Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 16
EDIL3 and VEGF Synergistically Affect Angiogenesis in Endothelial Cells
Authors Niu X, Li X, Feng Z, Han Q, Li J, Liu Y, Zhang K
Received 6 March 2023
Accepted for publication 12 May 2023
Published 18 May 2023 Volume 2023:16 Pages 1269—1277
DOI https://doi.org/10.2147/CCID.S411253
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 6
Editor who approved publication: Dr Anne-Claire Fougerousse
Xuping Niu,1 Xinhua Li,1 Zhipeng Feng,2 Qixin Han,1 Juan Li,1 Yanmin Liu,1 Kaiming Zhang1
1Shanxi Key Laboratory of Stem Cells for Immunological Dermatosis, Institute of Dermatology, Taiyuan Central Hospital of Shanxi Medical University, Taiyuan, Shanxi, People’s Republic of China; 2Department of Gastroenterology, Taiyuan Central Hospital of Shanxi Medical University, Taiyuan, Shanxi, People’s Republic of China
Correspondence: Kaiming Zhang, Email [email protected]
Background: Angiogenesis is one of the histologically predominant characteristics of psoriasis. Vascular endothelial growth factor (VEGF) and epidermal growth factor-like repeats and discoidin I-like domains 3 (EDIL3) have critical effects on angiogenesis. Both these proteins are vital proangiogenic factors in tumor occurrence and progression; however, the relationship between EDIL3 and VEGF with psoriasis remains unclear.
Objective: We aimed to elucidate the role of EDIL3 and VEGF and the involved mechanisms in psoriasis-associated angiogenesis.
Methods: EDIL3 and VEGF expression in cutaneous tissue was determined by immunohistochemical assay. The effects of EDIL3 on VEGF, VEGFR2, and the growth, migration, and tube formation of human umbilical vein endothelial cells (HUVECs) were analyzed by Western blotting assay, cell counting kit-8 assay, Transwell assay, and Matrigel tube formation assay.
Results: EDIL3 and VEGF levels in psoriatic lesions significantly increased as compared to those in normal individuals and showed a positive relationship with the Psoriasis Area and Severity Index. The downregulation of EDIL3 decreased VEGF and VEGFR2 expression in HUVECs. Moreover, the decreased expression of EDIL3 and VEGF reduced the growth, invasion, and tube formation abilities of HUVECs, while EDIL3 resistance to VEGF and VEGFR2 was restored by using the EDIL3 recombinant protein.
Conclusion: These results suggest that psoriasis is also characterized by EDIL3 and VEGF-mediated angiogenesis. Thus, EDIL3 and VEGF could serve as novel targets for treating psoriasis.
Keywords: psoriasis, angiogenesis, EDIL3, VEGF, endothelial cells
Introduction
Psoriasis vulgaris is a common immune-mediated inflammatory skin disorder that affects both skin and nails, and it shows a strong genetic susceptibility background.1 The disease remarkably affects patients’ quality of life and causes an immense economic burden on their families. Extensive research conducted on psoriasis demonstrates that Th1/Th17-weighted immunity has a critical effect on the pathogenic mechanism with increased dermal vascularity and immune cell infiltration. Microvascular modifications in psoriasis are characterized by angiogenesis and vascular expansion in both the superficial dermis and dermal papillae, which can help to diagnose psoriasis.2 Proinflammatory cytokines could promote the division and invasion of vascular endothelial cells (VECs) and induce angiogenesis. Moreover, endothelial cells (ECs) from the endothelium overexpress the high-affinity receptor for VEGF.3 This manifestation in psoriasis occurs through the involvement of TGF-α (transformation growth factor-α) and EGF (epidermal growth factor) secreted by keratinocytes and macrophages.4,5 To date, abundant proangiogenic factors have been investigated, including VEGF, basic fibroblast growth factor (bFGF), matrix metalloproteinases (MMPs), angiogenin (Ang), interleukin-17 (IL-17), and tumor necrosis factor-α (TNF-α).6–8 These growth factors and cytokines play crucial roles not only in angiogenesis but also in inflammation, and they form a feedback loop, which aggravates the interaction and complexity between inflammation and angiogenesis. The production of these abundant proangiogenic factors by VECs leads to the initiation of angiogenesis at the early development stage of psoriasis. The evaluation of cutaneous vascularity is regarded as a vital parameter for disease prognosis and recovery for patients with psoriasis.9 In recent years, psoriasis has been treated by inhibiting keratinocyte proliferation and targeting two major pathogenic pathways, namely inflammation, and angiogenesis.
The occurrence of wounds, tumors, and inflammatory diseases is accompanied by angiogenesis, which refers to the formation of new blood vessels based on the existing ones.10 Angiogenesis mainly involves three steps: proliferation, invasion, and new vessel generation by VECs.11 The angiogenetic process is tightly regulated by abundant proangiogenic factors and diverse antiangiogenic inhibitors. Effector cells of cutaneous inflammation, such as macrophages, lymphocytes, and mast cells, are the most important sources of the vast array of angiogenic factors. The precise pathogenetic mechanism of angiogenesis in psoriasis has been fully elucidated. According to previous studies, VEGF is highly elevated in lesions and peripheral blood of patients with psoriasis, and the plasma VEGF level is markedly associated with the severity of the clinical presentation of psoriasis.12,13 VEGF is the most important factor in the occurrence of many angiogenic-related diseases. Previous studies have confirmed a close correlation between many other proangiogenic mediators and VEGF levels in the skin lesions of patients with psoriasis.14 These factors could induce EC growth, accompanied by the generation of additional proangiogenic factors, including Ang-2, bFGF, and MMP-2. Several studies have also shown that Ang-2 expression is remarkably elevated in psoriatic lesions relative to that in healthy individuals and that serum bFGF level is distinctly higher in patients with psoriasis.15 The content of VEGF and bFGF in blood samples consistently decline following therapy with angiogenesis inhibitors that target vasoproliferation. Ang-2, another essential proangiogenic factor, together with VEGF, could destabilize the capillaries at the primary stage of vessel formation by binding to its receptor Tie-2.16 Collectively, these findings indicate the pathogenetic role of the potential relationship between VEGF and other angiogenic factors in a VEGF-dependent manner in psoriasis. In recent years, epidermal growth factor-like repeats and discoidin I-like domains 3 (EDIL3), a novel angiogenic factor, have been increasingly investigated in the progression and prognosis of many tumors, and it also related to inflammation and angiogenesis.17,18 Some studies have shown that EDIL-3 and VEGF exert a capillary destabilization effect to initiate angiogenesis by binding to the corresponding receptor integrin.
As an extracellular matrix protein, EDIL3 contains the Arg-Gly-Asp (RGD) motif that binds to the specific receptor integrin.19 This protein interacts with integrins and phospholipids and then regulates the host inflammatory disorders (from immune cell recruitment to the alleviation of inflammation).20 Recent findings indicate that EDIL3 participates in various biological processes, including angiogenesis, cellular proliferation and migration, and regulation of immunity, and thus, it could be a valuable disease biomarker in inflammatory diseases and tumors.21,22 In recent years, our studies, for the first time, showed increased EDIL3 mRNA and protein expression levels in dermis mesenchymal stem cells (DMSCs) in psoriatic patients;23 however, additional investigations are required to obtain further insights. The present study aimed to investigate the effect of EDIL3 on other angiogenic mediators and the relevant mechanism in psoriasis-associated angiogenesis.
Materials and Methods
Subjects
The study sample comprised 30 patients with psoriasis and 30 breast cancer patients after surgical resection without skin disease as controls. Samples were obtained from outpatients with psoriasis in the Taiyuan Central Hospital of Shanxi Medical University. We collected normal human umbilical cords from 8 patients with cesarean sections. All subjects provided informed consent before sampling. The study received ethical approval from the Ethics Committee of Taiyuan Central Hospital (Approval No. 2018010). We performed punch skin biopsies (4–6 mm) on the affected psoriatic skin (PP) and normal skin (NN). No topical or systemic treatment was applied to the PP skin in the 12 weeks before sampling.
Immunohistochemical Assay of Human Skin Lesions
Biopsy samples of healthy control skin tissues and subjects with psoriasis were subjected to immunohistochemical assay. After dewaxing with xylene and gradient ethanol, paraffin sections were blocked with 3% hydrogen peroxide to block endogenous peroxidase activity. This was followed by sequential incubation with antibodies (anti-EDIL3, 1:500; anti-VEGF, 1:500) (Abcam, UK), polymer helper, and poly-HRP anti-mouse IgG. The sections were later dehydrated and sealed in accordance with the protocol for hematoxylin and eosin (HE) staining. A microscope (Olympus, Japan) was used to capture images.
Five fields (100x) were selected randomly to determine the mean positively stained cell percentage, which was graded as 0–4, representing 0%, 1–25%, 26–50%, 51–75%, and 76–100%, respectively. The mean percentage of brown or dark yellow particles within the cytoplasm was rated as 0–3, indicating no staining, light brownish yellow, brownish yellow, and tan staining, respectively. These two scores were multiplied to acquire the final scores as follows: 0 (negative), 1–3 (weakly positive), 4–6 (positive), and 8–12 (strongly positive).
Cell Culture
Human umbilical vein endothelial cells (HUVECs) were obtained from the 5- to 8-cm-long human umbilical cord. HUVECs were isolated and cultured in endothelial basal medium (EBM, Lonza, Switzerland) containing EGM-2 (Lonza) until passage 3, followed by incubation in a 5% CO2 incubator with 100% humidity at 37 °C. To conduct experiments, we collected HUVECs of passage 3.
RNA Interference Assay
EDIL3 expression in HUVECs was inhibited in vitro by RNA interference. A small interference RNA (siRNA) targeting human EDIL3 was constructed. At 24 h before transfection, 1×105 cells/well were seeded onto 6-well plates for transfection using Lipofectamine 2000 reagent in accordance with the manufacturer’s protocol (Invitrogen, USA). Briefly, 100 µL of siRNA and 10 µL of Lipofectamine 2000 reagent were incubated in 100 µL of EBM medium for 30 min at 37 °C before treatment. Negative control (NC) sequence was transfected into the scramble group, while no treatment was applied to the blank control group. Cell morphology was observed using a confocal microscope. The cells were harvested after transfection for 24 h for subsequent analyses. EDIL3 siRNA sequences were as follows: forward (F) 5ʹ-GGUGAUAUUUGUGAUCCCATT-3ʹ, reverse (R) 3ʹ-UGGGAUCACAAAUAUCACCTT-5ʹ; NC RNA: (F) 5ʹ-UUCUCCGAACGUGUCACGUTT-3ʹ, (R) 3ʹ-ACGUGACACGUUCGGAGAATT-5ʹ.
Cell Proliferation Assay
Cell counting kit-8 (CCK-8, Boster, China) was used to assess the viability of HUVECs. A 100 µL suspension with 1000 HUVECs was obtained and added to a 96-well plate. The cells were then cultured in a 5% CO2 incubator with 100% humidity at 37 °C. After overnight incubation, CCK-8 solution (10 µL/well) was added, and HUVECs were incubated at 37 °C for a 2-h period. Optical density (OD) values in all wells were determined with the enzyme-marked analysis system (PerlonQ Instruments, China) at 450 nm. This assay was conducted thrice.
Transwell Assay
The 24-well Transwell chambers (polyester membrane, pore size: 8 µm; Corning, USA) were used for HUVEC migration assay. The cells were resuspended in serum-free DMEM/F12 (100 µL) at the density of 4×104 cells and the suspension was introduced into the top chamber. EBM (600 µL) containing EGM-2 as the chemoattractant was added to the bottom chamber. After 24 h of incubation in 5% CO2 at 37 °C, the migration of HUVECs through the polyester membrane was observed. Next, one cotton swab was used to remove cells from the top membrane surface, while 4% paraformaldehyde was added to fix cells that migrated into the lower membrane for 30 min, followed by 0.1% crystal violet staining for 20 min at ambient temperature. Finally, an inverted microscope (Olympus, Japan) was used to visualize and capture images of the migrating cells. The number of migrating cells in three randomly selected fields was determined, and ImageJ software (NIH, USA) was used for data analysis.
Tube Formation Assay in vitro
Under the in vitro condition, the Matrigel basement membrane matrix thawed under 4 °C (mimicking the natural basement membrane matrix in ECs; BD Biosciences, USA) was used to assess tube formation in HUVECs. Each procedure was conducted in a sterile environment on ice. After Matrigel thawing, it was pipetted in precooled 96-well plates and incubated for 120 min at 37 °C. After Matrigel polymerization, 2×104 cells were suspended in 100 µL EC medium prior to seeding onto the Matrigel. A microscope (Olympus, Japan) was used to capture images after 6 h, followed by analysis with ImageJ software.
Western Blotting Assay
RIPA buffer (Beyotime, China) and the protease inhibitor PMSF (Solarbio, China) (RIPA: PMSF ratio = 100:1) were used to extract total proteins. The extracted proteins were centrifuged at 13,000 g and 4 °C for 10 min. The BCA protein assay kit (Solarbio, China) was used to quantify the protein content. An automatic protein analyzer (Wes & Jess, USA) was used for the Western blotting assay in accordance with the specific protocols, and β-actin was used as a reference. The blots were then incubated with rabbit anti-human antibodies against β-actin (CST, USA), EDIL3, VEGF, and VEGFR2 (all from Abcam, UK).
Statistical Analysis
SPSS 22.0 was used for statistical analysis. The experimental results were expressed as mean difference ±standard deviation. Independent sample t-test was conducted to compare two groups, and ANOVA was used to compare several groups. A p-value of <0.05 was considered statistically significant.
Results
EDIL3 and VEGF Levels in Psoriatic Skin Lesions Correlated with the Psoriasis Area and Severity Index
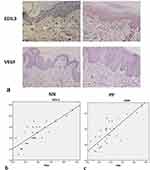
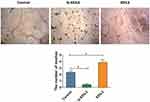
We compared the expression levels of VEGF and EDIL3 in the skin tissues of the two groups. The results showed intense immunostaining of VEGF and marked overexpression of EDIL3 in the biopsy samples of patients with psoriasis as compared to those in control individuals (Figure 1a). To further understand the relationship between proangiogenic factors and psoriasis, the expression levels of VEGF and EDIL3 and the Psoriasis Area and Severity Index (PASI), which is a frequently used method to assess the severity of psoriasis, were analyzed. The results showed that the EDIL3 level was correlated with the PASI (p < 0.05, r = 0.811) (Figure 1b). The elevated expression level of VEGF was also positively correlated with the PASI (p < 0.05, r = 0.784) (Figure 1c). These results may suggest that the two proangiogenic factors play an important role in the progression of psoriasis.
EDIL3 Promoted VEGF and VEGFR2 Expression in ECs
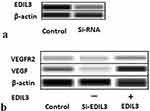
EDIL3 is a new angiogenic molecule expressed specifically in early embryonic ECs. We used siRNA and the recombinant EDIL3 protein to investigate the relationship between EDIL3 and VEGF. Western blotting assay (Figure 2a) revealed that the EDIL3 level of the siRNA group (si-EDIL3) remarkably decreased relative to the control group. VEGF and VEGFR2 levels are shown in Figure 2b. The results revealed that the decrease in EDIL3 level drastically reduced VEGF and VEGFR2 levels. The human recombinant EDIL3 protein induced VEGF and VEGFR2 expression in ECs (Figure 2b). These findings indicate that EDIL3 may have a critical effect on psoriasis-associated angiogenesis through VEGF.
EDIL3 Enhances the Growth, Migration, and Tube Formation of ECs
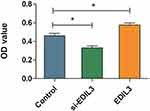
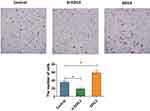
To determine whether EDIL3 is involved in psoriasis-associated angiogenesis, EDIL3 was silenced in HUVECs in vitro. siRNA knockout and recombinant EDIL3 were used to investigate the role of EDIL3. The results showed that silenced EDIL3 suppressed the proliferation (0.46 ±0.07 vs 0.33 ±0.05, p < 0.05) (Figure 3), migration (35.33 ±11.33 vs 18.70 ±7.60, p < 0.05) (Figure 4), and tube formation (2.34 ±0.67 vs 0.44 ±0.34, p < 0.05) (Figure 5) of ECs. In contrast, the exogenous recombinant EDIL3 protein induced the proliferation (0.46 ±0.07 vs 0.58 ±0.06, p < 0.05) (Figure 3), migration (35.33 ±11.33 vs 58.67 ±17.51, p < 0.05) (Figure 4), and tube formation (2.34 ±0.67 vs 3.87 ±1.16, p < 0.05) (Figure 5) of ECs. Collectively, EDIL3 successfully enhanced proliferation, migration, and neovascularization by ECs. The functional changes in ECs occurred parallelly with changes in the expression levels of EDIL3, VEGF, and VEGFR2. This finding indicated that EDIL3 and VEGF were closely associated with the angiogenesis process.
Discussion
The pathogenetic mechanism underlying the development of psoriasis is aberrant keratinocyte growth and differentiation, infiltration of inflammatory cells, and aberrant dermal microvascular changes, which interact with each other through cytokines and thus affect each other24,25 in a manner similar to the positive feedback loop. EDIL3 can enhance angiogenesis and suppress leukocyte recruitment into the inflammation sites.26,27 Thus, EDIL3 is not only a proangiogenic factor but also an endogenous anti-inflammatory molecule. Psoriasis is suggested to be a lymphocyte-driven disorder with the major manifestation of dermal microvascular expansion within the lesions, thus, indicating the angiogenesis-dependent nature of psoriasis;28,29 the dilatation of dermal capillaries is a characteristic histological finding in psoriasis.30 Microvascular alterations detected during psoriasis have been thought to be related to angiogenesis. Numerous known angiogenic factors, such as VEGF, are overexpressed in psoriasis lesions.31 Presently, many immunosuppressive drugs, such as calcipotriol and CyA can inhibit angiogenesis induced by VEGF in psoriasis.28 Hence, antiangiogenic strategies may represent a valid approach for drug development for treating psoriasis.28 Our results were consistent with previous reports regarding VEGF expression in psoriasis. Interestingly, increased EDIL3 expression was also observed in the present study. We also found that EDIL3 and VEGF levels in psoriatic lesions showed a positive correlation with disease severity. Collectively, EDIL3 plays a critical role in the pathogenesis of psoriasis, and there is a close relationship between VEGF and EDIL3, the mechanism of which remains unknown.
As an extracellular matrix protein, EDIL3 can interact with integrins and phospholipids and then regulate host inflammatory disorders.19,20 Recent findings indicate that EDIL3 participates in various biological processes,21 including angiogenesis, cell proliferation and migration, and regulation of immunity, and thus, it could be a valuable disease biomarker in inflammatory diseases and tumors.22 In the angiogenesis process during early embryonic development32 and in tumor33 and ischemic tissues,26 EDIL3 functions as a critical factor that regulates EC activities. In the present study, EDIL3 affected VEGF expression, and VEGF was positively correlated with EDIL3. Furthermore, the overexpression of EDIL3 and VEGF enhanced EC growth, migration, and tube formation in vitro. VEGF and EDIL3 exerted a synergistic effect on angiogenesis in psoriasis. The interaction of VEGF and EDIL3 with each other suggests that EDIL3 could be used as a novel complementary prognostic marker and a therapeutic target to inhibit psoriasis-associated angiogenesis.
Conclusions
Psoriasis is not only a disorder between inflammation and proinflammatory regression, but it also indicates an imbalance between angiogenesis and inflammation. The mutual modulation of inflammation and angiogenesis could help to achieve the optimal treatment of psoriasis. Based on our present study, we speculate that EDIL3 is involved in this imbalance and could be a key molecule. More investigations focused on EDIL3 are required, as it seems to be a critical factor related to both angiogenesis and proinflammatory regression.
Data Sharing Statement
The data utilized in this work can be found in the article.
Ethical Statement
This work gained approval from the institutional ethics committee of Taiyuan City Central Hospital and carried out following the Declaration of Helsinki.
Acknowledgments
Our thanks should go to the National Natural Science Foundation of China and every volunteer for their efforts in the present work.
Funding
The present work was funded by grants from the National Natural Science Foundation of the People’s Republic of China (No. 81803146).
Disclosure
The authors declare no competing interest.
References
1. Lebwohl M. Psoriasis. Ann Intern Med. 2018;168(7):ITC49–ITC64. doi:10.7326/AITC201804030
2. Akhtar T, Wani WY, Kamal MA, et al. Role of angiogenic growth factors in psoriasis: a review. Curr Drug Metab. 2018;19(11):910–916. doi:10.2174/1389200219666180416162942
3. Mousavi SA, Skjeldal F, Fønhus MS, et al. Receptor-mediated endocytosis of VEGF-A in rat liver sinusoidal endothelial cells. Biomed Res Int. 2019;2019:599–619. doi:10.1155/2019/5496197
4. Li T, Gu M, Liu P, et al. Clinical significance of decreased interleukin-35 expression in patients with psoriasis. Microbiol Immunol. 2018;62:454–461.
5. Chen W, Wu L, Zhu W, et al. The polymorphisms of growth factor genes (VEGFA & EGF) were associated with response to Acitretin in psoriasis. Per Med. 2018;15(3):181–188. doi:10.2217/pme-2017-0085
6. Glazewska EK, Niczyporuk M, Lawicki S, et al. ROC analysis of selected matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs) in psoriatic patients. Postepy Dermatol Alergol. 2018;35(2):167–173. doi:10.5114/pdia.2017.66621
7. Yu AR, Hao P, Hao S, et al. MiR-876–5p suppresses cell proliferation by targeting angiopoietin-1 in the psoriasis. Biomed Pharmacother. 2018;103:1163–1169. doi:10.1016/j.biopha.2018.04.145
8. Khandia R, Munjal A, Dhama K, et al. Bacterial toxins: a hope toward angiogenic ailments. Curr Drug Metab. 2017;18(10):926–941. doi:10.2174/1389200218666170911150948
9. Mehta NN, Shin DB, Joshi AA, et al. Effect of psoriasis treatments on vascular inflammation and novel inflammatory cardiovascular biomarkers: a randomized placebo-controlled trial. Circ Cardiovasc Imaging. 2018;11(6):e007394. doi:10.1161/CIRCIMAGING.117.007394
10. Li S, Xu HX, Wu CT, et al. Angiogenesis in pancreatic cancer: current research status and clinical implications. Angiogenesis. 2019;22(1):15–36. doi:10.1007/s10456-018-9645-2
11. Schröder K. Redox control of angiogenesis. Antioxid Redox Signal. 2019;30(7):960–971. doi:10.1089/ars.2017.7429
12. Luengas-Martinez A, Hardman-Smart J, Rutkowski D, et al. Vascular endothelial growth factor blockade induces dermal endothelial cell apoptosis in a clinically relevant skin organ culture model. Skin Pharmacol Physiol. 2020;33(3):110–118. doi:10.1159/000508344
13. Kuang YH, Lu Y, Liu YK, et al. Topical Sunitinib ointment alleviates Psoriasis-like inflammation by inhibiting the proliferation and apoptosis of keratinocytes. Eur J Pharmacol. 2018;824:57–63. doi:10.1016/j.ejphar.2018.01.048
14. Patel AB, Tsilioni I, Weng Z, et al. TNF stimulates IL-6, CXCL8 and VEGF secretion from human keratinocytes via activation of mTOR, inhibited by tetramethoxyluteolin. Exp Dermatol. 2018;2(2):135–143. doi:10.1111/exd.13461
15. Chung SW, Bae SM, Lee M, et al. LHT7, a chemically modified heparin, inhibits multiple stages of angiogenesis by blocking VEGF, FGF2 and PDGF-B signaling pathways. Biomaterials. 2015;37:271–278. doi:10.1016/j.biomaterials.2014.10.004
16. Lin QH, Qu W, Xu J, et al. 1-methoxycarbonyl-β-carboline from picrasma quassioides exerts antiangiogenic properties in HUVECs in vitro and zebrafish embryos in vivo. Chin J Nat Med. 2018;16(8):599–609. doi:10.1016/S1875-5364(18)30097-9
17. Lee SJ, Lee J, Kim WW, et al. Del-1 expression as a potential biomarker in triple-negative early breast cancer. Oncology. 2018;94(4):243–256. doi:10.1159/000485658
18. Yan S, Chen L, Zhao Q, et al. Developmental endothelial locus-1 (Del-1) antagonizes interleukin-17-mediated allergic asthma. Immunol Cell Biol. 2018;96(5):526–535. doi:10.1111/imcb.12023
19. Schürpf T, Chen Q, Liu JH, et al. The RGD finger of Del-1 is a unique structural feature critical for integrin binding. FASEB J. 2012;26(8):3412–3420. doi:10.1096/fj.11-202036
20. Jeong D, Ban S, Oh S, et al. Prognostic significance of edil3 expression and correlation with mesenchymal phenotype and microvessel density in lung adenocarcinoma. Sci Rep. 2017;2017(1):8649.
21. Lee J, Jeong JH, Jung JH, et al. Overcoming tamoxifen resistance by regulation of Del-1 in breast cancer. Oncology. 2019;97(3):180–188. doi:10.1159/000501340
22. Folwaczny M, Karnesi E, Berger T, et al. Clinical association between chronic periodontitis and the leukocyte extravasation inhibitors developmental endothelial locus-1 and pentraxin-3. Eur J Oral Sci. 2017;125(4):258–264. doi:10.1111/eos.12357
23. Niu X, Chang W, Liu R, et al. mRNA and protein expression of the angiogenesis-related genes EDIL3, AMOT and ECM1 in mesenchymal stem cells in psoriatic dermis. Clin Exp Dermatol. 2016;41(5):533–540. doi:10.1111/ced.12783
24. MacDonald A, Burden AD. Psoriasis: advances in pathophysiology and management. Postgrad Med J. 2007;83:690–697. doi:10.1136/pgmj.2007.061473
25. Costa C, Soares R, Soares R. Angiogenesis and chronic inflammation: cause or consequence? Angiogenesis. 2007;10:149–166. doi:10.1007/s10456-007-9074-0
26. Fan Y, Zhu W, Yang M, et al. Del-1 gene transfer induces cerebral angiogenesis in mice. Brain Res. 2008;1219:1–7. doi:10.1016/j.brainres.2008.05.003
27. Anne Klotzsche-von A, Cremer S, Hoffmann J, et al. Endogenous developmental endothelial locus-1 limits ischaemia-related angiogenesis by blocking inflammation. Thromb Haemost. 2017;117(6):1150–1163. doi:10.1160/TH16-05-0354
28. Creamer D, Sullivan D, Bicknell R, et al. Angiogenesis in psoriasis. Angiogenesis. 2002;5(4):231–236. doi:10.1023/A:1024515517623
29. Orciani M, Campanati A, Salvolini E, et al. The mesenchymal stem cell profile in psoriasis. Br J Dermatol. 2011;165(3):585–592. doi:10.1111/j.1365-2133.2011.10438.x
30. Hou R, Yin G, Peng A, et al. DNA methylation of dermal MSCs in psoriasis: identification of epigenetically dysregulated genes. J Dermatol Sci. 2013;72(2):103–109. doi:10.1016/j.jdermsci.2013.07.002
31. Creamer D, Allen M, Sousa A, et al. Altered vascular endothelium integrin expression in psoriasis. Am J Pathol. 1995;147(6):1661–1667.
32. Zhong J, Eliceiri B, Stupack D, et al. Neovascularization of ischemic tissues by gene delivery of the extracellular matrix protein Del-1. J Clin Invest. 2003;112(1):30–41. doi:10.1172/JCI17034
33. Kun Z, Xin G, Tao W, et al. Tumor derived EDIL3 modulates the expansion and osteoclastogenesis of myeloid derived suppressor cells in murine breast cancer model. J Bone Oncol. 2019;16:100–138. doi:10.1016/j.jbo.2019.100238
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.