Back to Journals » Local and Regional Anesthesia » Volume 16
Effect of 2% Topical Lidocaine Gel on Discomfort from Electrical Stimulation During Nerve Conduction Studies- A Prospective Double-Blind Placebo-Controlled Study
Authors Al-Hayk K, Smadi MM , Elsalem LM , Yassin A , Aqaileh S, Obiedat DH, Al-Hayk AK , Al Qawasmeh M , Kofahi R , El-Salem K
Received 19 June 2023
Accepted for publication 12 September 2023
Published 26 September 2023 Volume 2023:16 Pages 153—163
DOI https://doi.org/10.2147/LRA.S426076
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Stefan Wirz
Kefah Al-Hayk,1 Mahmoud M Smadi,2 Lina M Elsalem,3 Ahmed Yassin,1 Suha Aqaileh,1 Deema H Obiedat,1 Ahmad Kefah Al-Hayk,4 Majdi Al Qawasmeh1 ,† Raid Kofahi,1 Khalid El-Salem1
1Neurology Department, Faculty of Medicine, Jordan University of Science and Technology, Irbid, Jordan; 2Department of Mathematics and Statistics, Faculty of Science and Arts, Jordan University of Science and Technology, Irbid, Jordan; 3Department of Pharmacology, Faculty of Medicine, Jordan University of Science and Technology, Irbid, Jordan; 4Faculty of Medicine, Jordan University of Science and Technology, Irbid, Jordan
†Majdi Al Qawasmeh passed away on April 24, 2023
Correspondence: Kefah Al-Hayk, Neurology Department- Jordan University of Science and Technology, P.O. Box 3030, Irbid, 22110, Jordan, Tel +962-790606881, Fax +962 2-7095123, Email [email protected]
Purpose: Procedure discomfort can limit electrodiagnostic studies. Reducing discomfort can maximize the benefits of these diagnostic tools. This study targeted the discomfort associated with nerve conduction studies (NCS).
Patients and Methods: This was a prospective randomized double-blind placebo-controlled study comparing the effect of topical lidocaine gel (2%) versus analgesic-free lubricant gel (K-Y gel) on pain perception during NCS. Sequential patients (n=130) referred for routine NCS participated in the study. We applied 1 mL of lidocaine gel to one palm, and 1 mL of K-Y gel to the other as a control. After 20– 45 min of application, graded increments of electrical stimulation intensity were delivered to record the median and ulnar mixed palmar nerve responses. Patients were then asked to score the degree of pain felt from electrical stimulation over each palm using the Wong-Baker Faces Pain Scale (WBFPS) and the Numeric Rating Scale (NRS), independent of baseline pain.
Results: Mean WBFPS and NRS scores for lidocaine-treated palms were significantly lower than those for controls using parametric paired t-test (3.79 vs 4.37 and 3.35 vs 3.78 respectively, all p-values< 0.05). Subgroup analysis showed a significant decrease in mean scores in females, patients aged ≤ 50 years, patients without a history of previous NCS, and patients without comorbidities (all p-values< 0.05). Median scores using nonparametric Wilcoxon ranked test also showed statistically significant differences (all p-values< 0.05).
Conclusion: The results indicate that topical lidocaine 2% gel reduces discomfort associated with NCS. However, despite the statistical significance, clear clinical significance may be lacking. Clinical implementation may be considered for the subgroups that showed the greatest benefit. Further studies that incorporate more efficient drug delivery methods may yield better results.
Keywords: topical lidocaine, nerve conduction study, discomfort, electrodiagnostic
Introduction
Nerve conduction studies (NCS) and electromyography (EMG) are essential components of the electrodiagnostic examination for assessing many neuromuscular disorders.1 Pain has frequently been considered a major pitfall in EMG.2,3 NCS, on the other hand, are considered safe and well tolerated with no long-term adverse effects; however, they are frequently associated with discomfort (from electrical stimulation)4 that, from our experience, may occasionally lead to the premature termination of the procedure. Discomfort and pain can be significant contributors to suboptimal patient cooperation.2,5 This may also ultimately affect patients’ willingness to undergo the procedure a second time in the future and the likelihood that they will recommend the procedure to others. Many factors may contribute to the discomfort encountered during a procedure, including procedural anxiety level,2,6 sex,6 and the interaction between patients and the person performing the study.7 In general, pain expectation during electrodiagnostic testing is thought to be more exaggerated for EMG than for NCS procedures.8
Reviewing the literature revealed many studies investigating the reduction of pain and discomfort during EMG using different techniques and agents, such as oral and topical formulations.9–11 However, studies aiming at reducing the discomfort associated with NCS are limited. Topical lidocaine has been helpful in the management of neuropathic pain.12–14 Its limited systemic absorption is a particularly attractive option in elderly patients and in those taking multiple medications, as the risk/benefit ratio becomes more favorable in such patients. In this study, we aimed to evaluate whether topical lidocaine 2% gel can reduce the discomfort associated with NCS.
Methods
This was a prospective randomized double-blind placebo-controlled study. It was approved by the Institutional Review Board (IRB number 2/128/2019) and by the Human Research Ethics Committee and conformed to the Declaration of Helsinki. The study subjects were sequential patients aged ≥ 16 years who were referred to the clinical neurophysiology lab at our hospital for NCS. Excluded patients were those with significant comorbidities such as severe cardiac or pulmonary diseases; patients who were acutely ill; patients with a previous history of drug allergies; patients taking medications that affect pain perception; patients referred for a condition that does not require NCS of the hands; and patients with significant liver or renal diseases, as these may affect the metabolism of the study drug and potentially increase systemic adverse effects. The study aims and steps were explained to all patients, and those who agreed to participate were asked to sign an informed consent form. The main comorbidities that were present in the included patients were systemic arterial hypertension, diabetes mellitus (DM), mild ischemic heart disease, hypothyroidism, and degenerative disc disease. Most of the included patients were referred to rule out carpal tunnel syndrome (CTS). Other referral diagnoses were peripheral neuropathy, cervical radiculopathy, and brachial plexopathy. For all participants, we applied 1 mL of lidocaine 2% gel to one palm using a syringe and applied 1 mL of K-Y gel to the other palm as a control agent. The gel was applied to either the right or the left palms in a random fashion and was rubbed lightly to cover the entire palm. To help minimize early drying, plastic gloves were worn by the study subjects during the application period which ranged from 20–45 min, after which the gloves were removed, and the gel was wiped off with a dry gauze. Immediately after that, a second investigator performed the NCS and delivered electrical stimulation orthodromically to one palm with graded intensity increments of 3–5 mA at a constant pulse width of 0.1 msec until supramaximal stimulations of the median and ulnar mixed palmar nerves were reached. Recording electrodes were placed at the wrist 8 cm proximal to stimulation points. The same stimulation protocol was replicated on the contralateral palm until the same maximum stimulation intensity that was delivered to the first palm was reached. Both the patient and the second investigator performing the electrical stimulation were blinded to the type of gel that was applied to each palm. The patients were asked to score the discomfort/pain they felt from stimulating each palm using the Wong-Baker Faces Pain scale (WBFPS) and the Numeric Rating Scale (NRS) immediately after stimulating both sides and before completing the NCS for which they were referred. Standard safety measures were followed, according to our hospital and neurophysiology laboratory policies.
The sample size of the participants in the study was selected based on conducting a power analysis. First, a pilot study on 70 participants was conducted, and the difference in pain was computed for each patient. The results revealed a standard deviation in the differences scores of approximately 1.648 units. A detectable difference of 0.5 units with a 95% target test power of one-sided test of hypothesis at α =0.05 was selected. Power analysis provided a required sample size of 119 participants with an actual test power of 0.950094. Because of the factors affecting the errors in sample size determination such as sampling error in estimation, we have slightly increased the sample size of n=119 to n=130. Power curve is shown in Figure 1. The flow diagram of the statistical plan and design is shown in Figure 2.
 |
Figure 1 Sample size calculation and power curve. |
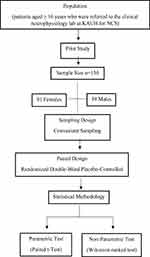 |
Figure 2 Flow diagram of design and statistical plan. |
Statistical Methodology
We used the parametric paired t-test and the nonparametric Wilcoxon ranked test for the comparison of the mean and median scores of WBFPS and NRS between palms treated with lidocaine gel (treatment) and palms treated with K-Y gel (control). All P-values presented were one-sided hypothesis to test if there was a significant decrease in the mean and median pain scores in the treatment group compared to the control group. Statistical significance was set at p < 0.05. Statistical Package for Social Sciences version 21 software (SPSS Inc., Chicago, IL, USA) was used to perform the statistical analyses. Jupyter Notebook version 6.0.3 was used to produce the bar chart plots.
Results
Study Subjects and Pain Scores
A total of 130 patients were enrolled in this study. Of those, 91 were females (70%) and 39 were males (30%). The patients’ age range was 19–72 years, with a mean ± SD of 44.65 ± 12.07 years. Summary of the demographic information of the subjects in the study is shown in Table 1. In the statistical analysis, both the parametric paired t-test and nonparametric Wilcoxon ranked test were used (Tables 2–4). The means and medians of the WBFPS and the NRS scores for lidocaine-treated palms were significantly lower than those for controls at different characteristics considered in this study (all p-values < 0.05).
 |
Table 1 Summary of the Demographic Information of the Subjects in the Study |
 |
Table 2 Summary Statistics and Results of Paired t-test and Wilcoxon Ranked Test of Treatment and Control Groups for the Study Subjects Using Wong-Baker Faces Pain Scale and Numeric Rating Scale |
Pain Scores and Other Variables
The paired t-test and the Wilcoxon ranked test were also used to calculate the differences in WBFPS and NRS scores between lidocaine and K-Y in relation to other variables such as sex, age, history of previous NCS, and presence of comorbidities to identify subgroups with the highest benefit/response to lidocaine gel. For both WBFPS (Table 3) and NRS (Table 4), the results showed a significant decrease in the mean and median scores for lidocaine compared to K-Y for the categories of females, patient age ≤ 50 years, negative history of previous NCS, and lack of comorbidities. A visual examination of the data using error bar plot using numeric rating scale of control and treatment is shown in Figure 3 and error bar plot using Wong-Baker faces pain scale of control and treatment is shown in Figure 4.
 |
Figure 3 Error bar plot using numeric rating scale of control and treatment in relation to (a) gender (b) history of NCS (c) age group (d) health status. |
 |
Figure 4 Error bar plot using Wong-Baker faces pain scale of control and treatment in relation to (a) gender (b) history of NCS (c) age group (d) health status. |
Neurophysiological Outcomes
The majority of participants had either unremarkable or non-lateralizing findings after completing their neurophysiological studies (Table 5). A total of 43 participants had neuropathic findings that were either unilateral or worse in one limb compared to the other. The more neuropathic side received lidocaine in 20 participants and K-Y in 23 participants. The electrical stimulation intensity applied to both sides was equal as per study design with a mean ± SD of 38.3 mA ± 16.05.
 |
Table 5 Final Neurophysiological Diagnosis of Participants at Study Completion |
Discussion
In this study, we used lidocaine 2% gel to assess whether it has beneficial effects on reducing discomfort encountered from electrical stimulation in NCS. To the best of our knowledge, this is the first study to evaluate the effects of topical lidocaine on NCS discomfort. Topical lidocaine is a local anesthetic agent that is widely used in multiple medical procedures and in pain management for a variety of conditions.15,16 It acts by binding to voltage-gated sodium channels on the inner surface of the neuronal cell membrane, leading to a decrease in the influx of sodium ions, which inhibits depolarization and ultimately leads to the blockage of conduction.15 Lidocaine gel has a rapid onset of action that is within 3–5 min when applied to mucus membranes, but has poor absorption through intact skin.15,16 Different topical formulations have variable skin absorption rates, and therefore, variable onsets of action ranging from about 25 min to more than 90 min. Additionally, the effect may vary with the site of application and the level of analgesia that is pursued.17 The adverse effects of lidocaine gel are essentially limited to mild local skin reactions if applied to intact skin, which seems to be related to the duration of the application and the concentration being used.16,18,19 In this study, we applied lidocaine gel as a treatment agent and K-Y gel as a control agent to the same study subject but on opposing palms and used the WBFPS and NRS to compare differences in perceived pain/discomfort between both sides. This approach helped eliminate most of the confounding factors that could have affected and limited the interpretation of the results, thereby increasing the power of the study.
The WBFPS and NRS are frequently used to evaluate the degree of pain. Usually, both scales are treated as ordinal scales in which nonparametric statistical methods are used in the statistical analysis. However, in pain research, there has been debate regarding whether the WBFPS should be treated as an ordinal scale or as an interval scale. In certain occupations, the WBFPS was assumed to be an interval scale, and the NRS was considered a ratio scale; thus, parametric statistics can be used in the statistical analysis.20–22 Some investigators argued that parametric statistics can be applied even if the data are composed of ordinal scales under the assumption that the data are normally distributed or when the sample size is large as per the central limit theorem.23,24 Others reported that the parametric t-test or the ANOVA test can be applied to the WBFPS without inflation of the type-1 error.25 In statistical analysis, statisticians and researchers prefer using parametric tests because, in general, they are more powerful than nonparametric tests. The statistical analysis in this study involved the use of both parametric and nonparametric tests. The results showed that both parametric and nonparametric tests revealed similar results in terms of statistical significance.
Both the WBFPS and NRS scores were lower in lidocaine-treated palms than in K-Y–treated palms (p < 0.05). This indicates that lidocaine has beneficial effects on reducing discomfort during NCS. Upon subgroup comparisons, we found that both scores were significantly lower for lidocaine-treated palms than for control palms in females, patients ≤ 50 years of age, patients without comorbidities, and patients undergoing NCS for the first time.
It has been reported that female skin is thinner than male skin and androgens account for why a man’s skin is about 20% thicker than that of a woman.26 Additionally, the dermis layer, which contains the free nerve endings responsible for pain sensation, is thinner in the palms of females compared to that in males.27 Therefore, these variations may have contributed to the clearer effect in females.
In this study, we relied on the patient’s ability to try to differentiate between the pain/discomfort felt over one palm from that over the other and from disease-related pre-procedure pain. This was particularly more difficult to clarify to elderly patients despite great attempts to explain the procedure in detail. Older age groups have a wide range of life experiences and cultural backgrounds that might affect their perception of diseases, willingness to follow medical protocols, and ability to communicate effectively.28,29 Additionally, the normal aging process may involve sensory loss, reduced memory, and slower processing of information.30 These factors may explain why the response was clearer in patients aged ≤ 50 years.
The scores were also lower in lidocaine-treated palms than in control palms in patients without comorbidities. Previous studies have shown that certain chronic diseases might affect pain perception and influence a patient’s response.31–33 It has also been reported that lidocaine efficacy decreases in the presence of inflammation or in conditions with increased production of inflammatory mediators.34 These findings may explain why the benefit was more evident among patients without comorbidities.
In patients undergoing NCS for the first time, it is difficult to ascertain the cause of the beneficial effect of lidocaine. A lower percentage of this group had a premorbid condition than those who had a previous NCS (31% vs 38%). Additionally, it is reasonable to assume that patients who had a previous NCS suffered from a more chronic disease course or had other comorbidities, as evidenced by the need to repeat their NCS. These factors would favor a beneficial response to lidocaine, as explained above. However, more data pertaining to pre-procedure pain, anxiety level, and patients’ experiences during previous NCS should be looked into for a better assessment.
While EMG studies are generally considered uncomfortable/painful,2,3 it was found that patients had a wide range of tolerance for the needle exam during EMG studies as the reported pain ranged from 0 (none) to 9 (10 being most severe) in one study.7 Similarly, the reported pain in our study using the NRS ranged from 0–10 for the control side and 0–9 for the treatment side; the range was 0–10 for both sides using the WBFPS. This indicates that for some patients, NCS can be as uncomfortable as EMG studies, which may be related to patients’ expectations of pain prior to the procedure,8 the intensity of maximal stimulation reached, and the total examination time.4 Interestingly, the mean NRS scores were slightly lower in females than in males (3.27 vs 3.51) (Table 4). This is different from what has been reported in previous EMG-related studies, where the female sex was associated with higher reported pain scores.6,35
Other factors that impact pain/discomfort perception are the number and intensities of electrical stimuli delivered to each palm. By applying the same incremental stimulation protocol to both palms, we ensured that each side received a fairly comparable number of stimulations. Moreover, the maximum stimulation intensity delivered was identical for both sides for the purpose of pain scoring. We reached supramaximal stimulation on the first tested palm and reached the same maximum intensity value on the contralateral side, regardless of being supra- or submaximal. This ensured that the scoring of pain was based on a comparable stimulation intensity experience irrespective of that from the post-scoring completion of the NCS procedure for which the patients were referred.
Limitations
Although our findings regarding the effect of topical lidocaine in reducing discomfort during NCS were statistically significant, clear clinical significance may be lacking. The minimum clinically significant difference in NRS scores was previously estimated to be in the range of 1.39–1.5.36–38 This lack of clinical significance may be attributed to numerous factors. The application of lidocaine to the palm, which has thick skin, is more likely to make absorption less efficient than if lidocaine is applied to thinner skin such as the forearm or leg skin. In addition, the thenar skin has a higher perception sensitivity to pain than the forearm skin.10 This higher sensitivity may have affected the patients’ ability to discriminate minor differences in pain levels between both palms. Additionally, the study subjects’ baseline pain and anxiety levels were not recorded prior to the procedure, which may have influenced the subjects’ responses and potentially contributed to a reduction in the expected analgesic effect of lidocaine. Other investigators showed that 8% lidocaine yielded a more robust effect on pain threshold over face and hand skin than the yield of 2% lidocaine.39 Therefore, the use of higher concentrations of topical lidocaine may result in greater pain relief. We could have attempted to increase the waiting time after the application of lidocaine beyond 45 min, but this would make it less applicable in everyday practice. Another concern is that topical lidocaine gel, with its conduction-blocking ability, may interfere with the nerve conduction parameters that we measure. However, the limited skin absorption coupled with the relatively deep location of the nerves undergoing stimulation and the ultimately diluted lidocaine concentrations reaching these nerves make this interference a remote possibility. It is reasonable to expect that the skin treated with topical lidocaine gel would become significantly anesthetized before a relevant impact on the deeper nerve trunks is detected. Significant skin anesthesia was not reported in our cohort of patients and is not typically observed in clinical practice.
Conclusion
The findings of this study demonstrated a significant decrease in pain scale scores for topical lidocaine-treated palms versus control palms, indicating that lidocaine 2% gel reduces discomfort associated with NCS. This reduction was most evident in the female sex, in the age group of 50 years or younger, in patients without comorbidities, and in patients undergoing the NCS for the first time. However, despite the statistical significance, the results may not be sufficient to be translated into clinical significance. Consideration of clinical implementation may be warranted for apprehensive patients from any of the abovementioned subgroups that showed the greatest benefit. Further studies using higher concentrations of lidocaine, different formulations with higher absorbability, different durations of analgesic application, and the application of lidocaine to non-palmar skin sites may yield better results.
Ethic Statement
The study was approved by the Institutional Review Board (IRB) of Jordan University of Science and Technology (IRB number 2/128/2019) and by the Human Research Ethics Committee of King Abdullah University Hospital and conformed to the Declaration of Helsinki.
Disclosure
The authors report no conflicts of interest in this work.
References
1. O’Bryan R, Kincaid J. Nerve conduction studies: basic concepts and patterns of abnormalities. Neurol Clin. 2021;39(4):897–917. doi:10.1016/J.NCL.2021.06.002
2. Jan MMS, Schwartz M, Benstead TJ. EMG related anxiety and pain: a prospective study. Can J Neurol Sci. 1999;26(4):294–297. doi:10.1017/S031716710000041X
3. Kothari MJ, Preston DC, Plotkin GM, Venkatesh S, Shefner JM, Logigian EL. Electromyography: do the diagnostic ends justify the means? Arch Phys Med Rehabil. 1995;76(10):947–949. doi:10.1016/S0003-9993(95)80072-7
4. Sasaki T, Nimura A, Kuroiwa T, Koyama T, Okawa A, Fujita K. Assessment of pain during nerve conduction studies in patients with carpal tunnel syndrome. J Hand Surg Glob Online. 2022;4(2):89–92. doi:10.1016/j.jhsg.2021.12.004
5. Delimar V, Miloš O, Hanevi M, et al. Factors contributing to the reduction of pain during electromyography and nerve conduction studies. Psychiatr Danub. 2019;31(2):52–58.
6. Khoshbin S, Hallett M, Lunbeck R. Predictors of patients’ experience of pain in EMG. Muscle Nerve. 1987;10(7):629–632. doi:10.1002/MUS.880100708
7. Strommen JA, Daube JR. Determinants of pain in needle electromyography. Clin Neurophysiol. 2001;112(8):1414–1418. doi:10.1016/S1388-2457(01)00552-1
8. Yalinay Dikmen P, Ilgaz Aydinlar E, Karlikaya G. [Expected and experienced pain levels in electromyography]. Elektromiyografide beklenen ve yaşanan aǧri{dotless} düzeyleri. Noropsikiyatri Ars. 2013;50(4):364–367. Turkish. doi:10.4274/npa.y6699
9. El-Salem K, Shakhatreh M. Prospective double-blind crossover trial of ibuprofen in reducing EMG pain. Muscle Nerve. 2008;38(2):1016–1020. doi:10.1002/mus.21017
10. Lamarche Y, Lebel M, Martin R. EMLA partially relieves the pain of EMG needling. Can J Anaesth. 1992;39(8):805–808. doi:10.1007/BF03008292
11. Digala LP, Govindarajan R. Topical lidocaine hydrochloride 4% spray on pain perception during the needle electromyography: a prospective study. Clin Neurophysiol Pract. 2021;6:170–171. doi:10.1016/j.cnp.2021.04.001
12. Likar R, Demschar S, Kager I, Neuwersch S, Pipam W, Sittl R. Treatment of localized neuropathic pain of different etiologies with the 5% lidocaine medicated plaster – a case series. Int J Gen Med. 2014;8:9–14. doi:10.2147/IJGM.S74802
13. Delorme C, Navez ML, Legout V, Deleens R, Moyse D. Treatment of neuropathic pain with 5% lidocaine-medicated plaster: five years of clinical experience. Pain Res Manag. 2011;Vol 16:259–263. doi:10.1155/2011/359591
14. Derry S, Wiffen PJ, Kalso EA, et al. Topical analgesics for acute and chronic pain in adults - an overview of Cochrane reviews. Cochrane Database Syst Rev. 2017;2017(5). doi:10.1002/14651858.CD008609.pub2
15. Voute M, Morel V, Pickering G. Topical lidocaine for chronic pain treatment. Drug Des Devel Ther. 2021;15:4091–4103. doi:10.2147/DDDT.S328228
16. Gudin J, Nalamachu S. Utility of lidocaine as a topical analgesic and improvements in patch delivery systems. Postgrad Med. 2020;132(1):28–36. doi:10.1080/00325481.2019.1702296
17. Friedman PM, Mafong EA, Friedman ES, Geronemus RG, Ronald §, Perelman O. Topical anesthetics update: EMLA and beyond. Dermatol Surg. 2001;27(12):1019–1026. doi:10.1046/j.1524-4725.2001.01855.x
18. Campbell BJ, Rowbotham M, Davies PS, Jacob P, Benowitz NL. Systemic absorption of topical lidocaine in normal volunteers, patients with post-herpetic neuralgia, and patients with acute herpes zoster. J Pharm Sci. 2002;91(5):1343–1350. doi:10.1002/JPS.10133
19. Sobanko JF, Miller CJ, Alster TS. Topical anesthetics for dermatologic procedures: a review. Dermatol Surg. 2012;38(5):709–721. doi:10.1111/J.1524-4725.2011.02271.X
20. Huskisson EC. Measurement of pain. J Rheumatol. 1982;9(5):768–769.
21. Price DD, McGrath PA, Rafii A, Buckingham B. The validation of visual analogue scales as ratio scale measures for chronic and experimental pain. Pain. 1983;17(1):45–56. doi:10.1016/0304-3959(83)90126-4
22. Ghasemi A, Zahediasl S. Normality tests for statistical analysis: a guide for non-statisticians. Int J Endocrinol Metab. 2012;10(2):486–489. doi:10.5812/IJEM.3505
23. Yim KH, Nahm FS, Han KA, Park SY. Analysis of statistical methods and errors in the articles published in the Korean journal of pain. Korean J Pain. 2010;23(1):35–41. doi:10.3344/KJP.2010.23.1.35
24. Norman G. Likert scales, levels of measurement and the “laws” of statistics. Adv Health Sci Educ Theory Pract. 2010;15(5):625–632. doi:10.1007/S10459-010-9222-Y
25. Dexter F, Chestnut DH. Analysis of statistical tests to compare visual analog scale measurements among groups. Anesthesiology. 1995;82(4):896–902. doi:10.1097/00000542-199504000-00012
26. Kopera D. Impact of testosterone on hair and skin. Diabetol Metab Syndr. 2015;4(3):1–4. doi:10.4172/2161-1017.1000187
27. IMSEAR. Gender differences in the microanatomy of skin of palm in humans. Available from. https://pesquisa.bvsalud.org/portal/resource/pt/sea-188821.
28. Robinson Ii TE, White GL, Houchins JC. Improving communication with older patients. Family Pract Manag. 2006;13(8):73.
29. Park DC, Morrell RW. The Challenge of Communicating Health Information to Elderly Patients: a View From Geriatric Medicine. In: Processing of Medical Information in Aging Patients. Psychology Press; 1999:35–40. doi:10.4324/9781410601070-7
30. Harada CN, Natelson Love MC, Triebel KL. Normal Cognitive Aging. Clin Geriatr Med. 2013;29(4):737. doi:10.1016/J.CGER.2013.07.002
31. Akintoye OO, Owoyele B, Fabunmi OA, et al. Diabetic neuropathy is associated with increased pain perception, low serum beta-endorphin and increase insulin resistance among Nigerian cohorts in Ekiti State. Heliyon. 2020;6(7):e04377. doi:10.1016/J.HELIYON.2020.E04377
32. Cristina de Oliveira N, Alfieri FM, Lima ARS, Portes LA. Lifestyle and pain in women with knee osteoarthritis. Am J Lifestyle Med. 2019;13(6):606. doi:10.1177/1559827617722112
33. de Goeij M, van Eijk LT, Vanelderen P, et al. Systemic inflammation decreases pain threshold in humans in vivo. PLoS One. 2013;8(12):e84159. doi:10.1371/JOURNAL.PONE.0084159
34. Beecham GB, Nessel TA, Goyal A. Lidocaine. Encyclop Toxicol. 2021;71–73. doi:10.1016/B978-0-12-386454-3.00742-9
35. Walker WC, Keyser-Marcus LA, Johns JS, Seel RT. Relation of electromyography-induced pain to type of recording electrodes. Muscle Nerve. 2001;24(3):417–420. doi:10.1002/1097-4598(200103)24:3<417::AID-MUS1015>3.0.CO;2-R
36. Kendrick DB, Strout TD. The minimum clinically significant difference in Patient-Assigned 11-Point numeric pain scale scores for pain. Ann Emerg Med. 2004;44(4):S86–S87. doi:10.1016/J.ANNEMERGMED.2004.07.283
37. Kendrick DB, Strout TD. The minimum clinically significant difference in patient-assigned numeric scores for pain. Am J Emerg Med. 2005;23(7):828–832. doi:10.1016/J.AJEM.2005.07.009
38. Bijur PE, Chang AK, Esses D, Gallagher EJ. Identifying the minimum clinically significant difference in acute pain in the elderly. Ann Emerg Med. 2010;56(5):517–521.e1. doi:10.1016/J.ANNEMERGMED.2010.02.007
39. Okayasu I, Komiyama O, Ayuse T, de Laat A. Effect of 8% lidocaine spray on the sensory and pain thresholds of the skin of the face and hands evaluated by quantitative sensory testing. J Dent Anesth Pain. 2018;18(6):361–365. doi:10.17245/jdapm.2018.18.6.361
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


