Back to Journals » Clinical Ophthalmology » Volume 16
Early Real-World Physician Experience with an Intracanalicular Dexamethasone Insert
Authors Matossian C , Stephens JD, Rhee MK, Smith SE, Majmudar PA, Gollamudi SR , Patel RH, Rosselson ME, Bauskar A , Montieth A, Silva FQ, Vantipalli S, Gibson A, Metzinger JL, Goldstein MH
Received 27 April 2022
Accepted for publication 22 July 2022
Published 6 August 2022 Volume 2022:16 Pages 2429—2440
DOI https://doi.org/10.2147/OPTH.S372440
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Cynthia Matossian,1 John D Stephens,2 Michelle K Rhee,3 Stephen E Smith,4 Parag A Majmudar,5 Subba Rao Gollamudi,6 Ravi H Patel,7 Maria E Rosselson,5 Aditi Bauskar,8 Alyssa Montieth,8 Fabiana Q Silva,8 Srilatha Vantipalli,8 Andrea Gibson,8 Jamie Lynne Metzinger,8 Michael H Goldstein8
1CM Associates, LLC, New Hope, PA, USA; 2Tyson Eye, Fort Myers, FL, USA; 3Icahn School of Medicine at Mount Sinai, New York, NY, USA; 4Eye Associates, Fort Myers, FL, USA; 5Chicago Cornea Consultants, Ltd., Chicago, IL, USA; 6Eye Specialty Group, Memphis, TN, USA; 7Eye Associates of Central Texas, Round Rock, TX, USA; 8Ocular Therapeutix, Inc., Bedford, MA, USA
Correspondence: Srilatha Vantipalli, Ocular Therapeutix, Inc, 24 Crosby Drive, Bedford, MA, 01730, USA, Tel +1 413-230-7242, Email [email protected]
Purpose: To describe the early real-world experience of physicians with an intracanalicular dexamethasone insert (DEX) in patients undergoing cataract surgery and to capture the clinical impact of adopting this therapy.
Patients and Methods: 23 United States sites including Ambulatory Surgical Center Setting (ASC) and Outpatient Clinical settings. Respondents were physicians who had early experience with DEX in cataract surgery patients. This was a Phase 4 experiential cross-sectional survey study comprised of 3 sequential online physician surveys. Descriptive statistics summarized the surveys’ responses to determine the early impressions of the respondents.
Results: Forty-two physicians completed surveys. On average, physicians reported feeling comfortable administering DEX after placing 3 inserts (mean 2.7; standard deviation 1.9). Most physicians (92%) were satisfied with DEX, and all physicians (100%) reported that DEX improved patient compliance. Most physicians (62.5%) indicated they would highly prefer DEX over traditional steroid eyedrops for the management of post-surgical inflammation and pain.
Conclusion: The surveys exploring the early use of DEX suggest that DEX is a clinically effective treatment with a rapid initial learning curve and integrates well into clinical use. Physicians had a very positive early experience with DEX, including comfort with insertion and satisfaction. DEX shows promise as a primary treatment choice of physicians for ocular inflammation and pain following cataract surgery by offering patients a hands-free innovative therapy that delivers a preservative-free steroid to the ocular surface over approximately 30 days.
Keywords: intracanalicular dexamethasone insert, phacoemulsification, hands-free therapy, ocular pain, ocular inflammation, sustained-release drug delivery
Introduction
Corticosteroid ophthalmic therapy is commonly used in eye care to manage inflammation after ophthalmic surgeries.1 It is also prescribed for a broad range of conditions, such as ocular surface disease (eg allergic conjunctivitis) and uveitis.1 For postoperative care, eye care professionals typically prescribe steroids in the form of topical ophthalmic eyedrops with a tapered regimen approach as part of the standard of care.1
Corticosteroid drops are fast-acting, effective medications when dosed properly, but they can present challenges for both physicians and patients that may hinder their effectiveness.1 Common adverse events typically associated with the chronic use of corticosteroid drops include elevation of intraocular pressure (IOP) and cataract formation. All FDA-approved corticosteroid eyedrops currently on the market in the United States contain a preservative that can cause toxicity to the ocular surface. Physicians and practices are familiar with many factors that can limit the treatment outcome of topical corticosteroids, such as patient compliance, patient self-administration issues, and burden to patients, physicians, and staff.2–4
Patient compliance is more challenging with corticosteroid drops than other types of drops due to the complex dosing regimen and repeated daily administration.2 For example, in the postoperative period for cataract surgery, patients self-administer 70 corticosteroid eyedrops while tapering over 4 weeks.5 Adding to compliance issues, patients view any eyedrop as a burden.4 A study that used electronic compliance monitoring found that dose compliance in patients on a topical fixed-combination ophthalmic drop averaged ~50% and was <25% in about one-fifth of patients.6 One might assume that compliance is lower with topical corticosteroids after cataract surgery due to the complex tapered dosing regimen and the number of required drops and ophthalmic medications after cataract surgery. Thus, poor patient compliance and increased patient burden with steroid eyedrops are common barriers to ensuring effective treatment outcomes.
Another limiting factor is difficulty with self-administration of eyedrops, particularly in elderly patients.4 Incorrect self-administration occurs in more than 90% of patients after cataract surgery, which can potentially injure the ocular surface and negatively impact surgical recovery. Further, incorrect administration may result in contamination of the bottle, increasing the risk of infection.3 A prospective, cross-sectional study of 54 patients found that 92.6% of patients overestimated their level of compliance and of drop-instillation technique.3
The burden on the practice for time used to educate patients on correct usage is high. Lindstrom et al estimated that 3000 staff hours are spent annually on phone calls related to cataract surgery eyedrops.7 Physician and practice time is devoted to confirming that patients adhere and comply to the dose schedule, training patients on correct and safe self-administration of eyedrops, and addressing potential ocular complications (eg, corneal edema, suboptimal vision, photophobia, increased pain) from non-adherence or poor instillation technique.1,4,8
In recent years, new therapies have become commercially available that address the above-mentioned challenges with corticosteroid eyedrops,9–11 as well as their potential for ocular toxicity due to preservatives12,13 and for limited bioavailability due to quick precorneal elimination and short corneal residence times.14–16 In this study, we focus on DEX, a sustained-release dexamethasone hydrogel-based insert (Dextenza, dexamethasone ophthalmic insert, 0.4 mg; Ocular Therapeutix, Inc., Bedford, MA), that was FDA-approved for intracanalicular use for treating ocular inflammation (2019) and pain (2018), after ophthalmic surgery.10 DEX was designed to replace traditional corticosteroid eyedrops.17 DEX may eliminate patient issues of adherence, compliance, and potential for misuse or overuse with steroid eye drops as it is administered by the physician.
In clinical trials, DEX showed robust inflammation and pain control and clinically relevant efficacy and safety.10 Three Phase 3 randomized trials had positive results in patients who received DEX compared with those who received placebo vehicle immediately upon completion of cataract surgery.19,20 In all three studies, a significantly higher proportion of the group randomized to DEX were pain free on post-operative day 8 compared with the vehicle group.10,19,20 In two of the three studies, the DEX group also had a significantly higher proportion of patients who had an absence of anterior chamber cells on post-operative day 14 compared with the vehicle group.10 In a pooled analyses of a Phase 2 and the three phase 3 trials, anterior chamber inflammation including iritis and iridocyclitis (10% incidence) and increased IOP (6%) were the two most common reactions reported in the DEX group.21 No patient required removal of DEX due to increased IOP.21 DEX was well tolerated, and the severity of the adverse events (AEs) was mild to moderate with <1% of patients withdrawing from the trials due to an AE.21
While the clinical trial data are compelling, actual data on the real-world usage of DEX are important to gather to better understand its use in actual clinical practice. Results from a small real-world study including 12 patients undergoing cataract surgery who received DEX on post-operative day 1 in an office setting were consistent with the data of the phase 3 clinical trials.22 The objective of the present study was to describe the early real-world experience of physicians with DEX in patients undergoing cataract surgery and to capture the impact of adopting this therapy into clinical practice. Focus was given to identifying surgeon expectations, procedural experience, preferences, practice burden, and overall satisfaction with DEX.
Materials and Methods
Intracanalicular Dexamethasone Insert (DEX)
DEX is a hydrogel-based insert that is resorbable and dispenses a preservative-free steroid.17 The insert is approximately 0.55 mm in width and 3.0 mm in length. A physician inserts DEX through the lacrimal punctum into the canaliculus, which occludes the punctum. The insert can be visualized through its conjugation with fluorescein and is removable, if needed.17 It continuously releases a tapered dose of dexamethasone over approximately 30 days onto the surface of the eye,17 which can potentially increase bioavailability (Figure 1). After the drug is eluted over 30 days, the hydrogel insert begins to slowly hydrolyze, eventually liquefying, then clearing the nasolacrimal system by flushing through the nasolacrimal duct.18 In the rare case where removal of the insert is needed, manual expression or saline irrigation can be administered.17
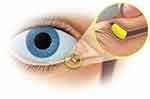 |
Figure 1 Placement of the Dexamethasone Intracanalicular Insert in the canaliculus of the eye. |
Study Development
We considered sites across the United States representative of “standard” cataract surgery practices for this phase 4 study. The inclusion criteria for site selection included geographical diversity, high volume of cataract surgeries, history of industry engagement, and ≥ 2 practicing cataract surgeons. Practices with a single surgeon were excluded. We identified a total of 301 eligible sites. Of those sites, 146 did not respond or declined. Of the remaining 155 sites who responded, 45 sites agreed to participate. From these sites, 23 sites participated. The final sample represented the first 42 physicians to agree and consent to participate. All physicians who used DEX intracanalicular inserts following cataract surgery as part of the study received an online survey. The intracanalicular DEX inserts used by physicians were part of a sampling program.
The study included quantitative surveys for physicians. This study was IRB approved. Advarra, a central institutional review board, approved the surveys, informed consent, and all patient-facing recruiting materials. Electronic informed consent was obtained from all study participants before they received the survey online. HIPAA regulations were followed. Personally identifiable information was not shared as part of the survey; all respondents received a fair market value honorarium ($100 for completed web survey) for their participation. The current study analyzes data from the physician arm, and a separate study will analyze clinical staff and patient data.
Study Design and Survey Intervals
The physician portion of the study consisted of 3 surveys intended to capture evolving DEX experience. Survey 1 was given at baseline, prior to physicians’ first DEX case, and included questions to determine practice profiles and baseline expectations for DEX, including whether patients with comorbidities would benefit from DEX. Survey 2 was distributed to the physicians one day after they completed a minimum of 5 DEX insertions following cataract surgery and included questions to obtain feedback on the initial user experience, including ease of use and satisfaction. Survey 3 was distributed 30 days after the physicians completed at least 5 DEX insertions following cataract surgery and consisted of questions to determine the physicians’ overall experience and satisfaction with DEX regarding patient outcomes and to obtain opinions on conditions and surgeries for which the physician felt DEX was appropriate.
Physicians completed each survey and examined the patients who received DEX at each designated visit. No comanaging doctors participated in the surveys or examined study patients at any pre- or post-surgery visits.
The full design of the study is presented in Figure 2.
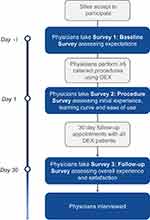 |
Figure 2 Design of the Phase 4 study. |
Survey Sections and Data Analysis
Each physician survey included a screener section to confirm study eligibility and a main section including questions to assess product and product use perceptions. Most survey questions used a 7- or 11-point Likert scale. Treatment satisfaction questions were evaluated on a 7-point scale, typically anchored as 1 = extremely dissatisfied and 7 = extremely satisfied. A minority of questions used multiple-choice, open-ended, or ranking formats. Respondents typically completed the surveys within 15–25 minutes.
The planned analysis included only descriptive statistics that summarized the responses on each item. With no planned comparisons, power calculations were not completed.
Results
Profile of Participating Physicians
A total of 42 ophthalmologists from 23 sites completed Survey 1, 37 completed Survey 2, and 40 completed Survey 3. All surveys were completed sequentially between September 2019 and February 2020. The ophthalmologists were experienced, reporting a mean of 17.0 years in practice, and had completed fellowships in various specialties (Table 1). The mean number of ophthalmologists included in the practices of study physicians was 10.4. Overall, physicians performed a mean of 14.4 cataract surgeries on a typical surgical day (Table 1).
 |
Table 1 Participating Physician Demographics |
The mean percentage of patients who are comanaged with other eye care professionals was 19.8%. Importantly, all patients included in this study were not comanaged. Of surveyed physicians, 83.3% comanaged with an optometrist outside of their practice. Practices that comanaged with an eye care professional had similar visit schedules for cataract patients, including a pre-surgery visit and approximately 4 post-surgery visits (immediately after, on the first postoperative day, 1 week, and 1 month).
In Survey 1 at baseline, physicians were likely (ie, mean score of 5, 6, or 7 on a 7-point Likert scale) to use DEX in patients with challenges taking eye drops, such patients with central nervous system conditions, memory problems (98.0% of physicians report), patients with motor impairment such as shaking (92.9%), and in patients without a care provider at home (95.0%). However, physicians were also likely (83.0%) to use DEX in patients who do not have these situations/comorbidities.
Physician Experience with Administration of DEX
Physicians without prior experience using DEX were surveyed about their experience with administering DEX following a minimum of 5 insertions, which were performed by the physicians either in the operating room or in office at their clinic. Physicians dilated the punctum in almost every patient before insertion of DEX.
Surgeons felt comfortable administering DEX after a mean of 2.7 insertions (range 1 to 10; standard deviation 1.9) (Figure 3A). Most physicians (90%) rated their experience using DEX as comfortable (a score of 5, 6, or 7 on a 7-point Likert scale) (Figure 3B).
Most physicians (87%) also rated their overall experience with DEX administration after initial experience (Survey 2) as easy (a score of 5, 6, or 7 on a 7-point Likert scale) (Figure 3B). Only one physician (3%) rated the overall experience with DEX administration to be difficult, which was due to the insert swelling immediately after contact with moisture.
The physician surveys included questions about the practice’s incremental time burden from DEX. According to most physicians (86%), the use of DEX had little to no incremental burden on staff time and logistics (a score of 5, 6, or 7 on a 7-point Likert scale, with 7 being no burden at all).
After initial experience with DEX insertion (Survey 2), 92% of surveyed physicians were satisfied with using DEX inserts (a score of 5, 6, or 7 on a 7-point Likert scale). Similar perceptions were noted at the conclusion of the study (Survey 3), in which 91% of surveyed physicians were satisfied (Figure 3B). In Survey 2, 92% of physicians described patients as being accepting of receiving DEX (a score of 5, 6, or 7 on a 7-point Likert scale).
Most physicians (78.4%) inserted DEX in the ASC at the end of surgery, while a minority placed DEX at the beginning of surgery (8.1%), immediately before the surgery in the office setting (2.7%), or the next day in the office setting (10.8%) (Table 2).
Patient Demographics
Physicians reported using DEX in patients with various ocular comorbidities. At least one comorbidity was present in 58.0% (155 of 267) of the patient population. The most common comorbidity was history of dry eye disease (30%, n=80). Five patients (2%) who had glaucoma or elevated IOP received DEX (Figure 4).
Physician Preferences for Future Use of DEX
From their initial experience with DEX, physicians noted potential applicability in use of DEX in a variety of ocular surgeries and patients. Most commonly, physician respondents perceived that the patients undergoing pterygium surgery (37.5%), corneal surgery (30.0%), and refractive surgery (27.5%) would benefit from the use of DEX to treat post-surgical inflammation and pain (Table 2).
Attributes ranked as most important when choosing an intracanalicular corticosteroid therapy after cataract surgery in both Survey 1 (at baseline) and Survey 3 (30 days after using DEX) were delivers efficacious steroid, well tolerated, single administration delivers steroid over 30 days, and biodegrades without the need for removal. However, the attributes for which the perceived importance increased by the greatest percentage between Surveys 1 and 3 included benefits of punctal occlusion for the ocular surface (increase of 15% in mean Likert score before and after the DEX experience), steroid can be visualized and monitored (increase of 11% in mean Likert score), steroid treats ocular surface (increase of 10% in mean Likert score), and preservative free (increase of 8% in mean Likert score) (Figure 5).
Based on their early experience with DEX application, physicians were asked to rate various attributes of DEX at the conclusion of the study in Survey 3. Physicians rated DEX as positive (mean score of 5, 6, or 7 on a 7-point Likert scale) regarding several qualities, with patient compliance (mean score of 6.6), FDA approval providing evidence of good safety and tolerability (mean score of 6.6), patient convenience of recovery (mean score of 6.3), and ability to use DEX in a variety of cataract patients (mean score of 6.3) ranking among the highest (Table 3).
 |
Table 3 Rating of the Characteristics of an Intracanalicular Dexamethasone Insert 30 Days After Insertion as Reported by Physicians |
In Survey 3, physicians indicated a preference for DEX to manage post-surgical pain and inflammation over traditional steroid eyedrops, 62.5% of physicians preferred DEX, 22.5% preferred traditional steroid eyedrops, and 15.0% preferred another option (eg, combination treatment, non-steroidal anti-inflammatory agents, or no treatment). Overall, 92.5% of the physicians planned to insert DEX in upcoming cataract surgeries.
Discussion
The data presented here demonstrate that DEX provides real-world practice benefits for physicians. On average, physicians with no prior experience using DEX in cataract surgery patients reported becoming comfortable with inserting DEX after 3 insertions. DEX appears to have a negligible time burden on physicians and staff based on most physicians who reported using DEX was easy (87%) and created little to no incremental burden (86%). Most physicians (91%) new to DEX also reported satisfaction with their experience using DEX. Overall, DEX appears to integrate well into clinical use.
Physicians in our survey expressed a positive shift towards using DEX as their first choice for corticosteroid therapy and were highly satisfied with DEX from the time of insertion to the end of the post-operative period (Figure 3B). Interestingly, physicians placed a higher value on most of the unique attributes of intracanalicular inserts after their experience with DEX compared with before (Figure 5). For example, physicians considered the benefit of punctal occlusion for the ocular surface was “important” before using DEX (average score of 4.8 on a 7-point Likert scale, with 1 being not important at all and 7 being extremely important) but considered this attribute to be even more important after using DEX (average score of 5.5 on a 7-point Likert scale).
DEX offers flexibility for patient selection, timing of insertion (before or after surgery), and type of surgery. The survey results indicate that physicians may choose to use DEX in patients with a variety of comorbidities (eg, dry eye disease, diabetes, diabetic retinopathy), including high-risk patients (eg, with glaucoma or elevated IOP) (Figure 4, Table 2). Importantly, physicians may insert DEX in the operating room or clinic and either before or after surgery, allowing individualization of treatment plans (Table 2).19,20,22 In addition to cataract patients, physicians most often indicated that on-label use of DEX could benefit patients undergoing pterygium surgery, corneal surgery, and refractive procedures (which have been studied in LASIK and refractive lens exchange) (Table 2).23,24 One of the greatest perceived values of DEX according to physicians was its ability to treat the ocular surface with a preservative-free formulation. As might be expected based on the high prevalence of dry eye in the United States,25,26 dry eye was the most common comorbidity of the cataract surgery patients in our study. Given the lack of preservatives, DEX may be of particular utility to manage inflammation and pain in patients with dry eye disease undergoing ophthalmic surgery.20
Eyedrops require a time investment of health care professionals to train patients on proper self-administration techniques, to follow up to ensure compliance, and to address complications that may arise from imperfect technique or noncompliance, the sum of which can be burdensome.4 Patient and pharmacy questions regarding use of substitutions of post cataract surgery eyedrop prescriptions are major drivers of calls to physician offices.7 Additionally, physicians often have difficulty identifying nonadherent patients and may overestimate adherence in clinical trials or clinical practice.28 The difficulty physicians have in accurately identifying nonadherent patients may be due, in part, to patients under-reporting nonadherence.28 Despite the existence of many methods to identify nonadherence, there are no quantitative standards.28 Self-instillation of eyedrops presents with challenges, especially in the elderly population who live alone and are dependent on others to instill eyedrops.29 Issues associated with improper application of eyedrops amongst this population include inability to abduct the shoulder sufficiently, struggle in squeezing the bottle due to arthritis, and difficulty aiming due to tremor and blinking.29
The intracanalicular insertion of DEX represents a modality of ocular drug delivery with a targeted approach that uses a sustained-release platform. It has the potential to become the new standard-of-care for management of post-surgical inflammation and pain, thus making it a true paradigm shift for this indication. DEX is not limited by a number of factors that can affect traditional topical steroid eyedrops, such as poor patient adherence, identification of non-adherent patients, compliance, and self-administration; increased treatment burden on patients, physicians, and staff; potential toxicity of preservatives.2–4,12 They may improve clinic efficiency by decreasing the need for filling a prescription, which is time-efficient for both healthcare providers as well as patients.24
As DEX is physician-administered, it eliminates the potential for improper drop installation techniques, including missing the eye, and bottle tip contamination due to contact with ocular surface, eyelid or lashes.27,30 Their use thus shifts the reliance of administration from patient to physician reducing risks associated with improper instillation technique, and eliminating non-adherence, resulting in better efficacy, and safety outcomes.31 Utilizing sustained-release steroid products has been advocated by multiple surgeons as an important step forward in optimizing therapeutic efficacy and postoperative quality of life in patients undergoing cataract surgery.31
After placement by the physician, DEX continuously delivers a tapered-dose, preservative-free steroid regimen directly to the ocular surface throughout the postoperative period to manage inflammation and pain, likely improving patient compliance by reducing missed doses or incorrect instillation.17 Following a quick learning curve, most physicians quickly gained proficiency and confidence with DEX insertion (Figure 3A). In addition, physicians appreciated that DEX can be visualized after insertion through its conjugation with fluorescein,17 and can be removed if necessary.
As with any survey-based research, our study has limitations. First, the study results presented here represent the early experiences with surgeons following FDA approval but prior to product launch. We limited this study to a select number of ophthalmologists or physicians who had agreed to participate in online surveys. Additionally, site selections for the study were not random. Therefore, the surveyed physicians do not represent the entire population of ophthalmologists in the United States. Our study involved respondents who used DEX in cataract surgery only, so findings may not be generalizable to other surgery types and other ocular conditions. In addition, physicians in this study each treated a relatively low number of patients with DEX. We collected data from three surveys completed by physicians, and any claims about patients or staff in this study were gathered from the perceptions of the surveyed physicians only. Additionally, survey responses may have been limited by the ability of respondents to accurately recall and report data. Future surveys with a larger sample size of physicians and a greater diversity in patient population would be beneficial to the field. As surgeons gain additional experience with the commercially available insert, studies to collect real-world data on the usage of DEX will be useful.
Conclusion
Our findings from short surveys on the preliminary use of DEX demonstrate the perceived convenience and ease of use after a short initial learning curve. Through the experiences of the physicians, the survey brought to light the versatility of DEX in various practice settings, the utility of different insertion timings, and their views on potential use in different surgeries and patient profiles.
Acknowledgments
Study support and data collection was provided by Clinical SCORE. Editorial assistance in the preparation of this article was provided by Mindy Nash, OD, of PenLight Communications and Cecelia Wall, of Wall Medical Writing, LLC. Support for this assistance was provided by Ocular Therapeutix, Inc.
Funding
Funding/support provided by Ocular Therapeutix, Inc.
Disclosure
Funding/support provided by Ocular Therapeutix, Inc. Cynthia Matossian, John D Stephens, Steven E Smith, Parag A Majmudar, Subba Rao Gollamudi, Ravi H Patel, and Maria E Rosselson received support from Ocular Therapeutix, Inc. for their participation in the study. Aditi Bauskar, Alyssa Montieth, Fabiana Q Silva, Srilatha Vantipalli, Andrea Gibson, Jamie Lynne Metzinger, and Michael H Goldstein are employees of Ocular Therapeutix, Inc. Michelle K Rhee reports grants from Ocular Therapeutix, on advisory board for NovaBay and Nevakar, outside the submitted work; and Research support from Ocular Therapeutix. The authors report no other conflicts of interest in this work.
References
1. Weiner G. Savvy steroid use. Eyenet Web site. Available from: https://www.aao.org/eyenet/article/savvy-steroid-use.
2. Newman-Casey PA, Robin AL, Blachley T, et al. The most common barriers to glaucoma medication adherence: a cross-sectional survey. Ophthalmology. 2015;122(7):1308–1316. doi:10.1016/j.ophtha.2015.03.026
3. An JA, Kasner O, Samek DA, Levesque V. Evaluation of eyedrop administration by inexperienced patients after cataract surgery. J Cataract Refract Surg. 2014;40(11):1857–1861. doi:10.1016/j.jcrs.2014.02.037
4. Cataract Surgeons for Improved Eyecare. Analysis of the economic impacts of dropless cataract therapy on Medicare, Medicaid, state governments, and patient costs. Available from: http://stateofreform.com/wp-content/uploads/2015/11/CSIE_Dropless_Economic_Study.pdf.
5. Durezol ® (difluprednate ophthalmic emulsion) [package insert]. East Hanover, New Jersey: Novartis Pharmaceuticals; 2020.
6. Hermann MM, Ustundag C, Diestelhorst M. Electronic compliance monitoring of topical treatment after ophthalmic surgery. Int Ophthalmol. 2010;30(4):385–390. doi:10.4103/2230-973X.100036
7. Lindstrom RL, Galloway MS, Grzybowski A, Liegner JT. Dropless cataract surgery: an overview. Curr Pharm Des. 2017;23(4):558–564. doi:10.2174/1381612822666161129150628
8. Juthani VV, Clearfield E, Chuck RS. Non-steroidal anti-inflammatory drugs versus corticosteroids for controlling inflammation after uncomplicated cataract surgery. Cochrane Database Syst Rev. 2017;7:CD010516. doi:10.1002/14651858.CD010516.pub2
9. Bausch Health Companies Inc. Bridgewater, New Jersey: Bausch + Lomb announces FDA approval of LOTEMAX® SM (lotedprednol etabonate ophthalmic gel) 0.38% for the treatment of postoperative inflammation and pain following ocular surgery [press release]. Available from: https://www.prnewswire.com/news-releases/bausch–lomb-announces-fda-approval-of-lotemax-sm-loteprednol-etabonate-ophthalmic-gel-0-38-for-the-treatment-of-postoperative-inflammation-and-pain-following-ocular-surgery-300800968.html.
10. Ocular Therapeutix. Bedford, MA: ocular Therapeutix announces FDA approval of supplemental new drug application (sNDA) for DEXTENZA® (0.4 dexamethasone intracanalicular insert for ophthalmic use) for the treatment of ocular inflammation following ophthalmic surgery [p ress release]. Available from: https://ocutx.gcs-web.com/node/9551/pdf.
11. EyePoint Pharmaceuticals. Watertown, MA: eyePoint Pharmaceuticals announces U.S. commercial launch of DEXYCU (dexamethasone intraocular suspension) 9% [press release]. Available from: https://www.globenewswire.com/news-release/2019/03/12/1752163/0/en/EyePoint-Pharmaceuticals-Announces-U-S-Commercial-Launch-of-DEXYCU-dexamethasone-intraocular-suspension-9.html.
12. Porela-Tiihonen S, Kokki H, Kaarniranta K, Kokki M. Recovery after cataract surgery. Acta Ophthalmol. 2016;94(Suppl 2):1–34. doi:10.1111/aos.13055
13. Goldstein MH, Silva FQ, Blender N, Tran T, Vantipalli S. Ocular benzalkonium chloride exposure: problems and solutions. Eye. 2021:1–8. doi:10.1038/s41433-41021-01668-x
14. Gaudana R, Ananthula HK, Parenky A, Mitra AK. Ocular drug delivery. AAPS J. 2010;12(3):348–360. doi:10.1208/s12248-010-9183-3
15. Kushwaha SK, Saxena P, Rai A. Stimuli sensitive hydrogels for ophthalmic drug delivery: a review. Int J Pharm Investig. 2012;2(2):54–60. doi:10.4103/2230-973X.100036
16. Saettone M. Progress and problems in ophthalmic drug delivery. In: Business Briefing: Pharmatechnology. World Markets Research Centre; 2002.
17. DEXTENZA [package insert]. Bedford, MA: Ocular Therapeutix, Inc; 2018.
18. Tyson SL, Campbell P, Biggins J, et al. Punctum and canalicular anatomy for hydrogel-based intracanalicular insert technology. Ther Deliv. 2020;11(3):173–182. doi:10.4155/tde-2020-0010
19. Tyson SL, Bafna S, Gira JP, et al. Multicenter randomized phase 3 study of a sustained-release intracanalicular dexamethasone insert for treatment of ocular inflammation and pain after cataract surgery. J Cataract Refract Surg. 2019;45(2):204–212. doi:10.1016/j.jcrs.2018.09.023
20. Walters T, Bafna S, Vold S, et al. Efficacy and safety of sustained release dexamethasone for the treatment of ocular pain and inflammation after cataract surgery: results from two phase 3 studies. J Clin Exp Ophthalmol. 2016;7(4):572. doi:10.4172/2155-9570.1000572
21. Lee A, Blair HA. Dexamethasone intracanalicular insert: a review in treating post-surgical ocular pain and inflammation. Drugs. 2020;80(11):1101–1108. doi:10.1007/s40265-020-01344-6
22. Stephenson PDG. Real-world evaluation of postoperative in-office Dexamethasone intracanalicular insert administration for control of postoperative inflammation following cataract surgery. J Clin Ophthalmol. 2020;4(3):273–278.
23. Greenwood MD, Gorham RA, Boever KR. A randomized fellow-eye clinical trial to evaluate patient preference for dexamethasone intracanalicular insert or topical prednisolone acetate for control of postoperative symptoms following bilateral femtosecond laser in site keratomileusis (LASIK). Clin Ophthalmol. 2020;14:2223–2228. doi:10.2147/OPTH.S265311
24. Larsen J, Whitt T, Parker B, Swan R. A randomized, controlled, prospective study of the effectiveness and safety of an intracanalicular dexamethasone ophthalmic insert (0.4 mg) for the treatment of post-operative inflammation in patients undergoing refractive lens exchange (RLE). Clin Ophthalmol. 2021;15:2211–2217. doi:10.2147/OPTH.S311070
25. Farrand KF, Fridman M, Stillman IO, Schaumberg DA. Prevalence of diagnosed dry eye disease in the United States among adults aged 18 years and older. Am J Ophthalmol. 2017;182:90–98. doi:10.1016/j.ajo.2017.06.033
26. Stapleton F, Alves M, Bunya VY, et al. TFOS DEWS II epidemiology report. Ocul Surf. 2017;15(3):334–365. doi:10.1016/j.jtos.2017.05.003
27. Cegielska O, Sajkiewicz P. Targeted drug delivery systems for the treatment of glaucoma: most advanced systems review. Polymers. 2019;11(11):1742. doi:10.3390/polym11111742
28. Robin AL, Muir KW. Medication adherence in patients with ocular hypertension or glaucoma. Expert Rev Ophthalmol. 2019;14(4–5):199–210. doi:10.1080/17469899.2019.1635456
29. Burns E, Mulley GP. Practical problems with eye-drops among elderly ophthalmology outpatients. Age Ageing. 1992;21(3):168–170. doi:10.1093/ageing/21.3.168
30. Tatham AJ, Sarodia U, Gatrad F, Awan A. Eye drop instillation technique in patients with glaucoma. Eye. 2013;27(11):1293–1298. doi:10.1038/eye.2013.187
31. Foster B. Same-day versus next-day dexamethasone intracanalicular insert administration for inflammation and pain control following cataract surgery: a retrospective analysis. Clin Ophthalmol. 2021;15:4091–4096. doi:10.2147/OPTH.S335764
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.