Back to Journals » ClinicoEconomics and Outcomes Research » Volume 14
Economic Burden of Non-Alcoholic Steatohepatitis (NASH) Among Diabetic Population in Italy: Analysis and Perspectives
Authors Torre E , Di Matteo S, Bruno GM , Martinotti C , Valentino MC , Testino G, Rebora A, Bottaro LC, Colombo GL
Received 6 May 2022
Accepted for publication 10 August 2022
Published 14 September 2022 Volume 2022:14 Pages 607—618
DOI https://doi.org/10.2147/CEOR.S371778
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Samer Hamidi
Enrico Torre,1 Sergio Di Matteo,2 Giacomo Matteo Bruno,3 Chiara Martinotti,2 Maria Chiara Valentino,2 Gianni Testino,4 Alberto Rebora,1 Luigi Carlo Bottaro,5 Giorgio Lorenzo Colombo3
1Diabetology and Metabolic Diseases Unit - ASL3, Genoa, Italy; 2S.A.V.E. Studi Analisi Valutazioni Economiche S.r.l., Health Economics & Outcomes Research, Milan, Italy; 3Department of Drug Sciences, University of Pavia, Pavia, Italy; 4Unit of Addiction and Hepatology - ASL3, Genoa, Italy; 5General Direction, ASL3, Genoa, Italy
Correspondence: Giorgio Lorenzo Colombo, Department of Drug Sciences, School of Pharmacy, University of Pavia, Italy, Email [email protected]
Background: Aim of our study is to evaluate the economic impact of NASH among diabetic population in Italy and potential benefits of treatments that can slow the disease progression.
Methods: A Markov model was conducted from the Italian National Healthcare System perspective reporting results at 3, 5, 10 and 15 years. The model included NASH and T2DM patients with all stages of fibrosis (F0-F3), compensated cirrhosis (CC), decompensated cirrhosis (DCC), hepatocellular carcinoma (HCC), liver transplant (LT), post-LT and death. A 1-year model cycle length was considered, with each patient passing through the stages and exiting the model when reached one of mortality states. Transition probabilities and annual cost related to health states were derived from published literature. Moreover, the model made it possible to develop a scenario analysis to simulate the impact of treatments capable of slowing the disease progression in phases F0-F4 (CC).
Results: The results highlighted an economic burden of NASH in T2DM patients of approximately € 1.4 billion, € 3.1 billion, and € 9.4 billion, respectively, after 3, 5 and 10 years, reaching about € 17.3 billion after 15 years. The slowing down of the progression in the early stages of the disease (fibrosis F0-CC) has led to significant savings corresponding to € 2.3 billion at 15 years. These savings were generated by the reduction of the patients in the advanced stages of the disease, which is linked to a reduction in deaths, equal to 92,208 deaths avoided over a 15-year time horizon.
Conclusion: Patients with NASH and T2DM reported an important burden in Italy. It is important to investigate the potential clinical and economic benefits of antidiabetic drugs that have been shown to be effective in preventing the transition to advanced disease, simultaneously acting on the therapeutic goals of diabetic disease.
Keywords: non-alcoholic steatohepatitis, type 2 diabetes mellitus, burden of illness, treatment perspectives
Introduction
NAFLD and his progressive more aggressive form, non-alcoholic steatohepatitis (NASH), will represent a new public health challenge in the coming years, ranging their prevalence between 20% and 30% among adults in western world (estimated prevalence of 2–3% in the general population), and with a growing trend as well.1 A 2016 study, performing a projection of the development of the disease in several countries, identified for Italy, all ages, a prevalence of 25.4% rising to 29.5% up to 2030, as well as an analogous pattern for NASH prevalence, from 4.4% to 6.3, in the same period.2 NAFLD is therefore recognized as the most prevalent chronic liver disease worldwide. It is defined by the presence of hepatic steatosis, diagnosed by either imaging or histology, and concomitant lack of secondary causes of liver disease (ethanol abuse, long-term use of a steatogenic medication, monogenic hereditary disorders). In the Mediterranean area, NAFLD is more common in men in the third and fourth decade of life (70%) than in women, but the women’s protection is lost after the onset of menopause. The clinical importance of NAFLD is also linked to the independent effect of promoting cardiovascular damage, which is added to one of the metabolisms itself. Furthermore, NAFLD seems to be associated with an increased risk of cancer (hepatocellular cancer), not only in the liver. Finally, the presence of steatosis in itself represents an additional factor of hepatic suffering in patients with acute or chronic liver disease of other etiology, conditioning its evolution and often interfering with treatments.
NASH is distinguished from NAFLD by the presence of inflammation and hepatocyte ballooning, and later of fibrosis.3,4 The fibrosis stage and its evolution are related to the clinical risk of progression to cirrhosis and long-term liver-related outcomes and mortality, as well as to the increase in healthcare and social costs of the disease.5 The pathology accounts for about 75% of all chronic liver disease in USA and represents a potentially underlying cause of hepatocellular carcinoma (HCC) in 14.1% of all cases.6,7 In the near future, NALFD and NASH are expected to become the most common liver transplant indication. The management of hepatic steatosis and advanced nonalcoholic steatohepatitis is costly and early diagnosis need to be improved to avoid the economic burden associated with disease progression. The economic burden of the disease is very relevant: the GAIN study, a real-world data analysis that collected direct medical, direct non-medical, and indirect costs associated with NASH among Europe and USA, detected mean total annual per patient cost, respectively, of € 2763, € 4917, and € 5509.8 As far as Italy is concerned, a recent study has evaluated the healthcare resource utilization and costs associated with patients with NAFLD/NASH and advanced liver disease (defined as NAFLD/NASH patients with compensated cirrhosis (CC), decompensated cirrhosis (DCC), liver transplant (LT), or hepatocellular carcinoma (HCC)). The researchers looked at data from 9729 Italian patients with NAFLD/NASH who were hospitalized between 2011 and 2017 and identified 131 individuals (1.3%) with CC, 303 (3.1%) with DCC, 11 (0.1%) with LT and 79 (0.8%) with HCC.9 NAFLD/NASH patients with advanced liver disease were hospitalized on average 4.2–4.4 times per year, compared with 2.9 times for those without advanced disease (p ≤ 0.05). Within the study cohort, the increase in annual health-care costs per NAFLD/NASH patient due to the progression of the disease towards advanced stages was observed: this increase varied from 1.9 times in case of hospitalization for diagnosis of CC, to 4.2 times for patients diagnosed with HCC; moreover, a 6.2-fold increase in costs was observed when comparing the patient with transplant versus the uncomplicated one.9
NASH is firmly related to type 2 diabetes mellitus (T2DM). Patients that suffer both T2DM and NASH tend to be more inclined to risk for adverse clinical outcomes, which results in a higher risk for mortality and morbidity.10–14 T2DM is a relevant public health problem worldwide due to its growing prevalence and socioeconomic burden. In 2019 in Italy there were more than 3.6 million diabetic patients (prevalence: 6.1%) with a mean diabetes-related health expenditure per person of $2849.15 In addition, the prevalence of diabetes is also increasing year-on-year, with an estimated 4 million patients by 2030.15 Diabetes represents the most important risk factor for both NAFLD and NASH: the estimated global prevalence among diabetic patients is 55.48% for NAFLD, and 37.3% for NASH.16 A recent study was able to develop a model that predicts significant clinical and economic burden due to population with NASH and type 2 diabetes over the next 20 years in the USA. It is highly likely that interventions reducing morbidity and mortality in NASH patients with T2DM could potentially reduce this projected clinical and economic burden.10 The association between diabetes and NASH therefore represents a threat to sustainability of chronic care costs in the coming years and it will therefore be necessary to intervene, also pharmacologically, as early as possible to prevent its development.
Aim of our study is to evaluate the economic impact of non-alcoholic steatohepatitis among diabetic population in Italy from the Italian National Healthcare System (INHS) perspective and evaluate the benefits of a possible disease progression slowing in the early stages.
Materials and Methods
Model Design
A burden of illness analysis based on a Markov model with a 15-year time horizon was conducted with the aid of an analytical support developed in MS Excel® and in compliance with the methodological guidelines published by the International Society for Pharmacoeconomics and Outcomes Research (ISPOR).17 The Italian National Healthcare System (INHS) perspective has been adopted with results at 3, 5, 10 and 15 years. This study is based on a simulation model starting from previously published literature data. For this reason, it did not require review and approval by an institutional review board or ethics committee.
A prevalence module was used to define the overall target population of patients with NASH and T2DM in Italy. The Markov model was then constructed considering specific health states referring to the natural evolution of the disease. The model included NASH patients with all stages of fibrosis along with the advanced stages of disease. In detail, the following health states were used in the Markov model: NASH with no fibrosis (F0), fibrosis stage 1 (F1), fibrosis stage 2 (F2), fibrosis stage 3 (F3), compensated cirrhosis (CC), decompensated cirrhosis (DCC), hepatocellular carcinoma (HCC), liver transplant (LT), year-LT (yLT), post-LT (PLT), and death. Three absorbing mortality states were considered: liver related (LRM), cardiovascular (CVM) and background mortality (BM). A 1-year model cycle length was considered, with each patient passing through the stages and exiting the model when reached one of mortality states. Within each cycle, the transition between health states was assumed to take place in the sixth month. For economic evaluation, the cost of one state was therefore adopted for the first half of the cycle and the cost of the subsequent state for the second half.
Death health state was managed in the same way, the dead patients abandoned the model; however, their economic evaluation took place considering the death occurred in the middle of the year thus using the health state cost prior to the death for six months. The economic impact was expressed for the total target population and per patient. The results were expressed at 3,5,10 and 15 years, reporting the clinical effects of the disease evolution in these intervals and the estimated cumulative costs. The quality of life correlated to different health states and how the disease progression affects the patient over time was also considered. The model also made it possible to develop a scenario analysis to simulate the clinical and economic impact of the use of therapies capable of slowing the disease progression in phases F0-F4 (CC), before the development of more advanced complications.
Population and Transition Probabilities
Starting from the total Italian population in 2021, based on recent national epidemiological data,18,19 the T2DM diagnosed population was obtained. The prevalence rate of NAFLD (67.97%) and NASH (37.3%) in the diabetic patients were then applied, leading to the target population of interest for the study.16 The prevalent NASH population with T2DM as of 2021 in Italy was inserted in the model, and NASH prevalence was considered for all stages of fibrosis. As for prevalence module the NAFL state was not considered, patients entered the model at NASH F0 and remained in or transitioned to another health state at the end of the cycle, with the possibility of progressing through the model to the stages of fibrosis NASH F1-F3, CC, DCC, HCC, year of liver transplant (yLT), and PLT; three absorbing mortality states (liver related, cardiovascular, and background mortality were adopted). The Meta-analysis of Histological Data in Viral Hepatitis (METAVIR) scale was adopted to define the prevalence of NASH, considering any degree of fibrosis from stages 0–4, where stage 4 is represented in the model as CC.20 Model predicted that every year patients transitioned to a different state (or remained in the same state) based on the associated transition probability and that they exited the model upon reaching one of three mortality states, Table 1. Transition probabilities were drawn from the literature.10,16 These probabilities were obtained by balancing the relative risk of advanced fibrosis, cirrhosis, cardiovascular mortality for patients with type 2 diabetes compared to the general population.10,21
 |
Table 1 Annual Transition Probabilities for Prevalence of NASH Patients with T2DM |
Patients would then progress through the model according to appropriate transition probabilities until reaching one of the three absorbing mortality states. For the analysis of the quality of life associated with the different health states and the evolution over time, utilities data from the literature were used.8
Cost Inputs
Adopting the INHS perspective, the model considered as input data only the direct health-care costs, with the aim of obtaining the annual direct average cost associated with each health state and the burden of NASH among diabetic population in Italy. Annual cost related to health states was derived from published literature specific to the Italian context. In detail, the costs referred to the NASH F0 were calculated based on national cost data referred to the diagnosis and monitoring of this stage in Italy,22 cost of F1-F3 states and CC state were taken from multinational cost of illness studies,1,8 including Italy, while the costs referred to DCC, HCC, 1LT, PLT from Italian studies.23–25 In the literature studies, the health state costs per-patient were estimated using national unit price, costs were calculated considering the consumption of health resources related to the management and monitoring of the NASH patients (medication, hospitalizations, consultations, tests, and procedures used for the diagnosis and follow-up of the disease and surgical interventions).
Table 2 shows the health state costs considered for the development of the analysis.
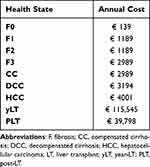 |
Table 2 Health State Cost Inputs |
Utilities Inputs
The model also considered the health-related quality of life associated with the evolution of the disease. To conduct this evaluation, data was sought in the literature. In the absence of specific data for the Italian population, utility values were adopted from international literature, considered applicable to the Italian context.1 Table 3 shows the health state utility inputs adopted in the model.
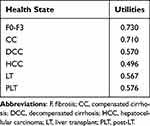 |
Table 3 Health State Utility Inputs |
Simulation Scenario and Sensitivity Analysis
The analysis involved the development of a simulation scenario in which the impact on clinical and economic outcomes of any future treatment capable of slowing the progression of the disease was assessed. Specifically, the effect of a 20% variation in the transition probabilities referred to fibrosis states, up to CC (F4), was hypothesized, considering the possibility of intervening with pharmacological treatments in the phases preceding the onset of DCC to be plausible. The effect of slowing the disease progression through use of suitable therapies was thus tested. In addition, in order to assess the robustness of simulation scenario analysis, a one-way sensitivity analysis has been carried out, varying by ±20% cost data and utilities values adopted, and considering the effect of alternative variations in the health states transition probabilities, respectively, of 15% and 25%.
Results
The results of the analysis estimated a prevalence of diagnosed T2DM in Italy equal to 3,673,969 patients in 2021. The prevalence of NAFLD in the diabetic population was estimated to be 2,248,469 patients, and the prevalence of NASH 1,233,351 patients. The latter population was considered as a target for the model. Table 4 shows the evolution of the health states of the population over the 15 years of analysis, with data referring to the end of each year. The model calculated the burden of NASH in T2DM population considering a 15-year time horizon. Starting from the estimated target population in 2021, after 15 years 620,540 NASH patient with F0-F3 were observed, of which 27% in F0, 38% in F1, 23% in F2 and 11% in F3. At the end of the 15th year 61,790 patients developed CC, 13,047 DCC and 4870 HCC, Table 4. The Markov model based on the prevalence module assumed 21,613 transplants over a 15-year time horizon. Liver-related mortality was estimated to be the cause of 89,410 deaths and cardiovascular diseases the cause of 142,136 deaths, Table 4. Over time, it has been observed that the disease progresses towards advanced health impairment and increase in mortality.
 |
Table 4 Clinical Impact of NASH in T2DM Population Over a 15-Year Time Horizon |
As regards the quality of life, the study showed a reduction in 15 years from 0.729 to 0.726. Table 3 shows the economic outcomes associated with the evolution of the disease estimated for the time horizon.
The results highlighted the economic burden of NASH in patients with type 2 diabetes in a time horizon of 3, 5 and 10 years, respectively, equal to about € 1.4 billion, € 3.1 billion, and € 9.4 billion, reaching an economic impact of about € 17.3 billion after 15 years, Table 5. These results, applied to each time horizon and live population, correspond to an average annual cost per patient ranging from a minimum of € 385 at 3 years to a maximum of € 1188 at 15 years, Table 5. Over time, the target population decreases due to mortality, however the costs increase due to the disease progression. Overall, the annual cost per patient necessarily increases.
 |
Table 5 Economic Impact of NASH in T2DM Population Over a 15-Year Time Horizon |
The results of the simulation scenario highlighted the potential benefits of slowing disease progression. Specifically, the hypothesis of a 20% reduction in the early health states transition probabilities (F0-CC) has allowed to determine a significant reduction in the INHS expenditure due to a slower progression of the stages with greater severity and cost. Tables 6 and 7 show the clinical effects of this slowdown overtime, while Tables 8 and 9 shows the economic implications. The slowing down of the progression in the early stages of the disease has led to significant savings corresponding to € 2.3 billion at 15 years, equivalent to € 204 per patient, Table 9. These savings were generated by the reduction of the patients in the advanced stages of the disease, which is linked to a reduction in deaths, equal to 92,208 deaths avoided over a 15-year time horizon, Table 7.
 |
Table 6 Clinical Impact of NASH in T2DM Population Over a 15-Year Time Horizon: Simulation Scenario (20% Reduction in the F0-CC Health States Transition Probabilities) |
 |
Table 7 Simulation Scenario Results Regarding Clinical Impact: Comparison with Basecase (20% Reduction in the F0-CC Health States Transition Probabilities) |
 |
Table 8 Economic Impact of NASH in T2DM Population Over a 15-Year Time Horizon: Simulation Scenario (20% Reduction in the F0-CC Health States Transition Probabilities) |
 |
Table 9 Simulation Scenario Results Regarding Economic Impact: Comparison with Basecase (20% Reduction in the F0-CC Health States Transition Probabilities) |
The sensitivity analysis has shown the robustness of the results. Figure 1 shows the results of the sensitivity analysis referred to the difference in live patients and total costs. The ±20% variation of cost and utilities data in scenario analysis has not resulted in significant deviations from the variation obtained considering the starting inputs. Keeping the other parameters unchanged, sensitivity analysis related to the simulation scenario showed how an alternative variation in the health states transition probabilities (assuming a slowing of progression of 15% and 25%) affects the results more significantly, Figure 2. Slowdown in the progression of the disease in its characteristic health states would be able to determine significant effects from a clinical and economic point of view.
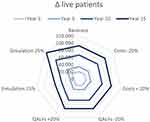 |
Figure 1 Sensitivity analysis results for scenario analysis: variation in the number of patients alive over time. |
 |
Figure 2 Sensitivity analysis results for scenario analysis: variation in the total costs over time. |
Discussion
Relationship and double causality between T2DM and NAFLD/NASH are now evident. As for these reasons it is important to know how to manage this patient and the natural evolution of the disease. NASH is a serious public health challenge that is predicted to become increasingly prevalent, and the simultaneous presence of diabetes complicates the patient’s metabolic state, making him a subject for which a diagnosis is required as early as possible, to allow clinical action in the early phase of the disease, characterized by lower risks and costs. Our goal is to analyze the burden of illness of the NASH and diabetes population in Italy, adopting NHS prospective and a 15-year time horizon. The study involved the creation of a Markov model based on health states able to simulate the disease evolution and to assume the economic and clinical impact of the disease over time.
It was highlighted that over time, due to the progression of the disease towards the most advanced states, the costs of the target population are inevitably destined to increase, resulting in an expense of € 1.4 billion at 3 years, which increases to about € 17.3 billion at 15 years. A significant increase in consideration of a reduction in the sample from 1,133,374 patients at 3 years to 713,636 at 15 years, due to mortality. The analysis also made it possible to highlight the reduction in the quality of life over time and the potential benefits of a slowdown in the disease progression. The results of the simulation scenario, in fact, showed how a 20% reduction of the progression within the transition states (F0-CC) can lead to a significant decrease in costs by the procrastination of advanced stages, characterized by significant costs. In addition, this slowdown would have a better impact on the quality of life and allow for a reduction in mortality in the target population. In detail, this variation would result in a minimum saving of € 162.2 million at 3 years, which would increase to € 406.1 and € 1.3 billion at 5 and 10 years, respectively, up to € 2.3 billion at 15 years. The 20% reduction was chosen based on assumptions, the economic model is able to evaluate the effects of other variations as well, and it is desirable that future studies allow to clearly identify the potential effects of therapies on the disease initial stages slowing.
Despite the burden of the disease, there are still few studies evaluating its clinical and economic impact.2,8,10 We believe that findings of this study are of great value and may be the basis for future observations and insights. There is currently no specific pharmacological treatment for NASH and the current standard of care comprises lifestyle changes and medication for comorbidities, such as diabetes. Individuals with early NAFLD are generally managed in primary care, with lifestyle modification, while patients with NASH and more advanced fibrosis require a multidisciplinary approach. Although there are no specific treatments, the benefit of using some antidiabetic therapies, capable of acting positively on NASH, is becoming evident. Based on the benefits brought by the disease evolution reduction highlighted in the simulation scenario, the importance of investigating the effects of these therapies and possible future applications emerges. In particular, the simulation scenario showed how over time the slowdown in the disease progression, hypothesized in the analysis through the reduction of the health states transition probabilities, allow to reduce the number of patients in the most advanced disease and mortality. From the economic point of view, it translates into lower management costs for the advanced stages of the disease, and greater resources available for the management of the patient without advanced liver disease. The reduction in overall mortality leads to an increase in the population in the simulation scenario (92,208 deaths avoided), resulting in more patients requiring healthcare, but, overall, a lower annual cost per patient.
This is the first study conducted for Italy evaluating the burden of NASH in T2DM patients and the potential benefits of slowing the progression of the disease, and we believe that the availability of real world data would be fundamental to support the results, provide further information on the study population, NHS costs and to monitor the disease evolution and response to drugs acting on both NASH and diabetes. The developed Markov model allowed a 15-year projection of the clinical and economic consequences of disease progression, with the typical limitations of this type of assessment, based on literature and assumptions. Italian real-world data collection would make it possible to remove the limit of international literature data adoption, used as a reference for the development of this study, in the absence of national data.
The lack of prospective real-world natural history data to determine the true incidence and progression of NASH represents an important limit, however we have tried to refer to up-to-date and relevant literature for the development of the model. The model considers only prevalence data, starting from the analysis of the current available data as cannot precisely estimate the NASH and diabetes evolution in Italy. It also does not take into account the costs associated with complications usually found in advanced liver disease and diabetes or the implementation of future costly treatment and, in line with the NHS perspective, it does not consider indirect costs.
As far as costs are concerned, although a recent Italian study was available,9 it was decided to adopt other bibliographic sources referring to Italy, due to their easier interpretation and extrapolation. The sensitivity analysis made it possible to verify the robustness of the results, and the choice to focus this analysis on the variation between the simulation scenario and the base case was made to verify the solidity of the simulation scenario as well.
The results of the analysis are in line with a similar USA study, from which information on health states transition probabilities was drawn.10 Both studies highlight that the clinical and economic burden of NASH in patients with T2DM is currently substantial, and as the prevalence of T2DM increases globally, this burden will continue to rise. NAFLD and its clinical evolution, NASH, represent the new frontier of diabetes management, both from clinic and health-care costs point of view. Considering that globally over 55% of diabetic patients are interested by NAFLD, and over 37% by NASH, the clinical implications of the disease among diabetic population are important in terms of both survival and quality of life.
Recent studies demonstrated that several antidiabetic drugs such as pioglitazone, GLP1 agonists and SGLT2 inhibitors can be effective in preventing the transition from early to final stages of the disease, always and simultaneously acting on the treatment goals of diabetic disease.3,26–29
It is critical that clinicians, payers, policy makers, and the pharmaceutical industry not only understand the clinical burden of NASH in patients with T2DM, but also the economic, patient reported outcomes burden and potentials of effective therapies for the disease control.
Conclusion
The economic burden of the diseases in the Italian diabetic population ranges from € 1.4 billion over a period of three years, up to € 17.3 billion reaching a time span of fifteen years. This last amount resembles a typical financial maneuver in our country. The greater burden of these costs is due to the irreversible final stages of the disease (advanced cirrhosis, hepatocarcinoma and liver transplantation); therefore, also from an economic point of view it is a priority to prevent the evolution of the disease. As shown above, we can then affirm that, assuming a 20% slowdown of the progression of NASH employing appropriate drugs, we could obtain significant savings, ranging from € 162.2 million over a three-year period, to € 2.3 billion up to fifteen years, without a real increase in the global costs of treatment of the globally considered diabetic disease. Moreover, pharmacological treatment should also consider a dual treatment approach (ie: Pioglitazone and GLP1ra, or SLT2i, or both the last ones) that would be cost-effective for the simultaneous presence of diabetes and NASH.
Disclosure
This study was funded by unconditional and exclusive grant from NovoNordisk, Italy. The authors report no other conflicts of interest in this work.
References
1. Younossi ZM, Koenig AB, Abdelatif D, et al. Global epidemiology of nonalcoholic fatty liver disease—meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73–84. doi:10.1002/hep.28431
2. Estes C, Anstee QM, Arias-Loste MT, et al. Modeling NAFLD disease burden in China, France, Germany, Italy, Japan, Spain, United Kingdom, and United States for the period 2016–2030. J Hepatol. 2018;69(4):896–904. doi:10.1016/j.jhep.2018.05.036
3. Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of non-alcoholic fatty liver disease: practice guidance from the American association for the study of liver diseases. Hepatology. 2018;67(1):328–357. doi:10.1002/hep.29367
4. Dyson JK, Anstee QM, McPherson S. Non-alcoholic fatty liver disease: a practical approach to diagnosis and staging. Frontline Gastroenterol. 2014;5:211–218. doi:10.1136/flgastro-2013-100403
5. Adams LA, Lymp JF, St Sauver J, et al. The natural history of non-alcoholic fatty liver disease: a population-based cohort study. Gastroenterology. 2005;129:113–121. doi:10.1053/j.gastro.2005.04.014
6. Younossi ZM, Stepanova M, Afendy M, et al. Changes in the prevalence of the most common causes of chronic liver diseases in the United States from 1988 to 2008. Clin Gastroenterol Hepatol. 2011;9:524–30.e1. doi:10.1016/j.cgh.2011.03.020
7. Younossi ZM, Otgonsuren M, Henry L, et al. Association of non-alcoholic fatty liver disease (NAFLD) with hepatocellular carcinoma (HCC) in the United States from 2004 to 2009. Hepatology. 2015;62:1723–1730. doi:10.1002/hep.28123
8. O’Hara J, Finnegan A, Dhillon H, et al. Cost of non-alcoholic steatohepatitis in Europe and the USA: the GAIN study JHEP Reports. JHEP Rep. 2020;2(5):100142. doi:10.1016/j.jhepr.2020.100142
9. Petta S, Ting J, Saragoni S, et al.; a LHUs group. Healthcare resource utilization and costs of non-alcoholic steatohepatitis patients with advanced liver disease in Italy. Nutr Metab Cardiovasc Dis. 2020;30(6):1014–1022. doi:10.1016/j.numecd.2020.02.016
10. Younossi ZM, Tampi RP, Racila A, et al. Economic and clinical burden of non-alcoholic steatohepatitis in patients with type 2 diabetes in the U.S. Diabetes Care. 2020;43(2):283–289. doi:10.2337/dc19-1113
11. Dai W, Ye L, Liu A, et al. Prevalence of non-alcoholic fatty liver disease in patients with type 2 diabetes mellitus: a meta-analysis. Medicine. 2017;96(39):e8179. doi:10.1097/MD.0000000000008179
12. Younossi ZM, Gramlich T, Matteoni CA, Boparai N, McCullough AJ. Non-alcoholic fatty liver disease in patients with type 2 diabetes. Clin Gastroenterol Hepatol. 2004;2(3):262–265. doi:10.1016/S1542-3565(04)00014-X
13. Tada T, Toyoda H, Sone Y, et al. Type 2 diabetes mellitus: a risk factor for progression of liver fibrosis in middle-aged patients with non-alcoholic fatty liver disease. J Gastroenterol Hepatol. 2019;34(11):2011–2018. doi:10.1111/jgh.14734
14. El-Serag HB, Tran T, Everhart JE. Diabetes increases the risk of chronic liver disease and hepatocellular carcinoma. Gastroenterology. 2004;126(2):460–468. doi:10.1053/j.gastro.2003.10.065
15. Federation, ID. IDF Diabetes Atlas. Federation, ID.
16. Younossi ZM, Golabi P, De Avila L, et al. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: a systematic review and meta-analysis. J Hepatol. 2019;71(4):793–801. doi:10.1016/j.jhep.2019.06.021
17. Husereau D, Drummond M, Petrou S. Consolidated Health Economic Evaluation Reporting Standards (CHEERS)—explanation and elaboration: a report of the ispor health economic evaluation publication guidelines good reporting practices task force. Value Health. 2013;16(2):231–250. doi:10.1016/j.jval.2013.02.002
18. Geodemo Istat popolazione residente 1 gennaio 2021. Available from:https://demo.istat.it/popres/index.php?anno=2021&lingua=ita.
19. Osservatorio ARNO Diabete. Il profilo assistenziale della popolazione con diabete. Available from: https://www.siditalia.it/clinica/linee-guida-societari/send/80-linee-guida-documenti-societari/5025-rapporto-arno-diabete-2019.
20. Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology. 1996;24(2):289–293. doi:10.1002/hep.510240201
21. Hossain N, Afendy A, Stepanova M, et al. Independent predictors of fibrosis in patients with non-alcoholic fatty liver disease. Clin Gastro Enterol Hepatol. 2009;7:1224–1229. doi:10.1016/j.cgh.2009.06.007
22. Tariffe delle prestazioni di assistenza specialistica ambulatoriale. Allegato 3 Ministero della Salute - Direzione generale della programmazione sanitaria. Supplemento ordinario n. 8 alla GAZZETTA UFFICIALE; 2013. Available from: https://www.salute.gov.it/portale/temi/p2_6.jsp?id=3662&area=programmazioneSanitariaLea&menu=vuoto. Accessed 4 September, 2022.
23. Cammarota S, Citarella A, Bernardi FF, et al. Burden of compensated and decompensated cirrhosis: real world data from an Italian population-based cohort study. Eur Rev Med Pharmacol Sci. 2021;25(13):4490–4498. doi:10.26355/eurrev_202107_26240
24. Filipponi F, Pisati R, Cavicchini G, Ulivieri MI, Ferrara R, Mosca F. Cost and outcome analysis and cost determinants of liver transplantation in a European National Health Service hospital. Transplantation. 2003;75(10):1731–1736. doi:10.1097/01.TP.0000063828.20960.35
25. OSMAR [OsservatorioMalattieRare]. Extract from an oral conference report at 4° Congress of the Italian Society for Safety and Quality in Transplantation. Available from: https://www.osservatoriomalattierare.it/politiche-socio-sanitarie/2174-trapianti-dorgano-tanti-in-lista-dattesa-ma-lo-stato-ha-tagliato-il-70-per-cento-dei-fondi.
26. Bril F, Cusi K. Management of non-alcoholic fatty liver disease in patients with type 2 diabetes mellitus: a call to action. Diabetes Care. 2017;40:419–430. doi:10.2337/dc16-1787
27. Carretero Gómez J, Ena J, Seguí Ripoll JM, et al. Effect of newer antihyperglycemic drugs on liver steatosis indices in patients with diabetes and obesity. Curr Med Res Opin. 2021;37(11):1867–1873. doi:10.1080/03007995.2021.1965563
28. Cusi K. A diabetologist’s perspective of non-alcoholic steatohepatitis (NASH): knowledge gaps and future directions. Liver Int. 2020;40(Suppl 1):82–88. doi:10.1111/liv.14350
29. Newsome PN, Buchholtz K, Cusi K, et al. NN9931-4296 Investigators. A placebo-controlled trial of subcutaneous semaglutide in nonalcoholic steatohepatitis. N Engl J Med. 2021;384(12):1113–1124. doi:10.1056/NEJMoa2028395
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
