Back to Journals » Cancer Management and Research » Volume 11
Ectopic expression of HSDL2 is related to cell proliferation and prognosis in breast cancer
Authors Dong B, Yang Y, Han A, Zhang S, Lin Z, Wang Y, Piao J
Received 14 February 2019
Accepted for publication 24 May 2019
Published 12 July 2019 Volume 2019:11 Pages 6531—6542
DOI https://doi.org/10.2147/CMAR.S205316
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Antonella D'Anneo
Bing Dong,1 Yang Yang,1,2 Anna Han,1 Songnan Zhang,3 Zhenhua Lin,1,2 Yixuan Wang,1 Junjie Piao1,2
1Department of Pathology & Cancer Research Center, Yanbian University Medical College, Yanji 133002, People’s Republic of China; 2Key Laboratory of the Science and Technology Department of Jilin Province, Yanji 133002, People’s Republic of China; 3Department of Oncology, Affiliated Hospital of Yanbian University, Yanji 13302, People’s Republic of China
Purpose: Human hydroxysteroid dehydrogenase-like 2 (HSDL2) is a characterized SDR gene that not only catalyses the oxidation and reduction of multiple substrates but also regulates different metabolic and signalling pathways. Accumulating evidences suggest that HSDL2 play an important role in cancer progression. However, the role of HSDL2 in breast cancer has not yet been determined. Thus, this study aims to explore the relevance of HSDL2 in breast cancer progression.
Patients and methods: The location of HSDL2 protein was detected in MDA-MB-231 breast cancer cells by using immunofluorescence (IF) staining. The expression level of HSDL2 was evaluated by immunohistochemical (IHC) staining in 119 breast cancer tissues and 40 normal breast tissues. Then, the correlations between the overexpression of HSDL2 and clinicopathological features of breast cancer patients were evaluated by using the chi-square test, and the survival rates were calculated by the Kaplan-Meier method. In addition, the role of HSDL2 in breast cancer proliferation was assessed by MTT and colony formation assays, and cell cycle distribution was detected by flow cytometry analysis and Western blot.
Results: IF staining and IHC analysis consistently showed that HSDL2 was predominantly expressed in the cytoplasm of breast cancer cells. The positive rate of HSDL2 protein was significantly higher in breast cancer tissues (87.4%, 104/119) than in adjacent normal breast tissues (25%, 10/40) (p<0.01). A high expression of HSDL2 protein was significantly associated with high histological grades, late clinical stages and low survival rates. Moreover, multivariate analysis indicated that HSDL2 protein was an independent prognostic factor in breast cancer patients. Studies in vitro showed that HSDL2 depletion reduced cell proliferation and induced cell cycle arrest in breast cancer.
Conclusion: In conclusion, this study indicated that HSDL2 plays a role in promoting the development of breast cancer. HSDL2 could be a valuable prognostic biomarker and a potential therapeutic target for patients with breast cancer.
Keywords: HSDL2, breast cancer, growth, survival, prognosis, biomarker
Introduction
Breast cancer is one of the most common malignancies for women and is the leading cause of cancer death among females worldwide.1 Globally, up to 1.4 million new patients are diagnosed with breast cancer each year, which is the cause of death for one-third of these patients.2 Recently, molecular diagnosis and targeted therapy have led to great advances and progress in cancer treatment and have provided opportunities for patients to receive personalized treatment. For instance, the use of a humanized monoclonal antibody against HER2, such as trastuzumab and lapatinib, has been shown to improve the survival of breast cancer patients.3 Despite the significant progress in the treatment of breast cancer, the clinical prognosis of breast cancer patients is still poor. Therefore, breast cancer treatment requires more effective biomarkers and therapeutic targets.
The members of the short-chain dehydrogenase/reductase (SDR) superfamily catalyse the oxidation and reduction of multiple substrates, such as steroids, fatty acids, and xenobiotics.4 Some studies have shown that SDRs can regulate different metabolic and signalling pathways, and SDR enzyme dysfunctions can lead to diseases such as Alzheimer’s disease, malignant tumours and obesity-related diseases.5–7 Human hydroxysteroid dehydrogenase-like 2 (HSDL2) is located at the chromosome 9q32 loci and encodes the HSDL2 protein, which is characterized as an SDR.8 HSDL2, a member of the SDR family, was reported to be correlated with glioma and ovarian cancer.9,10 However, to date, the relations between HSDL2 and breast cancer have not yet been reported.
Thus, to gain insight into the role of HSDL2 in the initiation and progression of breast cancer, we detected the expression level of HSDL2 in normal breast tissues and breast cancer tissues and investigated the correlation between HSDL2 expression and clinicopathological features. We also evaluated the prognostic value of HSDL2 in breast cancer patients and explored the role of HSDL2 in breast cancer cell growth in vitro.
Materials and methods
Cell culture
The human breast cancer cell lines MDA-MB-231 and MDA-MB-468 were purchased from the Cell Bank of the Chinese Academy of Medical Science (Shanghai, China) and conserved by the Cancer Research Center of Yanbian University. Cells were cultured in Dulbecco’s Modifified Eagle Medium (DMEM) (Gibco, Gaithersburg, MD, USA) supplemented with penicillin (100 U/ml), streptomycin (100 µg) and 10% fetal bovine serum (FBS). All cells were maintained in 5% CO2 at 37 °C.
Transfection
HSDL2 siRNA (si-HSDL2) targeting the human HSDL2 gene and siRNA duplexes with non-specific sequences were used as negative controls (si-Control). All sequences (Table 1) were designed and synthesized by RiboBio (Guangzhou, China). pCMV6-HSDL2 overexpression plasmid was purchased from Origene (Rockville, USA). Transfections were performed by using Lipofectamine 3000 (Invitrogen, CA, USA) according to the manufacturer’s protocol.
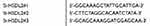 |
Table 1 The sense sequences of si-RNA |
Oncomine database
We utilized the Oncomine database (https://www. oncomine.org/resource/main.html) to evaluate the expression level of HSDL2 in breast cancer. We retrieved a breast cancer-related mRNA microarray dataset to assess correlations between HSDL2 and breast cancer.11 Then, we predicted the correlation between the mRNA expression of HSDL2 and the survival time of breast cancer patients by using KM plotter and the Protein Atlas database.
Ethics statement
This study was conducted in accordance with the Declaration of Helsinki and approved by the Human Ethics and Research Ethics committees of Yanbian University Medical College in China.
Immunofluorescence (IF)
The sub-cellular localization of HSDL2 protein in MDA-MB-231 cells was detected by using IF staining. Cells were grown on coverslips to 70–80% confluence and fixed in 4% paraformaldehyde for 10 min. Then, the cells were permeabilized with 0.5% Triton X-100 for 10 min. Blocking was performed using 3% Albumin Bovine V (A8020, Solarbio, Beijing, China) in PBS supplemented with 0.3% Triton X-100 for 1 h at RT. Anti-HSDL2 Antibodies (15631-1-AP, Proteintech) were diluted in 1% BSA/PBS/0.3% Triton X-100 and incubated with the cells at 4 °C overnight. After washing with PBS, the cells were incubated with fluorochrome-labelled secondary antibodies (Alexa Fluor® 568 goat anti-rabbit IgG (H+L)) (A11004, 1:1,000, Invitrogen, USA) for 1 h at RT. Next, the cells were counterstained with DAPI (C1006, Beyotime, Shanghai, China). Finally, the fluorescence signals were detected with a Leica SP5II confocal microscope (Heidelberg, Germany).
Immunohistochemistry (IHC)
IHC analysis was performed using the Dako LSAB Kit (Dako A/S, Glostrup, Denmark). Tissue sections were dewaxed with xylene and ethanol, followed by PBS washes and rehydration. Then, the endogenous peroxidase activity of the sections was quenched with 0.3% H2O2. Subsequently, the antigen was retrieved, followed by incubation with 1% BSA. The slides were then incubated with a primary antibody at 4 °C overnight. After incubation with a secondary antibody at RT for 30 min, the slides were then incubated with a streptavidin-peroxidase complex. The peroxidase reaction was developed with 3,3ʹ-diaminobenzidine, counterstained with Mayer’s haematoxylin, dehydrated and mounted. Rabbit IgG isotopes were used as a negative control, and positive tissue sections were processed by omitting the primary antibody as a further negative control.
Evaluation of IHC
Two pathologists independently evaluated all tissue specimens. In cases of discrepancies, the final score was established with a reassessment by both pathologists on a double-headed microscope. We first observed staining in the whole breast lesion. Only the cytoplasmic expression patterns were considered positive staining. The staining was scored as “−” (negative, no or less than 5% positive cells), “+” (5–25% positive cells), “++” (26–50% positive cells) or “+++” (more than 50% positive cells). The “strongly positive” descriptor (HSDL2 overexpression) was assigned to samples scored as “++” and “+++”. For survival analysis, the expression level was denoted as high HSDL2 expression (“++” and “+++”) and low HSDL2 expression (“−” and “+”).
Cell viability assay
The cells were plated (5,000 cells/well) in 96-well plates and incubated overnight at 37 °C in a humidified atmosphere containing 5% CO2. After 24, 48, 72 and 96 h of incubation, 20 μL of MTT (5 mg/mL) was added into each well and incubated for 4 h under the same conditions. The supernatants were carefully removed. Then, 200 μL of DMSO was added to each well, and the wells were thoroughly mixed for 10 min. The absorbance value (OD) at 490 nm of each well was measured using a microplate reader (LabSystems Multiskan Ascent).
Colony formation assay
Single-cell suspensions were plated in six-well plates (200 cells/well) and incubated for 14 days at 37 °C in an atmosphere containing 5% CO2. Then, the cells were fixed in 4% paraformaldehyde for 15 min. After washing with PBS, they were stained with Giemsa for 25 mins. Finally, images of the colonies were taken, and the numbers were calculated. Statistical significance was calculated from each of three independent experiments.
Western blot analysis
Total protein was extracted for Western blot. The proteins were lysed in IP lysis buffer with protease inhibitors and then incubated on ice for 30 min. Supernatants were collected by centrifugation (15,000 rpm for 30 min at 4 °C). Next, the protein samples were separated on SDS polyacrylamide gels and then transferred to PVDF membranes. The membranes were blocked for 2 h with 5% fat-free milk and incubated with primary antibodies at 4 °C overnight. Afterwards, primary antibodies were removed, and the membranes were washed again three times with TBST. Finally, the membranes were incubated with HRP-conjugated secondary antibody for 2 h at RT. Detection by enzyme-linked chemiluminescence (ECL) was performed according to the manufacturer’s protocol. The results were analysed quantitatively using the Chemiluminescent and Fluorescent Imaging System.
Flow cytometry analysis
Cells were harvested by trypsinization and washed twice with a PBS solution. Next, 500 µL of cold PI staining solution was added to each tube and incubated for 15 min in the dark on ice. Cells were collected and analysed using a flow cytometer (BD Accuri C6, MI, USA) according to the manufacturer’s instructions. The results were finally compared with those of the untreated control cells.
Statistical analysis
The data analysis was performed using SPSS 17.0 software and GraphPad Prism 6.0 software. The effect of HSDL2 on the clinical stage of breast tumours and histological grade was investigated by the Pearson chi-square test. Survival rate was analysed by the Kaplan-Meier method, and differences in the survival curves were analysed by log-rank tests. Cox proportional hazards regression was employed to assess the prognostic significance of factors in univariate and multivariate models. All observations were confirmed by at least 3 independent experiments. The results are presented as the mean ± SD. A value of p<0.05 was considered statistically significant.
Results
HSDL2 protein is overexpressed in breast cancer
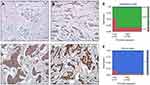
To investigate the role of HSDL2 in breast cancer, we evaluated the mRNA expression level of HSDL2 in breast cancer samples from microarray data obtained from the Oncomine database. The results showed that the expression level of HSDL2 mRNA was upregulated in breast cancer tissues compared with normal breast tissues (Figure 1A and B). We then predicted the correlation between the mRNA expression of HSDL2 and survival time of breast cancer patients by using KM plotter and the Protein Atlas database. A high expression of HSDL2 mRNA was correlated with short survival time in breast cancer patients (Figure 1C and D).
To verify the location of HSDL2 in breast cancer cells, we performed IF staining on MDA-MB-231 breast cancer cells. The results showed that HSDL2 protein was mainly located in the cytoplasm (Figure 2). In addition, we performed IHC staining on 119 breast cancer tissue samples and 40 normal breast tissue samples. Consistently, IHC staining of HSDL2 in breast cancer tissues also showed that HSDL2 was mainly located in the cytoplasm (Figure 3A–D). The positive rate of HSDL2 protein was significantly higher in breast cancer tissues (87.4%, 104/119) than in adjacent normal breast tissues (25%, 10/40) (p<0.01). Similarly, the strongly positive rate of HSDL2 protein was also significantly higher in breast cancer tissues (48.7%, 58/119) than in normal breast tissues (12.5%, 5/40) (p<0.01) (Table 2).
 |
Table 2 HSDL2 protein expression in breast cancer |
 |
Figure 2 IF staining of HSDL2 in MDA-MB-231 breast cancer cells. Notes: HSDL2 protein is mainly located in the cytoplasm of MDA-MB-231 cells. (Blue for DAPI, green for HSDL2). |
Correlations between HSDL2 and the clinicopathological features of breast cancer
To assess the association between HSDL2 protein expression and the clinicopathological parameters of breast cancer, statistical analysis was performed based on the IHC staining of HSDL2 in breast cancer samples. The expression of HSDL2 protein was significantly associated with the histological grade and clinical stage of breast cancer. The strongly positive rate of HSDL2 expression in grade 1 breast cancer (17/25, 68.0%) was significantly lower than that in grade 2 (85/92, 92.3%) and grade 3 (2/2, 100%) (p<0.01) breast cancer. Similarly, for the clinical stage, the strongly positive rate of HSDL2 protein expression in breast cancer patients with clinical stage II-III was 89.2% (99/111), which was significantly higher than that in patients with clinical stage 0-I (62.5%, 5/8) (p<0.05) (Figure 3E and F; Table 3). However, the positive rate of HSDL2 expression was not associated with age, lymph node metastasis, ER, PR or HER2 expression status.
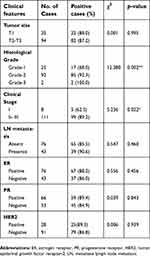 |
Table 3 Correlation between HSDL2 expression and clinicopathological parameters of breast cancer patients |
High expression of HSDL2 is an independent biomarker for poor prognosis of breast cancer patients
To further substantiate the importance of high HSDL2 expression in breast cancer progression, we analysed the correlation between HSDL2 expression and the overall survival (OS) rates of 119 breast cancer patients by using the Kaplan-Meier method. We found that patients with high HSDL2 expression had lower OS rates than those with low HSDL2 expression (p=0.043, log-rank=4.091). Remarkably, for PR (-) breast cancer patients, OS rates were significantly higher in patients with low HSDL2 protein expression than in those with high HSDL2 expression (p=0.046, log-rank=3.998) (Figure 4A and B). However, the expression status of HSDL2 protein was not correlated with OS in PR (+) breast cancer patients (p=0.223, log-rank=1.485) (Figure 4C).
Univariate analysis demonstrated that clinical stage (p=0.013), ER status (p=0.008), PR status (p=0.002), and HSDL2 expression status (p=0.047) were significantly associated in patients with breast cancer. Further multivariate analysis using the Cox proportional hazards model revealed that HSDL2 overexpression was a significant independent prognostic factor for survival along with clinical stage (p=0.007) and PR status (p=0.027) in breast cancer patients (Table 4). These data suggested that HSDL2 could be a valuable prognostic factor in breast cancer.
 |
Table 4 Univariate survival analyses (Cox regression model) of various factors in patients with breast cancer |
Knockdown of HSDL2 inhibits the proliferation of breast cancer cells in vitro
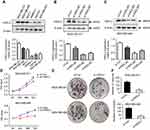
To examine whether HSDL2 could affect breast cancer cell growth, we detected HSDL2 expression in different breast cancer cell lines (Figure 5A). Then, MDA-MB-231 and MDA-MB-468 cells were transfected with si-HSDL2. Western blot analysis showed that si-HSDL2 effectively downregulated the protein expression level of HSDL2 in MDA-MB-231 and MDA-MB-468 cells (Figure 5B and C).
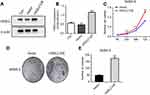
Then, we detected cell viability by using the MTT assay. The results showed that the efficient knockdown of HSDL2 in cells significantly decreased cell viability in vitro, suggesting the important function of HSDL2 in cell viability (Figure 5D). We next assessed the colony formation abilities before and after HSDL2 silencing and found that the knockdown of HSDL2 remarkably reduced cell survival and decreased breast cancer cell proliferation (Figure 5E and F). In addition, SKBR-3 cells were transfected with HSDL2 overexpression plasmid. Then, MTT assay and colony formation assay were performed. The results showed that HSDL2 overexpression promotes cell proliferation (Figure S1). These data suggest that HSDL2 is an essential regulator of breast cancer growth.
Knockdown of HSDL2 induced cell cycle arrest in breast cancer
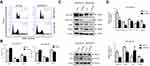
To investigate the mechanism underlying the anti-proliferative effect of HSDL2 silencing in breast cancer cells, we determined the cell cycle phase distribution in the efficient knockdown of HSDL2 by using flow cytometry. The results showed that HSDL2 silencing increased the percentage of cells in G0/G1 phase arrest, which was accompanied by a decrease in the G2/M phase and S phase (Figure 6A and B). Then, we performed a Western blot assay to detect the expression of cell cycle regulatory proteins in breast cancer cells. The results showed that cyclin-dependent kinase 1 (CDK1), cyclin B1, and cyclin D1 expression were decreased after HSDL2 silencing. In contrast, cyclin-dependent kinase inhibitors p21, known as a tumor suppressor, was increased in the si-HSDL2 group, while the expression of p53 was not altered (Figure 6C and D). In consideration of off-target effect, another si-RNA against HSDL2 was transfected in MDA-MB-231 cells, and cell proliferation and cell cycle distribution were detected (Figure S2). These results indicated that HSDL2 knockdown suppressed breast cancer cell proliferation through the induction of cell cycle arrest.
Discussion
The HSDL2 protein contains a D-terminal sterol carrier protein domain, indicating that HSDL2 belongs to the short-chain dehydrogenase (SDR) family. The HSDL2 sequence is found in the 32 regions of the long arm of chromosome 9, and the cDNA length is 3211 bp. The protein consists of 418 amino acids and contains a sterol carrier protein-2 (SCP2) domain.12,13
Recently, researchers have focused on studying the biological functions of the HSDL2 protein in oncogenesis. Sun et al reported that HSDL2 was deregulated in ovarian cancers, and a high expression of HSDL2 protein was correlated with poor outcomes.10 Ruokun et al also reported that HSDL2 was significantly overexpressed in glioma.9 Though the association between HSDL2 expression and oncogenesis has been reported, to our knowledge, this is the first report of the prognostic value of HSDL2 expression in breast cancers. We found that HSDL2 was significantly upregulated in breast cancers and associated with histological grade and clinical stage. The most evident impact of HSDL2 expression was seen for overall survival, both in the full cohort and in the subgroup analysis of patients with PR-negative tumours. These results suggest that HSDL2 could be a prognostic marker for breast cancer patients.
Numerous studies have indicated that the expression levels of the HSDL2 gene are significantly increased after high cholesterol feeding in mice.14 Because other subfamilies of steroid dehydrogenase can promote cholesterol metabolism and steroid hormone synthesis, HSDL2 is believed to be involved in the cholesterol metabolism pathway and control cholesterol synthesis. Since cholesterol is the precursor of steroid hormones involved in cancer promotion and inhibition, deregulation of cholesterol metabolism was frequently occurred in cancer development.15 Not only that, metabolic productions and enzymes at different points in the cholesterol metabolism has different functions in breast cancer progression. For example, 27-hydroxycholesterol (27HC), one of the cholesterol metabolites, suppresses breast cancer growth through interacting with the estrogen receptor (ER), while 11β-hydroxysteroid-dehydrogenase-type-2 (11βHSD2) promotes breast cancer cell growth.16,17 Since the opposing properties of cholesterol metabolites and enzymes, it is important to quantitatively profile sterols and oxysterols in tumor cells and also measure the expression of genes and enzymes controlling the metabolism in cancers.15
In this study, we found that the knockdown of HSDL2 suppressed breast cancer cell proliferation and induced cell cycle arrest in the G1 phase. Current studies also reported that the knockdown of HSDL2 suppressed cell proliferation and induced cell cycle arrest in both ovarian cancer and glioma.9,10 However, an opposite result was also reported by Zhang et al They found that the expression of HSDL2 was downregulated in cholangiocarcinoma and functioned as a tumour suppressor.18 These data highlight the important role of HSDL2 in breast and other cancers, and the role of HSDL2 in cancer may be cell- and tissue-specific.
Cholesterol and its metabolites are essential components of plasma membranes and plays a key role in cell mitosis and intracellular signal transduction.19,20 Additionally, recent studies also found that the cholesterol metabolites could bind to the nuclear receptor, and lead to breast cancer cell proliferation.17 We hypothesized that HSDL2 knockdown caused cell growth reduction and cell cycle arrest partially due to an inhibition of cholesterol metabolism. However, further studies are required to confirm whether HSDL2 could regulate cholesterol metabolism in breast cancers, and the underlying molecular mechanisms also need to be discussed.
Conclusion
In conclusion, these findings suggest a possible relevance between HSDL2 expression and the prognosis of breast cancer patients. HSDL2 may serve as a potential target for breast cancer and provide new options for physicians in clinical therapy.
Acknowledgments
This research was supported by the National Natural Science Funds of China (no. 31760313), the Youth Scientific Research Fund of Jilin Province (20170520015JH, 20150520048JH), the Funds of Changbai Mountain Scholar Project and Key Laboratory of the Science and Technology Department of Jilin Province.
Author contributions
All authors contributed toward data analysis, drafting and revising the paper, gave final approval of the version to be published and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Arveux P, Bertaut A. Epidemiology of breast cancer. Rev Prat. 2013;63:1362–1366.
2. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi:10.3322/caac.21262
3. Smith I, Procter M, Gelber Richard D, et al. 2-year follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer: a randomised controlled trial. Lancet. 2007;369:29–36. doi:10.1016/S0140-6736(07)60028-2
4. Kowalik D, Haller F, Adamski J, Moeller G. In search for function of two human orphan SDR enzymes: hydroxysteroid dehydrogenase like 2 (HSDL2) and short-chain dehydrogenase/reductase-orphan (SDR-O). Steroid Biochem Mol Biol. 2009;117:117–124. doi:10.1016/j.jsbmb.2009.08.001
5. Oppermann UC, Salim S, Tjernberg LO, Terenius L, Jörnvall H. Binding of amyloid beta-peptide to mitochondrial hydroxyacyl-CoA dehydrogenase (ERAB): regulation of an SDR enzyme activity with implications for apoptosis in Alzheimer’s disease. FEBS Lett. 1999;451:238–242.
6. Chang N-S, Schultz L, Hsu L-J, Lewis J, Su M, Sze C-I. 17beta-Estradiol upregulates and activates WOX1/WWOXv1 and WOX2/WWOXv2 in vitro: potential role in cancerous progression of breast and prostate to a premetastatic state in vivo. Oncogene. 2005;24:714–723. doi:10.1038/sj.onc.1208124
7. Persson B, Kallberg Y, Bray JE, et al. The SDR (short-chain dehydrogenase/reductase and related enzymes) nomenclature initiative. Chem Biol Interact. 2009;178:94–98. doi:10.1016/j.cbi.2008.10.040
8. Dai J, Xie Y, Wu Q, et al. Molecular cloning and characterization of a novel human hydroxysteroid dehydrogenase-like 2 (HSDL2) cDNA from fetal brain. Biochem Genet. 2003;41:165–174.
9. Ruokun C, Yake X, Fengdong Y, Xinting W, Laijun S, Xianzhi L. Lentivirus-mediated silencing of HSDL2 suppresses cell proliferation in human gliomas. Tumour Biol. 2016;37:15065–15077. doi:10.1007/s13277-016-5402-6
10. Sun Q, Zhang Y, Su J, Li T, Jiang Y. Role of hydroxysteroid dehydrogenase-Like 2 (HSDL2) in human ovarian cancer. Med Sci Monit. 2018;24:3997–4008. doi:10.12659/MSM.909418
11. Rhodes Daniel R, Jianjun Y, Shanker K, et al. ONCOMINE: a cancer microarray database and integrated data-mining platform. Neoplasia. 2004;6:1–6.
12. Kallberg Y, Oppermann U, Jörnvall H, B. P. Short-chain dehydrogenases/reductases (SDRs). Eur J Biochem. 2002;269:4409–4417.
13. Cheng Z, Li Y, Sui C, Sun X, Xie Y. Synthesis, purification and crystallographic studies of the C-terminal sterol carrier protein type 2 (SCP-2) domain of human hydroxysteroid dehydrogenase-like protein 2. Acta Crystallogr F Struct Biol Commun. 2015;71:901–905. doi:10.1107/S2053230X15008559
14. Gallegos AM, Atshaves BP, Storey SM, et al. Gene structure, intracellular localization, and functional roles of sterol carrier protein-2. Prog Lipid Res. 2001;40:498–563.
15. Silvente-Poirot S, Poirot M. Cancer. Cholesterol and cancer, in the balance. Science. 2014;343:1445–1446. doi:10.1126/science.1252787
16. Nelson ER, Wardell SE, Jasper JS, et al. 27-Hydroxycholesterol links hypercholesterolemia and breast cancer pathophysiology. Science. 2013;342:1094–1098. doi:10.1126/science.1241908
17. Voisin M, de Medina P, Mallinger A, et al. Identification of a tumor-promoter cholesterol metabolite in human breast cancers acting through the glucocorticoid receptor. Proc Natl Acad Sci U S A. 2017;114:E9346–E9355. doi:10.1073/pnas.1707965114
18. Zhang D-Y, Liu Z, Lu Z, et al. Lentivirus-mediated overexpression of HSDL2 suppresses cell proliferation and induces apoptosis in cholangiocarcinoma. Onco Targets Ther. 2018;11:7133–7142. doi:10.2147/OTT.S176410
19. Yamauchi Y, Reid PC, Sperry JB, et al. Plasma membrane rafts complete cholesterol synthesis by participating in retrograde movement of precursor sterols. J Biol Chem. 2007;282:34994–35004. doi:10.1074/jbc.M703653200
20. Singh P, Saxena R, Srinivas G, Pande G, Chattopadhyay A. Cholesterol biosynthesis and homeostasis in regulation of the cell cycle. PLoS One. 2013;8:e58833. doi:10.1371/journal.pone.0058833
Supplementry materials
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.