have been industrial and cost much. However, given the lack of studies, it is recommended that similar studies conducted to make the findings more generalizable. Also, further studies on other natural products such as honey or olive oil in the form of prophylactic dressing for pressure injury prevention could be interesting issues.
Data Sharing Statement
Raw data are available if requested up to one year after publication of manuscript. All requests should be sent to the email of correspondence author.
Acknowledgment
Researchers need to appreciate the officials of the Qazvin Nursing and Midwifery Faculty, the staff working at the ICU of the Alborz Hospital in Karaj, especially Mina Abdollahi and Bakhtiar Mirloo and, patients and their relatives who cooperated with us in this study. We also thank Dr Akhavi (Seagull) and Dineh companies for their cooperation in providing the Aloe vera gel and Calendula officinalis ointment.
Disclosure
The authors report no conflicts on interest in this work.
References
1. AhmadiNejad M, Rafiei H. Pressure ulcer incidence in intensive care unit patients in Bahonar Hospital, Kerman. J Iran Soc Anaesthesiol Intensive Care. 2011;57:10–16.
2. Byrne J, Nichols P, Sroczynski M, et al. Prophylactic sacral dressing for pressure injury prevention in high-risk patients. Am J Crit Care. 2016;25(3):228–234. doi:10.4037/ajcc2016979
3. Iranmanesh S, Rafiei H, Sabzevari S. Relationship between Braden scale score and pressure injury development in patients admitted in trauma intensive care unit. Int Wound J. 2012;9(3):248–252. doi:10.1111/j.1742-481X.2011.00852.x
4. Tafti AA, Sajadi S, Rafiei H. Pressure injury stage IV caused by cervical collar in patients with multiple trauma in intensive care unit. Int Wound J. 2015;12(5):606–607. doi:10.1111/iwj.12158
5. Azimian J, Rafiei H, Alipoor Heydari M, Senmar M. Prevalence of pressure injury among patients who were admitted to open heart surgery intensive care unit. Int J Novel Res Healthcare Nurs. 2016;3(3):28–33.
6. Akbari Sari A, Doshmanghir L, Neghahban Z, Ghiasipour M, Beheshtizavareh Z. Rate of pressure ulcers in intensive units and general wards of Iranian hospitals and methods for their detection. Iran J Public Health. 2014;43(6):787–792.
7. Shokati Ahmadabad M, Rafiei H, Alipoor Heydari M, Bokharaei M, Amiri M. Incidence of pressure injury in patients who were admitted to open heart cardiac surgery intensive care unit. Int J Epidemiologic Res. 2016;3(1):12–18.
8. He M, Tang A, Ge X, Zheng J. Pressure ulcers in the intensive care unit: an analysis of skin barrier risk factors. Adv Skin Wound Care. 2016;29(11):493–498. doi:10.1097/01.ASW.0000494779.66288.c9
9. Cullen Gill E. Reducing hospital acquired pressure ulcers in intensive care. BMJ Quality Improv Rep. 2015;4(1):
10. Walsh NS, Blanck AW, Smith L, Cross M, Andersson L, Polito C. Use of a sacral silicone border foam dressing as one component of a pressure injury prevention program in an intensive care unit setting. J Wound Ostomy Continence Nurs. 2012;39(2):146–149. doi:10.1097/WON.0b013e3182435579
11. Santamaria N, Gerdtz M, Sage S, et al. A randomised controlled trial of the effectiveness of soft silicone multi-layered foam dressings in the prevention of sacral and heel pressure ulcers in trauma and critically ill patients: the border trial. Int Wound J. 2015;12(3):302–308. doi:10.1111/iwj.12101
12. Bishopp A, Oakes A, Antoine-Pitterson P, Chakraborty B, Comer D, Mukherjee R. The preventative effect of hydrocolloid dressings on nasal bridge pressure ulceration in acute non-invasive ventilation. Ulster Med J. 2019;88(1):17–20.
13. Kohta M, Sakamoto K, Oh-i T. Polyurethane film dressings and ceramide 2-containing hydrocolloid dressing reduce the risk of pressure ulcer development in high-risk patients undergoing surgery: a matched case-control study. Chron Wound Care Manage Res. 2015;2:23–30. doi:10.2147/CWCMR.S77087
14. Reid K, Ayello EA, Alavi A. Pressure ulcer prevention and treatment: use of prophylactic dressings. Chron Wound Care Manage Res. 2016;3:117–121. doi:10.2147/CWCMR.S78422
15. Karimi Z, Behnammoghadam M, Rafiei H, et al. Impact of olive oil and honey on healing of diabetic foot: a randomized controlled trial. Clin Cosmet Investig Dermatol. 2019;12:347–354. doi:10.2147/CCID.S198577
16. Kuo YS, Chien HF, Lu W. Plectranthus amboinicus and Centella asiática Cream for the treatment of diabetic foot ulcers. Evid Based Complement Alternat Med. 2012;2012(418679):1–9.
17. Carvalho AFM, Feitosa MCP, Coelho NPMF, et al. Low-level laser therapy and Calendula officinalis in repairing diabetic foot ulcers. Rev Esc Enferm USP. 2016;50(4):626–632. doi:10.1590/S0080-623420160000500013
18. Buzzi M, Freitas F, Winter MB. Therapeutic effectiveness of a Calendula officinalis extract in venous leg ulcer healing. J Wound Care. 2016;26(12):732–739. doi:10.12968/jowc.2016.25.12.732
19. Givol O, Kornhaber R. A systematic review of Calendula officinalis extract for wound healing. Wound Repair Regener. 2019;27(5):548–561.
20. Moriyama M, Moriyama H, Uda J, et al. Beneficial effects of the genus aloe on wound healing, cell proliferation, and differentiation of epidermal keratinocytes. PLoS One. 2016;11(10):e0164799. doi:10.1371/journal.pone.0164799
21. Surjushe A, Vasani R, Saple DG. Aloe vera: a short review. Indian J Dermatol. 2008;53(4):163–166. doi:10.4103/0019-5154.44785
22. Hashemi SA, Madani SA, Abediankenari S. The review on properties of aloe vera in healing of cutaneous wounds. Biomed Res Int. 2015;2015:714216. doi:10.1155/2015/714216
23. Shedoeva A, Leavesley D, Upton Z, Fan C. Wound healing and the use of medicinal plants. Evidence Based Complementary Altern Med. 2019;2019:30. doi:10.1155/2019/2684108
24. Shafeie N, Tabatabai Naini A, Kargar Jahromi H. Comparison of different concentrations of calendula officinalis gel on cutaneous wound healing. Biomed Pharmacol J. 2015;8(2):979–992. doi:10.13005/bpj/850
25. Buzzi M, de Freitas F, Winter M, Prospective A. Descriptive study to assess the clinical benefits of using calendula officinalis hydroglycolic extract for the topical treatment of diabetic foot ulcers. Ostomy Wound Manage. 2016;62(3):8–24.
26. Eghdampour F, Jahdie F, Kheyrkhah M, Taghizadeh M, Naghizadeh S, Hagani H. The impact of aloe vera and calendula on perineal healing after episiotomy in primiparous women: a randomized clinical trial. J Caring Sci. 2013;2(4):279–286. doi:10.5681/jcs.2013.033
27. National Pressure Ulcer Advisory Panel. European pressure ulcer advisory panel and pan pacific pressure injury alliance. In: Haesler E, editor. Prevention and Treatment of Pressure Ulcers: Quick Reference Guide. Perth, Australia: Cambridge Media; 2014.
28. Grap MJ, Munro CL, Wetzel PA, et al. Tissue interface pressure and skin integrity in critically ill, mechanically ventilated patients. Intensive Crit Care Nurs. 2017;38:1–9. doi:10.1016/j.iccn.2016.07.004
29. Hekmatpou D, Mehrabi F, Rahzani K, Aminiyan A. The effect of aloe vera clinical trials on prevention and healing of skin wound: a systematic review. Iran J Med Sci. 2019;44(1):1–9.
30. Zanaty MM, Sultan MA, Shebl AM, Soliman OA, Abdelrahman HA. The effect of aloe vera on the healing of second degree pressure cancers among critically ill patients. IOSR J Nurs Health Sci. 2017;6(6):23–34.
31. Matsuo K, Yagi A, Kabbash A. Case reports of bedsores using aloe vera gel powder with high molecular weight. Phcog Res. 2009;1:136–142.
32. Hekmatpou D, Mehrabi F, Rahzani K, Aminiyan A. The effect of aloe vera gel on prevention of pressure ulcers in patients hospitalized in the orthopedic wards: a randomized triple-blind clinical trial. BMC Complement Altern Med. 2018;18(1):264. doi:10.1186/s12906-018-2326-2
33. Esmaili R, Ebrahim Zadeh M, Khalilian A, et al. Study regarding the effect of calendula officinalis cream in healing of pressure sores. J Mazandaran Univ Med Sci. 2008;18(66):19–25.
34. Buzzi M, Freitas F, Winter Mde B. Pressure injury healing with Plenusdermax(R) Calendula officinalis L. Extract Revista Brasileira De Enfermagem. 2016;69(2):250–257. doi:10.1590/0034-7167.2016690207i
35. Oguwike N, Onubueze DPM, Ughachukwu P. Evaluation of activities of marigold extract on wound healing of Albino Wister rat. IOSR J Nurs Health Sci. 2013;8(5):67–70.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
