Back to Journals » Clinical Interventions in Aging » Volume 16
Economic Evaluation of a Reablement Training Program for Homecare Staff Targeting Sedentary Behavior in Community-Dwelling Older Adults Compared to Usual Care: A Cluster Randomized Controlled Trial
Authors Rooijackers TH , Metzelthin SF, van Rossum E, Kempen GIJM , Evers SMAA, Gabrio A , Zijlstra GAR
Received 29 September 2021
Accepted for publication 23 November 2021
Published 22 December 2021 Volume 2021:16 Pages 2095—2109
DOI https://doi.org/10.2147/CIA.S341221
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Maddalena Illario
Teuni H Rooijackers,1,2 Silke F Metzelthin,1,2 Erik van Rossum,1– 3 Gertrudis IJM Kempen,1,2 Silvia MAA Evers,1 Andrea Gabrio,4 GA Rixt Zijlstra1
1Department of Health Services Research, Care and Public Health Research Institute, Maastricht University, Maastricht, the Netherlands; 2Living Lab in Ageing and Long-Term Care, Maastricht, the Netherlands; 3Research Center for Community Care, Academy of Nursing, Zuyd University of Applied Sciences, Heerlen, the Netherlands; 4Department of Methodology and Statistics, Care and Public Health Research Institute, Maastricht University, Maastricht, the Netherlands
Correspondence: Teuni H Rooijackers
Department of Health Services Research, Care and Public Health Research Institute, Maastricht University, P.O. Box 616, Maastricht, 6200 MD, the Netherlands
Tel +31 43-388-1711
Email [email protected]
Purpose: Training and supporting homecare staff in reablement aims to change staff behavior from “doing for” to “doing with” older adults and is assumed to benefit the health and quality of life of older adults and reduce healthcare utilization and costs. This study evaluated the cost-effectiveness and cost-utility of the staff reablement training program “Stay Active at Home” (SAaH) from a societal perspective.
Participants and Methods: An economic evaluation was embedded in a 12-month cluster randomized controlled trial. Ten Dutch homecare nursing teams participated (n = 313 staff members), of which five teams were trained in reablement and the other five provided usual care. Cost and effect data were collected from 264 older adults at baseline, 6 and 12 months. Costs included “intervention,” “healthcare,” and “patient and family” costs (collectively, societal costs) and were assessed using questionnaires and client records or estimated by bottom-up micro-costing. Effects included sedentary behavior and quality-adjusted life years (QALYs). Multiple imputed bootstrapped data were used to generate cost-effectiveness planes and acceptability curves.
Results: No statistically significant differences were observed between the intervention and control group in terms of sedentary time (adjusted mean difference: 4.8 minutes [95% CI – 26.4, 36.0]), QALYs ( 0.01 [95% CI – 0.03, 0.04]), and societal costs ( € 2216 [95% CI – 459, 4895]), except lower costs for domestic help in the intervention group ( €– 173 [95% CI – 299, – 50]). The probability that SAaH was cost-effective compared to usual care ranged from 7.1% to 19.9%, depending on the willingness-to-pay (WTP) (€ 0‒€ 50,000)/minute of sedentary time averted and was 5.9% at a WTP of € 20,000/QALY gained.
Conclusion: SAaH did not improve outcomes or reduce costs and was not cost-effective from a societal perspective compared to usual care in Dutch older adults receiving homecare. Consequently, there is insufficient evidence to justify widespread implementation of the training program in its current form.
Trial Registration: ClinicalTrials.gov: NCT03293303.
Keywords: cluster randomized controlled trial, cost-effectiveness, cost-utility, home and community-based care services, independence, aged
Introduction
Older adults are among the most sedentary age group of society. They spend approximately 9.4 h per day sedentary, representing 65–80% of their waking day,1 with even higher sedentary times reported in older adults receiving long-term care.2 This can lead to numerous health problems, including functional limitations, loss of independence, and lower health-related quality of life (HRQoL), as well as economic problems due to higher healthcare utilization and costs.3–6 Interventions to reduce sedentary behavior in older adults have primarily focused on promoting physical activity (ie, structured exercise programs). Although participation in such interventions may be beneficial,7 the positive effects of being active a few times a week for a limited time may be small when older adults spend the rest of the day sedentary.8,9 Recent studies highlight older adults’ preference for integrating activity into daily routines and tasks.10 Thus, for older adults receiving long-term care (most of whom live at home), embedding such interventions into daily homecare practice may hold promise.7,11 For example, homecare staff can motivate and encourage older adults to perform daily and physical activities as independently as possible.12 Nevertheless, staff often view their role as task-oriented and have a well-intentioned tendency to take over activities,13 even when older adults could perform these activities themselves.14,15 This can lead to a downward spiral in older adults, with more sedentary behavior, greater loss of function and independence, and paradoxically, higher care consumption.15,16
A promising approach that can help homecare staff in this regard is reablement. Reablement is a holistic and person-centered approach that aims to enhance individual’s (physical) functioning, increase or maintain their independence in meaningful activities of daily living, and reduce their need for long-term care.17 It is a “doing with” approach, as opposed to traditional homecare, which tends to be a “doing for” approach. According to systematic reviews on reablement, there is no unequivocal evidence for the effect of reablement on health and quality of life outcomes, cost and cost-effectiveness,18,19 although there is growing support that it may lead to improved performance of daily activities,19–22 lower healthcare utilization, and similar or lower costs for home, health, or social care compared to usual care.23–28 These inconsistent findings are expected to be caused by variation in population and intervention characteristics, and to the often highly tailored and personalized nature of reablement.18 To date, only two studies have conducted a cost-effectiveness analysis comparing the relative costs and effects of reablement to those of usual care: a prospective longitudinal study that evaluated different reablement services as practiced targeting different populations (ie, those discharged from the hospital or recently referred to homecare), and a small-scale trial among older adults who applied for or were referred to homecare after hospitalization or gradual functional decline.26,27 Both studies concluded that reablement was cost-effective compared to usual care. Economic evaluations of trials integrating a reablement approach into usual homecare, targeting a general population of older adults with an indication for long-term care at home, are not yet available.
To contribute to the integration of reablement in Dutch homecare for older adults, the “Stay Active at Home” (SAaH) reablement training program was developed for homecare staff (ie, nurses, nurse assistants, nurse aides and domestic workers). SAaH aims to equip staff with knowledge, attitude, and skills on reablement, and to provide social and organizational support. In doing so, it aims to change staff behavior from “doing for” to “doing with” older adults, so that older adults participate more in daily and physical activities and exhibit less sedentary behavior.29 A previous pilot study and an early trial showed that it was feasible to implement SAaH in Dutch homecare.29,30 A cluster randomized controlled trial (c-RCT), consisting of a process, effect and economic evaluation, was then conducted, comparing SAaH with traditional homecare (hereafter referred to as usual care).31 The process evaluation found that SAaH was largely implemented as intended and that staff experienced positive changes in their knowledge, attitude, skills, and social and organizational support to implement reablement in practice.32 However, the effect evaluation found no differences between the study groups for sedentary behavior in older adults (primary care), implying that SAaH was as effective as usual care.33 SAaH may still be cost-effective, as no effect difference can be justified by lower costs.34 The current paper therefore describes the findings of the economic evaluation comparing SAaH with usual care in Dutch older adults from a societal perspective.
Materials and Methods
Study Design
This economic evaluation was embedded in the c-RCT and conducted in a Dutch healthcare organization in the Netherlands between September 2017 and July 2019. The study was approved by the Dutch Medical Research Committee Zuyderland (METC #17N110), registered at ClinicalTrials.gov (Identifier NCT03293303) and conducted in accordance with the Declaration of Helsinki. Details of the study design and sample size calculation have been published elsewhere.31 Reporting follows the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) guidelines.35
Setting
Dutch homecare includes personal care (ie, assistance with activities of daily living such as washing and dressing), nursing care (ie, medical assistance such as tending to wounds or administering injections), and domestic help (assistance with instrumental activities of daily living such as doing laundry and vacuuming). Personal and nursing care needs are assessed and coordinated by district nurses and reimbursed by health insurers; domestic help needs are assessed by municipalities and funded from general tax revenues, although clients pay a small income-dependent contribution.36 Homecare organizations typically provide personal, nursing, and domestic help. The organization involved in the current study has divided its region into seven working areas, with an average of 11 small-scale self-directed nursing teams operating in each area (range 3–28). Each team consists of about 10 nursing team members (ie, baccalaureate-educated and vocationally trained registered nurses, (certified) nurse assistants and nurse aides) who provide personal and nursing care. Each working area further includes a group of domestic workers who provide domestic support.
Participants
Ten nursing teams from five working areas (two teams per area) participated. These were pre-stratified by area and randomized into the intervention or control group, along with their clients and, if applicable, clients’ domestic workers. The current study focused only on clients. Clients were eligible to participate if they were ≥65 years old, not terminally ill or bedbound, had no serious cognitive or psychological problems, and were able to communicate in Dutch. Eligible clients were informed about the study through an information letter and flyer, a brief telephone call, and, if clients were willing to participate, a home visit. Clients who agreed to participate, provided written informed consent before the study began. They could withdraw from the study at any time and for any reason.31
Study Perspective and Time Horizon
This study was conducted from a societal perspective, meaning that all relevant costs (ie, intervention costs, healthcare costs, and patient and family costs) and effects to society as a whole were included in the analyses.37 Costs related to productivity losses were not taken into account because all participants were past the retirement age of 65 years. Cost and effect data were collected over a 12-month time horizon and were therefore not discounted.37
Intervention
SAaH is a 9-month reablement training for nursing and domestic staff, consisting of program meetings, practical assignments between meetings, and 20 weekly newsletters. The program meetings consisted of a joint kick-off meeting for nursing and domestic staff from the same working area (120 min), followed by five and three team meetings (60 min each) for nursing staff and domestic staff, respectively, over a 6-month period, and a joint booster session at 9 months (120 min). The kick-off meeting described why a reorientation of homecare is needed. During the team meetings, staff learned skills to (1) motivate clients; (2) increase clients’ engagement in daily and physical activities; (3) apply goal setting and action planning; (4) involve clients’ social network; and (5) assess clients’ capabilities. In the booster session, staff practiced conversational skills and situations that were still perceived as challenging in role-plays with professional actors. Staff received ongoing motivation and mentoring during the training, focused on staff knowledge, attitude and skills, and received social and organizational support from colleagues and team managers, with the goal of changing their practice behaviors from “doing for” to “doing with” older adults. Intervention details have been published elsewhere.29
Implementation
All program meetings (50 across 5 working areas) were organized by a group of four program trainers (two trainers from the healthcare organization and two researchers (authors THR and SFM)). One trainer from the organization and one researcher were present at each meeting. On average, staff attended 73.4% of the program meetings, conducted 56.7% of the practical assignments, and consulted 56.6% of the weekly newsletters. Details of implementation, potential mechanisms of impact (ie, knowledge, attitude, skills and support), and contextual factors can be found elsewhere.32 The control group received no training and delivered care as usual.
Data Collection
Baseline Characteristics
Data on sociodemographic characteristics were collected with a baseline questionnaire: age [years], sex, country of origin, educational level [low vocational or advanced elementary education; intermediate vocational or higher secondary education; higher vocational education or university], marital status [single; married; divorced; widowed], and living situation [living alone; living together]. Disability in (instrumental) activities of daily living was assessed with the Groningen Activity Restriction Scale (score range 18–72).38 Duration of homecare received [years] was extracted from client records of the healthcare organization.
Cost Outcome Measures
Intervention costs were estimated using bottom-up micro-costing and included labor costs of the program trainers, staff training costs, material costs, travel costs (home–work), and accommodation costs.36 Labor costs were based on an average time investment of 2 h per program meeting per program trainer (200 h in total). Training costs were based on full staff compliance with the program (ie, 20 h for nursing staff and 16 h for domestic workers). Labor costs, training costs, and travel costs were valued using gross hourly wages. For each discipline, the average number of years of work experience was used to determine the gross hourly wage. Material and accommodation costs were estimated from invoices. Costs were allocated to intervention group participants only by dividing the total cost of the intervention by the number of intervention group participants.
Healthcare costs and patient and family costs were derived from healthcare and informal care data assessed with an adapted version of the iMTA Medical Consumption Questionnaire at baseline, 6 and 12 months, and from client records.39 Healthcare costs included primary care costs (ie, visits to general practitioner and physiotherapist), hospital care costs (ie, outpatient hospital visits, emergency room visits, ambulance transportation, and hospitalization), and long-term care costs (ie, nursing care, personal care, domestic help, day care, and inpatient care use). Patient and family costs included only informal care costs and were based on the amount of time the participant received care from family and/or friends. Cost prices from the Dutch Manual for Costing in Economic Evaluations were used to value data on healthcare and informal care use (Supplementary Table 1).40 Costs were expressed in 2018 euros (€), and, if needed, prices were indexed to the reference year using a consumer price index.40
Effect Outcome Measures
Sedentary time was assessed with tri-axial wrist-worn accelerometers (ActiGraph GT9X Link, ActiGraph Inc, Pensacola, FL, USA) worn for 7 consecutive days, at baseline and 12 months. Raw acceleration data were collected at 30 Hz and aggregated to 60-second epochs using ActiLife software version 6.13.4. Activity counts per daily minute were derived for each axis and for their composite score (ie, vector magnitude). Subsequently, sleep time and non-wear time were identified and removed.41,42 Remaining minutes were labeled wake/wear time. Sedentary time during wake/wear time was determined using vector magnitude cut-points of Koster et al.43 Sedentary time was defined in two ways: first, as the average number of daily minutes, and second, as the average proportion of wake/wear time (in both cases averaging across days within each participant). Details of the data treatment have been published elsewhere.33
Quality-adjusted life years (QALYs) were derived from HRQoL data assessed with the EQ-5D-5L questionnaire at baseline, 6, and 12 months.44 Participants were asked to rate five quality of life domains (ie, mobility, self-care, usual activities, pain/discomfort and anxiety/depression) with five response levels.45 This resulted in an overall health state. Health states were first converted into utilities using the Dutch tariff;46 utilities ranged from –0.446 to 1, with negative values indicating “worse than death” and 1 indicating “perfect health”. Utilities were then used to calculate QALYs over the trial period by means of the area under the curve method (ie, multiplying the duration of a health state by the utility value related to that health state).37 In addition, participants were asked to rate their self-perceived health on a visual analogue scale (EQ-VAS, range 0–100).45
Data Analysis
All analyses were performed in R version 4.0.3 (R Core team, 2020).47 The base-case analyses were conducted according to the intention-to-treat principle, provided that participants had ≥1 accelerometer wear day of ≥10h of wake/wear time.31 Multiple imputation by chained equations was used to impute missing data, assuming data to be missing at random.48,49 Prior to fitting the imputation model, the association between observed variables and missing outcome data was examined via logistic regression to identify those variables that were substantially associated with missingness. These variables were included as predictors in the imputation model (ie, living situation), along with the stratification factor (ie, working area) and variables that were deemed a priori relevant to the outcomes (ie, age, sex, education, disability, and duration of homecare).31 Baseline values for the outcomes were also included to control for possible baseline differences between groups. We choose this subset of variables because they were the most relevant from a statistical and clinical perspective, while also achieving a balance in terms of model complexity. Imputation was performed separately for each treatment arm. A multilevel normal approach was used for the imputed outcome data, taking into account cluster effects (ie, participants), while predictive mean matching was used for all predictor variables not imputed via clustering (for which the proportion of missing variables was small (<1%)). Although the assumption of normality for cost data may not always be met, using non-parametric bootstrapping to derive mean incremental cost-effectiveness can yield robust estimates against parametric assumptions, even in small samples with skewed data.50 For each analysis, 20 imputed datasets were generated, and pooled estimates for the key parameters of interest for each fitted model were derived using Rubin’s rules.49
Cost Analysis
The mean incremental difference in societal costs and costs by cost category were calculated using mixed-effects linear regression via restricted maximum likelihood estimation and linear contrasts. By design, the hierarchical structure of our data consists of three levels (repeated measures nested in participants nested in nursing teams). However, two-level models with adjustment for working area were presented, as the small sample size of the third level led to instability of the random effect parameters. Treatment, time, their interaction, and working area were specified as fixed factors, and participants as random factors. Models were adjusted for age, sex, education, disability, duration of homecare, and baseline costs. Since we assumed a non-normal distribution of cost data, 95% bias-corrected and accelerated bootstrap confidence intervals (CIs) were derived, using 1000 bootstrap replications.51 A thousand replicates were justified, as changing the seed number in the models resulted in reasonably similar results. We additionally calculated the mean incremental difference in healthcare and informal care utilization.
Effectiveness Analysis
The mean incremental difference in sedentary time and QALYs was calculated similarly to the mean incremental difference in costs, using mixed-effect models with linear contrast and the same predictors and interactions, but with adjustment for baseline sedentary time and baseline EQ-5D-5L values, respectively, instead of baseline costs. Parameter estimates were derived based on multiple imputation methods and mixed effects linear regression for sedentary time and QALYs. For the latter, 95% CIs were derived using bootstrap methods assuming a non-normal distribution.
Cost-Effectiveness and Cost-Utility Analysis
A cost-effectiveness analysis, based on sedentary time and costs, and a cost-utility analysis, based on QALYs and costs, were conducted. Incremental cost-effectiveness ratios (ICERs) were calculated by dividing incremental costs by incremental effects between groups. The ICERs were considered as the incremental cost per unit of additional effect. Therefore, values for sedentary time were averted so that higher times reflected better effects. Non-parametric bootstraps with 1000 replications were used to estimate the uncertainty surrounding the ICERs, taking into account the correlation between costs and effects by fitting both models within the same bootstrap function. The bootstrapped cost-effect pairs were then plotted on cost-effectiveness planes (CE-planes), in which the vertical line represented the incremental costs and the horizontal line the incremental effects.52,53 Cost-effectiveness acceptability curves (CEACs) were also generated, reflecting the probability that the intervention was cost-effective compared to control for a range of willingness-to-pay (WTP) thresholds (ie, the amount of money society is willing to pay for a unit of effect gained).54,55 We reported the probability of the intervention being cost-effective compared to control at a WTP of €20,000 per QALY gained, which is a conservative estimate for the burden of disease for a relatively healthy population of older adults, according to the Dutch National Health Care Institute.56,57 Because the WTP for sedentary behavior is unknown, maximum probabilities were provided.
Sensitivity Analysis
Three sensitivity analyses were performed to assess the robustness of results: one from the healthcare perspective (including only healthcare costs); one using only complete cases (ie, participants with complete data for total societal costs, sedentary time, and QALYs); and one without participants with extreme cost outliers. Outliers were defined by a boxplot in which a point beyond the upper outer fence was considered an extreme outlier.58
Results
Participant Flow and Baseline Characteristics
Of the 742 potential participants screened for eligibility, 290 were not eligible, 156 declined to participate, and 32 dropped out before baseline measurements, leaving 264 participants who agreed to participate and were measured at baseline (n = 131 control, n = 133 intervention). Table 1 shows their baseline characteristics. Participants’ mean age was 82.1 (SD 6.9) years, 67.8% were female, and 67.4% had a low level of education. During follow-up, 23.9% (n = 63; 32 control, 31 intervention) dropped out, mainly due to institutionalization or death. Dropouts’ characteristics were comparable between groups; however, at baseline, dropouts were significantly more sedentary, had worse daily, physical, and psychological functioning, and fell more often than study completers.33
 |
Table 1 Baseline Characteristics of Participants in the Control and Intervention Groups (N = 264) |
Of all participants, 92.8% (n = 245; 120 control, 125 intervention) had ≥1 valid accelerometer wear day and were included in the base-case analyses (on average, participants had 7.0 ± 1.7 valid wear days, with an average daily wake/wear time of 1,056.4 ± 191.0 minutes (17.6 ± 3.2 h)). Complete data for societal costs and QALYs were obtained from 78.4% (n = 192; 95 control, 97 intervention); complete data for sedentary time were obtained from 70.6% (n = 173; 87 control, 86 intervention).
Costs
The cost of the intervention was estimated at €625/participant in the intervention group (Supplementary Table 2). Societal costs were €22,469 per participant in the intervention (intervention costs included) compared to €20,254 per participant in the control group (Table 2). There were no statistically significant differences between the study groups for societal costs and most cost categories, except for lower for domestic help in the intervention group (adjusted mean difference: 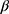 € –173 [95% CI –299, –50]). Volumes of healthcare and informal care use were also comparable between groups, except for lower domestic help utilization in the intervention group (
€ –173 [95% CI –299, –50]). Volumes of healthcare and informal care use were also comparable between groups, except for lower domestic help utilization in the intervention group (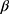 : –7.8 h [95% CI –13.3, –2.3]) (Table 3). Observed estimates for costs and volumes (rather than imputed estimates) are tabulated in Supplementary Tables 3 and 4, respectively.
: –7.8 h [95% CI –13.3, –2.3]) (Table 3). Observed estimates for costs and volumes (rather than imputed estimates) are tabulated in Supplementary Tables 3 and 4, respectively.
Effectiveness
Table 4 reports the effect outcomes in both study groups at baseline and 12 months, and the difference in effect outcomes between the groups over time. No differences were found for sedentary time and QALYs. More information on the effectiveness of SAaH compared to usual care has been published elsewhere.33
Cost-Effectiveness and Cost-Utility
For sedentary time expressed as daily minutes averted, most bootstrapped cost-effect pairs (74.2%) were in the northwest (NW) quadrant of the CE-plane (Table 5 and Figure 1A). This suggests that the intervention was less effective and more costly than the control. The CEAC shows that the probability that the intervention was cost-effective compared to control ranged from 7.1% to 19.9%, depending on the (WTP) (€0‒€50,000)/minute of sedentary time averted (Figure 1B). Similar findings were observed for sedentary time expressed as proportion of wake/wear time averted (Figure 1C and D). For QALYs, most cost-effect pairs (95.6%) were located in the northern quadrants of the CE-plane, roughly evenly distributed around the y-axis, indicating higher costs for the intervention compared to the control, but no clear difference in QALYs between groups (Figure 1E). The cost-utility was 5.9% at a WTP of €20,000/QALY gained (Figure 1F).
Sensitivity Analysis
Sensitivity analyses were conducted for the healthcare perspective, complete cases, and participants without extreme cost outliers, respectively. Overall, the results of the sensitivity analyses did not differ substantially from those of the base-case analyses, although the probability of cost-effectiveness seemed to increase slightly (Table 5). Nevertheless, most cost-effect pairs still fell in the NW quadrant of the CE-planes (range 34.0–68.8%), and CEACs barely exceeded 30% regardless of the WTP and outcome chosen, indicating that the intervention was still dominated by control (Supplementary Figure 1).
Discussion
The current study evaluated the cost-effectiveness and cost-utility of the SAaH reablement training program for homecare staff compared to usual care in Dutch older adults receiving homecare from a societal perspective. The average societal cost per participant was €20,254 in the control group and €22,469 in the intervention group, including €625 for the intervention. No differences were observed between groups for societal costs, sedentary time, and QALYs. The probability that the intervention was cost-effective compared to the control ranged from 7.1% to 19.9%, depending on the WTP/minute or proportion of sedentary time averted. The cost-utility was 5.9% at a WTP of €20,000/QALY gained. In the sensitivity analyses from the healthcare perspective, for complete cases, and for participants without extreme cost outliers, the probability of cost-effectiveness and cost-utility increased slightly, but still did not exceed 30.2%. Therefore, SAaH cannot be considered cost-effective compared to usual care in both the base-case and sensitivity analyses.
Interestingly, a statistically significant decrease in the cost of domestic help was observed in the intervention group compared to the control group but not in the other two categories targeted by the intervention (ie, personal and nursing care). This may be explained by differences in the level of education and experience of domestic and nursing staff. In the Netherlands, domestic staff typically do not require a formal domestic qualification, are generally low educated, and receive little training.30,59,60 Nursing staff, on the other hand, are generally higher educated and receive more training, with an increasing emphasis in recent years on promoting client independence. Thus, the standard of Dutch personal and nursing care is already at a relatively high level,30,59,60 so there may be more to gain from domestic staff. Another interesting finding was that more than a quarter of all costs came from informal care. This is in line with previous research on reablement indicating that informal care was a large cost driver.61 Since informal caregivers in general play a large role in the lives of, and care and support for, older adults, they may also fulfill a significant role in promoting older adults’ independence; this would argue for supplementing SAaH with a component for informal caregivers.62
In terms of healthcare use and costs, previous research on reablement often reported lower care use for reablement compared to usual care (eg, less personal care use,25 shorter homecare visits or episodes,23,24,26 fewer emergency department visits,23,24 and fewer hospitalizations.24 In addition, similar or lower home, health, or social care costs were often reported for reablement,23–28 in contrast to the findings in the current study. This may be related to differences in intervention and population characteristics. While SAaH was integrated into usual care, targeting older adults who had been receiving homecare for some time, previous research often focused on time-limited (usually up to 12 weeks) interventions for older adults who had recently experienced a health loss, had been discharged from hospital, or had recently been referred to homecare.23–28 These latter groups may have greater potential for improvement and thus may benefit more from reablement, particularly in terms of healthcare utilization and associated costs, as they generally require temporary rather than long-term support.63 In terms of effect¸ previous research on reablement has not yet examined sedentary behavior,64 and uncertainty has often been reported regarding the effect on HRQoL.18,19 This may be explained by the use of generic outcome measures, such as the EQ-5D, which do not account for benefits beyond health, such as well-being and independence.65,66 Although such outcome measures are often used in economic evaluations to compare the effects of different interventions for different health outcomes on a comparable scale, they may be insensitive to capturing subtle changes in quality of life in older adults.67,68 In terms of cost-effectiveness, a prospective longitudinal study evaluating different reablement services,27 a small-scale trial,26 and a systematic review on economic evaluations of social care interventions including reablement,69 concluded that reablement was cost-effective compared to usual care for different WTP values and outcomes. Yet clear comparisons of economic evaluations is difficult for several reasons. First, studies differ in terms of interventions (eg, content, intensity, duration, and delivery mode), participant groups (eg, those receiving long-term versus acute care), and settings (eg, homecare versus hospital care). Second, the type of economic evaluation, time horizon, analytic approach, and costs included differ across studies.69 Third, the results must be interpreted in light of the national context, as healthcare systems and available resources vary across countries.37
This study has a several strengths. First, it was conducted alongside a c-RCT, reflecting a real-life situation and allowing for prospective data collection. Second, the study was conducted from a societal perspective, which is the broadest perspective and is often advocated for use in evaluating publicly funded programs.70 Third, it is one of the few full economic evaluations in the aged care sector conducted according to standard guidelines.40,70 Some limitations should also be noted. First, the cost data included retrospective questions over a 6-month period, which may have led to recall bias. However, we assume that this bias is equally distributed across groups and thus will not affect differences between groups. Second, because some participants considered the baseline measurement too time-consuming, we shortened the follow-up measurements by removing the questions on visits to allied health professionals, except for visits to the physical therapist, which is the most commonly used allied health service among Dutch older adults.71 We therefore expect that this led to only a small underestimation of healthcare costs. Third, a substantial amount of data was imputed due to dropout. Nonetheless, the results of the sensitivity analyses for participants with complete data yielded similar results to the base-case analyses. Fourth, we assumed in the imputation that data were missing at random and did not explore missing not at random departures. Fifth, the results cannot be generalized to other populations due to the use of two-level multivariable models in which working area was treated as fixed effect instead of nursing team as random effect. Lastly, the study period of one year is relatively short for an economic evaluation. Therefore, the long-term costs and effects are still unclear.
The current findings show that SAaH was not cost-effective compared to usual care. Based on these and previous findings,33 wider implementation of the training program in its current form cannot be recommended. Future studies should investigate how the training program could be improved. Possible options for this are: a stronger emphasis on the role of domestic staff and the addition of a component for informal caregivers. However, reablement is a relatively new approach and there is still debate about its conceptualization, operationalization, and measurement,20,72 which may explain the inconsistent findings across studies. To avoid suboptimal use of public investment, more high-quality research is needed to support or refute whether reablement is (cost-) effective.69 First, research should provide more insight into how reablement is configured and operates in practice,20 and why it works, for whom, and under which conditions.28 Second, research should provide recommendations for conducting and reporting economic evaluations in the field of reablement, and for standardized outcome measures that represent quality of life domains that are most important to older people.66,69,70,73
Conclusion
The SAaH reablement training program, which aimed to change the behavior of homecare staff from “doing for” to “doing with” older adults so that older adults would exhibit less sedentary behavior, did not improve outcomes or reduce costs compared to usual care in a population of Dutch community-dwelling older adults who received homecare. Moreover, SAaH was not cost-effective from a societal perspective after 12 months compared to usual care. Consequently, there is insufficient evidence to justify widespread implementation of the training program in its current form.
Abbreviations
CEAC, cost-effectiveness acceptability curve; CE-plane, cost-effectiveness plane; c-RCT, cluster randomized controlled trial; HRQoL, health-related quality of life; ICER, incremental cost-effectiveness ratio; QALY, quality-adjusted life year; SAaH, stay active at home; WTP, willingness-to-pay.
Data Sharing Statement
Individual participant data and data dictionaries that underlie the results reported in this article are available from the corresponding author on reasonable request after de-identification. Data will be available beginning 9 months and ending 36 months following article publication for researchers who provide a methodologically sound proposal to achieve aims in the approved proposal. Proposals should be directed to [email protected]. To gain access, data requestors will need to sign a data access agreement.
Ethics Approval and Informed Consent
The study was approved by the Dutch Medical Research Committee Zuyderland (METC #17N110) and conducted in accordance with the Declaration of Helsinki. Participation was voluntary; older adults were informed about the study and asked for written informed consent prior to study commencement. They could withdraw from the study at any time for any reason.
Acknowledgments
The authors would like to thank all participating homecare clients, homecare staff, and team managers of the healthcare organization MeanderGroep South-Limburg. Furthermore, they would like to thank program trainers José Blezer and Thecla Terken and program champions Marijke Hennen and Mandy Boosten (MeanderGroep South-Limburg) for their contribution to the implementation of the training program; Henny Geelen and Maria Wetzels (Citizen Power Limburg) for their valuable practice insights as representatives of older adults and informal caregivers; Marja Veenstra (Citizen Power Limburg), José van Dorst (Dutch Nurses Association), Bem Bruls (General Practitioners Eastern South-Limburg), Wiro Gruisen (CZ health insurance), Lisette Ars (healthcare organization Envida), Roger Ruijters, Tessa Schreibers, Margreet Bruinsma, and Karin Pieters (Meandergroep Zuid-Limburg) for their guidance and advice as part of the steering group, and Astrid van den Bosch, Ine Hesdahl, Susanne Hanssen, Mariska Machiels, and Wendy Halbach for their contribution to the data collection and/or entry.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the Netherlands Organization for Health Research and Development [ZonMw 50–53120–98–014], The Hague, The Netherlands. They had no role in the study design, data collection, data analysis, decision to publish, or the preparation of the manuscript.
Disclosure
The authors report no conflict of interest in this work.
References
1. Harvey JA, Chastin SF, Skelton DA. How sedentary are older people? A systematic review of the amount of sedentary behavior. J Aging Phys Act. 2015;23(3):471–487. doi:10.1123/japa.2014-0164
2. Leung K-CW, Sum K-WR, Yang Y-J. Patterns of sedentary behavior among older adults in care facilities: a scoping review. Int J Environ Res Public Health. 2021;18(5):2710. doi:10.3390/ijerph18052710
3. Kehler DS, Hay JL, Stammers AN, et al. A systematic review of the association between sedentary behaviors with frailty. Exp Gerontol. 2018;114:1–12. doi:10.1016/j.exger.2018.10.010
4. Heron L, O’Neill C, McAneney H, Kee F, Tully MA. Direct healthcare costs of sedentary behaviour in the UK. J Epidemiol Community Health. 2019;73(7):625–629. doi:10.1136/jech-2018-211758
5. Cunningham C, O’Sullivan R, Caserotti P, Tully MA. Consequences of physical inactivity in older adults: a systematic review of reviews and meta‐analyses. Scand J Med Sci Sports. 2020;30(5):816–827. doi:10.1111/sms.13616
6. Paterson DH, Warburton DE. Physical activity and functional limitations in older adults: a systematic review related to Canada’s physical activity guidelines. Int J Behav Nutr Phys Act. 2010;7(1):1–22. doi:10.1186/1479-5868-7-38
7. Weber M, Belala N, Clemson L, et al. Feasibility and effectiveness of intervention programmes integrating functional exercise into daily life of older adults: a systematic review. Gerontology. 2018;64(2):172–187. doi:10.1159/000479965
8. Martin A, Fitzsimons C, Jepson R, et al. Interventions with potential to reduce sedentary time in adults: systematic review and meta-analysis. Br J Sports Med. 2015;49(16):1056–1063. doi:10.1136/bjsports-2014-094524
9. Prince S, Saunders T, Gresty K, Reid R. A comparison of the effectiveness of physical activity and sedentary behaviour interventions in reducing sedentary time in adults: a systematic review and meta‐analysis of controlled trials. Obes Rev. 2014;15(11):905–919. doi:10.1111/obr.12215
10. Burton E, Lewin G, Boldy D. Physical activity levels of older adults receiving a home care service. J Aging Phys Act. 2013;21(2):140–154. doi:10.1123/japa.21.2.140
11. Kerse N, Peri K, Robinson E, et al. Does a functional activity programme improve function, quality of life, and falls for residents in long term care? Cluster randomised controlled trial. BMJ. 2008;337:a1445. doi:10.1136/bmj.a1445
12. Eanes L. Ce: Too much sitting: a newly recognized health risk. Am J Nurs. 2018;118(9):26–34. doi:10.1097/01.NAJ.0000544948.27593.9b
13. Sharp S, Mcallister M, Broadbent M. The tension between person centred and task focused care in an acute surgical setting: a critical ethnography. Collegian. 2018;25(1):11–17. doi:10.1016/j.colegn.2017.02.002
14. Burton E, Farrier K, Galvin R, et al. Physical activity programs for older people in the community receiving home care services: systematic review and meta-analysis. Clin Interv Aging. 2019;14:1045. doi:10.2147/CIA.S205019
15. Resnick B, Boltz M, Galik E, Pretzer-Aboff I. Restorative care nursing for older adults: a guide for all care settings. New York: Springer Publishing Company; 2012.
16. Schuurmans M, Lambregts J, Grotendorst A. Beroepsprofiel verpleegkundige. Deel 1: Leren van de toekomst [Nursing Professional Profile. Part 1: Learning from the future]. Verpleegkundigen en Verzorgenden 2020; 2012. Dutch.
17. Metzelthin SF, Rostgaard T, Parsons M, Burton E. Development of an internationally accepted definition of reablement: a delphi study. Ageing Soc. 2020;1–16. doi:10.1017/S0144686X20000999
18. Cochrane A, Furlong M, McGilloway S, Molloy DW, Stevenson M, Donnelly M. Time‐limited home‐care reablement services for maintaining and improving the functional Independence of older adults. Cochrane Database Syst Rev. 2016;10:CD010825. doi:10.1002/14651858.CD010825.pub2
19. Sims-Gould J, Tong CE, Wallis-Mayer L, Ashe MC. Reablement, reactivation, rehabilitation and restorative interventions with older adults in receipt of home care: a systematic review. J Am Med Dir Assoc. 2017;18(8):653–663. doi:10.1016/j.jamda.2016.12.070
20. Aspinal F, Glasby J, Rostgaard T, Tuntland H, Westendorp RGJ. New horizons: reablement - supporting older people towards Independence. Age Ageing. 2016;45(5):574–578. doi:10.1093/ageing/afw094
21. Whitehead PJ, Worthington EJ, Parry RH, Walker MF, Drummond AE. Interventions to reduce dependency in personal activities of daily living in community dwelling adults who use homecare services: a systematic review. Clin Rehabil. 2015;29(11):1064–1076. doi:10.1177/0269215514564894
22. Tuntland H, Aaslund MK, Espehaug B, Førland O, Kjeken I. Reablement in community-dwelling older adults: a randomised controlled trial. BMC Geriatr. 2015;15(1):145. doi:10.1186/s12877-015-0142-9
23. Tinetti ME, Baker D, Gallo WT, Nanda A, Charpentier P, O’Leary J. Evaluation of restorative care vs usual care for older adults receiving an acute episode of home care. JAMA. 2002;287(16):2098–2105. doi:10.1001/jama.287.16.2098
24. Lewin G, Allan J, Patterson C, Knuiman M, Boldy D, Hendrie D. A comparison of the home-care and healthcare service use and costs of older Australians randomised to receive a restorative or a conventional home-care service. Health Soc Care Community. 2014;22(3):328–336. doi:10.1111/hsc.12092
25. Lewin GF, Alfonso HS, Alan JJ. Evidence for the long term cost effectiveness of home care reablement programs. Clin Interv Aging. 2013;8:1273. doi:10.2147/CIA.S49164
26. Kjerstad E, Tuntland HK. Reablement in community-dwelling older adults: a cost-effectiveness analysis alongside a randomized controlled trial. Health Econom Rev. 2016;6(1):15. doi:10.1186/s13561-016-0092-8
27. Glendinning C, Jones K, Baxter K, et al. Home Care Re-Ablement Services: Investigating the Longer-Term Impacts (Prospective Longitudinal Study). York/Canterburry: Social Policy Research Unit (SPRU)/Personal Social Service Research Unit (PSSRU); 2010.
28. Bauer A, Fernandez JL, Henderson C, Wittenberg R, Knapp M. Cost‐minimisation analysis of home care reablement for older people in England: a modelling study. Health Soc Care Community. 2019;27:1241–1250. doi:10.1111/hsc.12756
29. Metzelthin SF, Zijlstra GAR, Van Rossum E, et al. Doing with…’rather than ‘doing for…’ older adults: rationale and content of the ‘stay active at home’ programme. Clin Rehabil. 2017;31(11):1419–1430. doi:10.1177/0269215517698733
30. Smeets RGM, Kempen GIJM, Zijlstra GAR, et al. Experiences of home‐care workers with the ‘stay active at home’ programme targeting reablement of community‐living older adults: an exploratory study. Health Soc Care Community. 2020;28(1):291–299. doi:10.1111/hsc.12863
31. Metzelthin SF, Rooijackers TH, Zijlstra GAR, et al. Effects, costs and feasibility of the ‘stay active at home’ reablement training programme for home care professionals: study protocol of a cluster randomised controlled trial. BMC Geriatr. 2018;18(1):276. doi:10.1186/s12877-018-0968-z
32. Rooijackers T, Zijlstra G, van Rossum E, Veenstra M, Kempen G, Metzelthin S. Process evaluation of a reablement training program for homecare staff to encourage Independence in community-dwelling older adults. BMC Geriatr. 2021;21(1). doi:10.1186/s12877-020-01936-7
33. Rooijackers TH, Kempen GIJM, Zijlstra GAR, et al. Effectiveness of a reablement training program for homecare staff on older adults‘ sedentary behavior: a cluster randomized controlled trial. J Am Geriatr Soc. 2021;69(9):2566–2578. doi:10.1111/jgs.17286
34. Brouwer W, van Baal P, van Exel J, Versteegh M. When is It Too Expensive? Cost-Effectiveness Thresholds and Health Care Decision-Making. Springer; 2019. doi:10.1007/s10198-018-1000-4
35. Husereau D, Drummond M, Petrou S, et al. Consolidated health economic evaluation reporting standards (cheers) statement. Eur J Health Econ. 2013;14(3):367–372. doi:10.1186/1741-7015-11-80
36. Zorginstituut Nederland. Richtlijnen voor het uitvoeren van economische evaluaties in de gezondheidszorg [Guideline for conducting economic evaluations in healthcare]. Diemen, The Netherlands: Zorginstituut Nederland; 2016. Dutch.
37. Drummond MF, Sculpher MJ, Claxton K, Stoddart GL, Torrance GW. Methods for the Economic Evaluation of Health Care Programmes. Oxford University Press; 2015.
38. Kempen GI, Miedema I, Ormel J, Molenaar W. The assessment of disability with the Groningen activity restriction scale. Conceptual framework and psychometric properties. Soc Sci Med. 1996;43(11):1601–1610. doi:10.1016/s0277-9536(96)00057-3
39. Bouwmans C, Hakkaart-van Roijen L, Koopmanschap M, Krol M, Severens H, Brouwer W. Handleiding iMTA Medical Consumption Questionnaire [Manual iMTA Medical Consumption Questionnaire]. Rotterdam, The Netherlands: Institute for Medical Technology Assessment, Erasmus Universiteit Rotterdam; 2013. Dutch.
40. Hakkaart-van Roijen L, Van der Linden N, Bouwmans C, Kanters T, Tan SS. Kostenhandleiding: Methodologie van kostenonderzoek en referentieprijzen voor economische evaluaties in de gezondheidszorg [Costing manual: Methodology of costing research and reference prices for economic evaluations in healthcare]. Diemen, The Netherlands: Zorginstituut Nederlands; 2015. Dutch.
41. Cole RJ, Kripke DF, Gruen W, Mullaney DJ, Gillin JC. Automatic sleep/wake identification from wrist activity. Sleep. 1992;15(5):461–469. doi:10.1093/sleep/15.5.461
42. Choi L, Liu Z, Matthews CE, Buchowski MS. Validation of accelerometer wear and nonwear time classification algorithm. Med Sci Sports Exerc. 2011;43(2):357. doi:10.1249/MSS.0b013e3181ed61a3
43. Koster A, Shiroma EJ, Caserotti P, et al. Comparison of sedentary estimates between activpal and hip-and wrist-worn actigraph. Med Sci Sports Exerc. 2016;48(8):1514. doi:10.1249/MSS.0000000000000924
44. The EuroQol Group. Euroqol-A new facility for the measurement of health-related quality of life. Health Policy (New York). 1990;16(3):199–208. doi:10.1016/0168-8510(90)90421-9.
45. Herdman M, Gudex C, Lloyd A, et al. Development and preliminary testing of the new five-level version of eq-5d (eq-5d-5l). Qual Life Res. 2011;20(10):1727–1736. doi:10.1007/s11136-011-9903-x
46. Versteegh MM, Vermeulen KM, Evers SM, De Wit GA, Prenger R, Stolk EA. Dutch tariff for the five-level version of eq-5d. Val Health. 2016;19(4):343–352. doi:10.1016/j.jval.2016.01.003
47. R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2018.
48. Van Buuren S. Flexible Imputation of Missing Data. Boca Raton: CRC press; 2018.
49. Rubin DB. Multiple Imputation for Nonresponse in Surveys. Vol. 81. New York: John Wiley & Sons; 2004.
50. Rascati KL, Smith MJ, Neilands T. Dealing with skewed data: an example using asthma-related costs of Medicaid clients. Clin Ther. 2001;23(3):481–498. doi:10.1016/S0149-2918(01)80052-7
51. Tibshirani RJ, Efron B. An introduction to the bootstrap. Monog Stat Appl Probab. 1993;57:1–436.
52. Thompson SG, Barber JA. How should cost data in pragmatic randomised trials be analysed? BMJ. 2000;320:1197–1200. doi:10.1136/bmj.320.7243.1197
53. Black WC. The ce plane: a graphic representation of cost-effectiveness. Med Decis Making. 1990;10(3):212–214. doi:10.1177/0272989X9001000308
54. Fenwick E, O’Brien BJ, Briggs A. Cost‐effectiveness acceptability curves–facts, fallacies and frequently asked questions. Health Econ. 2004;13(5):405–415. doi:10.1002/hec.903
55. Fenwick E, Byford S. A guide to cost-effectiveness acceptability curves. Br J Psychiatry. 2005;187(2):106–108. doi:10.1192/bjp.187.2.106
56. Zorginstituut Nederland. Kosteneffectiviteit in de Praktijk [Cost-Effectiveness Analysis in Practice]. Diemen, The Netherlands: Zorginstituut Nederland; 2015. Dutch.
57. Zorginstituut Nederland. Ziektelast in de Praktijk: De Theorie En Praktijk van Het Berekenen van Ziektelast Bij Pakketbeoordelingen [The Theory and Practice of Calculating Burden of Disease in Package Assessments]. Diemen, The Netherlands: Zorginstituut Nederland; 2020. Dutch.
58. Croarkin C, Tobias P, Filliben J, Hembree B, Guthrie W. Nist/Sematech e-Handbook of Statistical Methods. Gaithersburg: Nist/sematech; 2006.
59. Genet N, Boerma W, Kroneman M, Hutchinson A, Saltman RB; World Health Organization. Home Care Across Europe: Current Structure and Future Challenges. Copenhagen: World Health Organization. Regional Office for Europe; 2012.
60. Kroneman M, Boerma W, van den Berg M, Groenewegen P, de Jong J, van Ginneken E. The Netherlands: Health System Review. Health System in Transition. Kopenhagen: WHO Regional Office for Europe; 2016.
61. Beresford B, Mayhew E, Duarte A, et al. Outcomes of reablement and their measurement: findings from an evaluation of English reablement services. Health Soc Care Community. 2019;27(6):1438–1450. doi:10.1111/hsc.12814
62. Lorthioir NT. Including relatives in the “stay active at home” program. Clin Rehabil. 2018;32(3):419–420. doi:10.1177/0269215517752489
63. Tessier A, Beaulieu M-D, Mcginn CA, Latulippe R. Effectiveness of reablement: a systematic review. Healthc Policy. 2016;11:49. doi:10.12927/hcpol.2016.24594
64. Mjøsund HL, Moe CF, Burton E, Uhrenfeldt L. Integration of physical activity in reablement for community dwelling older adults: a systematic scoping review. J Multidiscip Healthc. 2020;13:1291–1315. doi:10.21203/rs.2.17945/v1
65. Usman A, Lewis S, Hinsliff-Smith K, et al. Measuring health-related quality of life of care home residents, comparison of self-report with staff proxy responses for eq-5d-5l and howru: protocol for assessing proxy reliability in care home outcome testing. BMJ Open. 2018;8(8):e022127. doi:10.1093/ageing/afy191
66. Bulamu NB, Kaambwa B, Ratcliffe J. A systematic review of instruments for measuring outcomes in economic evaluation within aged care. Health Qual Life Outcomes. 2015;13(1):1–23. doi:10.1186/s12955-015-0372-8
67. van Dongen JM, van Wier MF, Tompa E, et al. Trial-based economic evaluations in occupational health: principles, methods, and recommendations. J Occup Environ Med. 2014;56(6):563. doi:10.1097/JOM.0000000000000165
68. Whitehead SJ, Ali S. Health outcomes in economic evaluation: the qaly and utilities. Br Med Bull. 2010;96(1):5–21. doi:10.1093/bmb/ldq033
69. Faria R, Kiss N, Aspinal F, Harden M, Weatherly H. Economic evaluation of social care Interventions: lessons drawn from a systematic review of the methods used to evaluate reablement. Health Econ Outcome Res. 2016;96(1):107. doi:10.1093/bmb/ldq033
70. Bulamu NB, Kaambwa B, Ratcliffe J. Economic evaluations in community aged care: a systematic review. BMC Health Serv Res. 2018;18(1):1–13. doi:10.1186/s12913-018-3785-3
71. Van Dijk C, Van den Burg M, Dik J-W, Heim N. Ouderenzorg 2013–2016 Deel 1 | Zorggebruik En Zorgkosten van Ouderen [Elderly Care 2013-2016 Part 1 | Care Use and Costs of the Elderly]. Diemen, The Netherlands: Zorginstituut Nederland; 2018. Dutch.
72. Clotworthy A, Kusumastuti S, Westendorp RG. Reablement through time and space: a scoping review of how the concept of ‘reablement’for older people has been defined and operationalised. BMC Geriatr. 2020;21:1–16. doi:10.21203/rs.2.21256/v3
73. Makai P, Brouwer WB, Koopmanschap MA, Stolk EA, Nieboer AP. Quality of life instruments for economic evaluations in health and social care for older people: a systematic review. Soc Sci Med. 2014;102:83–93. doi:10.1016/j.socscimed.2013.11.050
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.







