Back to Journals » International Journal of General Medicine » Volume 14
Early Prediction for Persistent Inflammation-Immunosuppression Catabolism Syndrome in Surgical Sepsis Patients
Authors Zhong M, Pan T, Sun NN, Tan RM, Xu W, Qiu YZ, Liu JL, Chen EZ, Qu HP
Received 28 July 2021
Accepted for publication 30 August 2021
Published 9 September 2021 Volume 2021:14 Pages 5441—5448
DOI https://doi.org/10.2147/IJGM.S331411
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Ming Zhong,1,* Tingting Pan,1,* Na-Na Sun,1 Ruo-Ming Tan,1 Wen Xu,1 Yu-Zhen Qiu,1 Jia-Lin Liu,1 Er-Zhen Chen,2 Hong-Ping Qu1
1Department of Critical Care Medicine, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, People’s Republic of China; 2Department of Emergency Medicine, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Hong-Ping Qu
Department of Critical Care Medicine, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, People’s Republic of China
Email [email protected]
Er-Zhen Chen
Department of Emergency Medicine, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, People’s Republic of China
Email [email protected]
Objective: To find the predictors for persistent inflammation-immunosuppression catabolism syndrome in ICU surgical septic patients.
Design: Single center observation study.
Participants: Inclusion: 1) patients ≥ 18, 2) admitted to the ICU after major surgery or transferred to the ICU within 48 hours after the diagnosis of sepsis following the definition of sepsis-3.0. Exclusion: 1) pregnant or lactating patients, 2) patients with severe immune deficiency, 3) patients that expired within 14 days after the diagnosis of sepsis.
Results: A total of 169 participants were included. After propensity score matching, PICS patients were found to have higher intensive care unit (ICU) mortality (32.4% vs 12.4%, p=0.046), 90-day mortality (32.4% vs 9.1%, p=0.006), and ICU-acquired infection rate (44.1% vs 12.7%, p< 0.001), and longer ICU stays (29 vs 11 days, p< 0.001) comparing to non-PICS patients. In multivariate logistic regression, it demonstrated that the SOFA score, Charlson co-morbidity index (CCI), albumin level on the ICU day 1, and lymphocyte count on the ICU day 3 were statistically significant. Sensitivity analysis was conducted with the receiver operating characteristic curve for a combination of the four parameters and the area under the curve was 0.838 (95% confidence interval 0.774– 0.901).
Conclusion: The chronic disease condition and decreased immunity in the early course of sepsis were crucial for PICS. The combination of CCI, SOFA score, albumin level on ICU Day 1 and lymphocyte count on ICU Day 3 can be early predictor for PICS.
Keywords: sepsis, persistent inflammation-immunosuppression catabolism syndrome
Introduction
Sepsis is one of the most devastating disease processes and contributes to a high mortality in the intensive care unit (ICU).1–3 Surgical sepsis, defined as sepsis that requires surgical intervention for source control or sepsis within 14 days of a surgical procedure, is recognized as an engine for the development of postoperative morbidity and mortality.4
The rate of surgical sepsis increased from 0.7% in 1997 to 1.3% in 2006, and the rate of severe surgical sepsis increased from 0.3% in 1997 to 0.9% in 2006.5 Although the incidence of sepsis in surgical patients is increasing,1,3 the overall outcome of sepsis has greatly improved over the last 20 years with the implementation of evidence-based ICU management of diseases.6,7 However, in a study on the long-term outcomes of sepsis, one-third of patients with sepsis developed a chronic critical illness, and these patients were at a high risk of poor function and death over 1-year period.8
Chronic critical illness is clinically characterized as persistent but manageable organ failure, impaired life activity, mechanical ventilation dependence, malnutrition, prolonged ICU stay, and eventually death.9 The underlying pathophysiologic syndrome was termed persistent inflammation-immunosuppressive catabolism syndrome (PICS).10 Patients surviving sepsis with PICS have impaired physical function, need high resources to support their lives, and are usually with irreversibly poor long-term outcomes.11 PICS has been reported in patients with major trauma, pancreatitis, and intestinal fistula, but minimal data exist on patients with sepsis associated with major surgery.12–14
Unfortunately, although PICS has received more attention regarding the management of surgical patients with septic complications, methods to predict its occurrence and change the course of the disease remain unclear. According to the definition, PICS are diagnosed over 14 days after sepsis onset, so it is crucial to find risk factors to recognize this population and apply strategy to prevention. The purpose of this report is to describe the character of PICS patients in the early stage of sepsis and to seeking risk factors to predict the PICS.
Methods
Study Design
We performed this study by review the electronic medical records involving surgical patients admitted to our 30-bed intensive care unit from January 2015 to June 2019 at a teaching hospital. This study was conducted in accordance with the Declaration of Helsinki. The study design and possible ethic issues related to this study were reviewed and approved by the ethics committee of Ruijin Hospital affiliated Shanghai Jiao Tong University School of Medicine.
Patient Eligibility
The inclusion criteria were set as: patients must be 18 years old or older and were admitted to the ICU after major surgery or transferred to the ICU within 48 hours after the diagnosis of sepsis following the definition of sepsis-3.0 from a surgical department. The exclusion criteria were any of the following: pregnant or lactating patients, patients with severe immune deficiency, and patients that expired within 14 days after the diagnosis of sepsis.
Severe immune deficiency15 was considered in this study if the patient received chemotherapy in the past 28 days, had a blood neutrophil count less than 1x109/L, took a dose of prednisone greater than 20 mg per day or an equivalent dose of other steroids for more than 14 days, had HIV infection, underwent transplantation, or had active tuberculosis.
Definition of Sepsis and PICS
In this study, sepsis was defined according to the Sepsis-3.0 guidelines16 and surgical sepsis was delimited as sepsis requiring surgical intervention for source control or sepsis within 14 days after a surgical procedure.4
PICS was diagnosed according to the following criteria: On the day of PICS diagnosis, the patient’s ICU stay ≥14 days with a body weight reduction of 10%, body mass index (BMI) of 18 or less, blood C-reactive protein level of 150 ug/dL or above, blood lymphocyte count of 0.8x109/L or less, pre-albumin level of 100 mg/L or less, and albumin level of 30 g/L or less.17 An ICU-acquired infection was defined as an infection (or suspected infection) that occurred 48 hours after ICU admission.
Data Collection
The ICU admission demography was collected from a review of medical records including the age of the patients, gender, body weight, diagnosis at ICU admission, reasons for ICU admission, chronic co-morbidities, and BMI. Laboratory parameters comprising the blood neutrophil count, blood lymphocyte count, C-reactive protein levels, albumin levels, pre-albumin levels, and procalcitonin levels measured on post-ICU admission days 1, 3, and 7 were also extracted from the medical records.
Upon admission to the ICU, the sequential organ failure assessment (SOFA) score, acute physiology and chronic health evaluation (APACHE II) score, and CCI were all calculated such that they could be obtained from the medical record review. For evaluation of the outcome, the data were further stratified into ICU mortality, ICU stay duration in days, hospital mortality, hospital stay duration in days, 90-day mortality, 28-day mortality, and the incidence of ICU-acquired infection.
Statistical Analysis
SPSS version 22.0 (IBM) was used for propensity score matching (PSM) and all calculations in this study. PSM was used to reduce the bias in measuring patient’s outcome. Data are presented as frequency, percentage, mean with standard deviation, median, or the 25th/75th percentiles. Fisher’s exact test and the Kruskal–Wallis test were used for comparison of the categorical and continuous variables, respectively. Statistical significance was defined as a two-tailed P value of less than 0.05.
In the PSM, the patients were matched at a ratio of one PICS patient to two non-PICS patients based on age, CCI, BMI, gender, and SOFA score. Multivariate stepwise logistic regression models were created to determine the independent risk factors (determinable within 72 hours of sepsis onset) for the development of PICS. The variables included in the models were those with statistical significance in the univariate analysis or clinical relevance. Diagnostic performance was evaluated using the area under the curve (AUC). Lastly, the Kaplan-Meier curve was used to evaluate survival.
Results
Demographics
Following the study design, a total number of 169 patients were enrolled in this study with a mean age of 65 years, a median BMI of 22.85, and a ratio of men versus women of 2.6:1. Among the 169 patients, 146 (86.4%) underwent gastrointestinal surgery before the development of PICS. The top three infection sources for sepsis were intra-abdominal infections (56%), lung infections (11.83%), and a combination of hematogenous infection and intra-abdominal infection (10.64%) (Table 1). The most common deteriorated organ in septic patients was the lung (60.95%), followed by the liver (41.42%). Malignancies were diagnosed in 46% of patients.
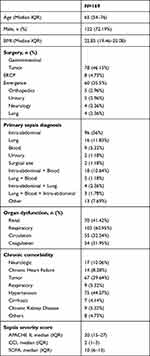 |
Table 1 Characteristics of Patients |
Mortality of PICS Patients
PICS was diagnosed in 27.8% (47/169) of the included patients. Before PSM, patients with PICS possessed higher APACHE II scores (22 vs 19.5), SOFA scores (11 vs 9), and CCI (4 vs 2) than non-PICS patients. Moreover, in comparison with non-PICS patients, PICS patients not only had higher ICU (36.2% vs 8.2%), 28-day (10.6% vs 1.6%), and 90-day (38.3% vs 6.6%) mortality, they also stayed in the ICU longer (29 vs 12 days). Using PSM, the patients were matched with a 1:2 ratio (PICS vs non-PICS) based on age, CCI, BMI, gender, and SOFA score. After matching, 89 patients were included in each cohort, and the PICS patients were found to have higher ICU mortality (32.4% vs 12.4%), 90-day mortality (32.4% vs 9.1%), and ICU-acquired infection rate (44.1% vs 12.7%) (Table 2). The Kaplan-Meier curve showed that the PICS patients had particularly high 90-day mortality (Figure 1).
 |
Table 2 Propensity Score Matching and Outcomes for Patients |
Laboratory Evidence for PICS
Patients with PICS displayed a lower albumin level (25 vs 28 g/L), a lower lymphocyte count (0.6 vs 0.7 x109/L) on the first day after ICU admission, and a lower lymphocyte count (0.6 vs 0.8 *109/L) and neutrophil to lymphocyte ratio (NLR) (16.9 vs 10.5) on the third day compared to the non-PICS patients (Table 3).
 |
Table 3 Laboratory Parameters of Patients |
Prediction of the Development of PICS
Univariate logistic regression was first performed to compare the laboratory results between patients with and without PICS. Afterward, multivariate logistic regression was performed on the APACHE II score, CCI, SOFA score, albumin level on day 1, lymphocyte counts on days 1 and 3, and NLR on day 3 after ICU admission to determine their statistical significance. The results showed that the albumin level on day 1, lymphocyte count on day 3, SOFA score, and CCI were statistically significant (Table 4). Lastly, sensitivity analysis was performed with the receiver operating characteristic (ROC) curve for the CCI, SOFA score, albumin level on day 1, lymphocyte count on day 3, and a combination of the four parameters. The results showed that the AUC of the combined predictors was 0.838 (95% confidence interval 0.774–0.901) (Table 4).
 |
Table 4 The Result of Logistic Regression and ROC Curve |
Patient and Public Involvement
Patients and members of the public were not involved in the design of this study.
Discussion
A substantial portion of surgical patients who were admitted to the ICU for septic complications progressed to CCI, which can subsequently develop into PICS. Gentile et al17 first characterized PICS clinically as: ICU stay > 14 days, C-reactive protein > 150 Kg/dL, total lymphocyte count < 0.80 × 109/L, weight loss > 10% during hospitalization or BMI < 18, creatinine height index < 80%, albumin level < 3.0 g/dL, pre-albumin level < 10 mg/dL, and retinol-binding protein level < 10 µg/dL. Following these criteria, we identified 47 PICS patients from among 1452 patients admitted to our ICU over a period of 4 years.
In this study, we found that PICS patients had higher incidences of ICU mortality, 28-day and 90-day mortality, and ICU-acquired infection. We further identified the CCI, SOFA score, albumin level on the first day, and lymphocyte count on the third day following ICU admission as risk factors for the development of PICS. We confirmed that the combination of the CCI, SOFA score, albumin level on day 1, and lymphocyte count on day 3 was a prognostic predictor for PICS. Taken together, PICS is a deteriorating syndrome that increases the mortality rate in surgical patients suffering from sepsis, which is consistent with the findings in previous reports involving septic patients with major trauma, pancreatitis, or intestinal fistula.9–11
One of the predominant pathophysiologies of PICS is multiple immunological determinants, which can subsequently promote adaptive immunosuppression. We found our PICS patients not only had a higher incidence of ICU-acquired infections, also displayed lower lymphocyte count on the third day after the onset of sepsis, indicating the existence of immune deficiency in the early stages of sepsis, which is consistent with the recent understanding of sepsis and PICS,7 and suggesting that the lymphocyte count on the third day can be accepted as a surrogate predictor to for the occurrence of PICS in the early stage of sepsis. Myeloid-Derived Suppressor Cells (MDSCs), a cluster of cells with immunosuppressive functions, are believed to play an important role in the development of PICS.17 Some unidentified genes involved in immune suppression may also be expressed early in the development of PICS.18 But neither MDCS nor genes are now available clinically in ICU. The lymphocyte count on the 14th day after ICU admission is one of the major criteria for PICS diagnosis. In our study, the lymphocyte count on the 3rd day can be used for predicting the PICS occurrence in the early stage of sepsis.
Charlson comorbidity index was widely used to describe the underline chronic comorbidities of patients.19 Chronic conditions had been considered to associate with immune insufficiency to infection in pathological backgrounds such as tumors, and diabetes,20 COPD,21 chronic heart disease.22 Thereby, septic patients with higher Charlson comorbidity index had more complicated outcome,23,24 which is reasonable in the case of PICS.
SOFA score was used as another mean to evaluate and stratify sepsis.16 The higher the score, the greater severity of sepsis was. In our study, SOFA score on ICU admission was suggested to be a predictor for the onset of PICS, indicating the extent of severity of sepsis projects to the dysfunction of organs and immune system in the early stage, which may expedite the complex pathologic process of PICS.
Albumin and pre-albumin levels are widely used to reflect catabolism, but pre-albumin may be more associated with inflammation.25 Our study showed a significantly lower level of albumin on the first day after ICU admission in the PICS group. This finding was consistent with the catabolic nature of PICS and indicated that catabolism occurs in the early phase of sepsis long before the onset of PICS.
In order to build a model to predict the onset, development, and prognosis of PICS, four parameters were selected to reflect the differences in the pathophysiological nature of the syndrome. SOFA indicates the severity of sepsis, the CCI reflects the comorbidities with the primary illness, the lymphocyte count indicates the status of early immunosuppression, and the albumin level is associated with the extent of catabolism. Each of these four parameters showed the ability to predict PICS, but the results from our study suggested that a combination of the four parameters has an even better predictive capacity for the development of PICS.
Our study has several limitations. First, it is a single-center cohort study with a small sample size, which can unavoidably generate sampling bias. Factually, only a few reported studies on PICS can be found in the literature, and none of them estimated the incidence of PICS in critically ill patients in the ICU. Thus, we were unable to predict the sample sizes for our study. Second, since the pathogenesis and pathophysiological nature of PICS still require further clarification, the parameters and biomarkers that were selected as surrogate predictors for the development of the disease may not truly reflect the complicated nature of PICS. Third, tumor cachexia have similar clinical manifestations to patients with PICS, and the inclusion of tumor surgery patients (nearly 40%) may have an impact. In our study, the BMIs of the patients were 22.85 (19.46–25.08). This showed there is few patient with tumor cachexia, and it seemed to have little impact on our result. We thought further study needed to further understand PICS in such population. Thus, we advocate more well-designed studies, such as randomized controlled trials with a larger number of participants to improve the understanding of the pathogenesis and pathophysiological characteristics of PICS as well as for other related diagnostic and therapeutic issues.
Conclusion
In this study, we found that the chronic disease condition and decreased immunity in the early course of sepsis were crucial contributing factors to the development of PICS. A combination of the CCI, SOFA score, albumin level on the first day, and lymphocyte count on the third day following ICU admission can be a potential predictor for the development of PICS.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding author Hong-ping Qu on reasonable request.
Ethics Approval and Consent to Participate
The study protocol was approved by the Ruijin Hospital Ethics Committee of Shanghai Jiaotong University School of Medicine, China.
Formal informed consent was obtained from the patients or their next of kin.
Consent for Publication
Consent for publication was obtained from the all the authors.
Acknowledgments
The study was conducted thanks to the helpful contributions of all the ICU staff and understanding and love of all the family members.
Funding
There is no funding to report.
Disclosure
The authors declare that they have no competing interests.
References
1. Rudd KE, Johnson SC, Agesa KM, et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: analysis for the Global Burden of Disease Study. Lancet. 2020;395(10219):200–211. doi:10.1016/S0140-6736(19)32989-7
2. Xie J, Wang H, Kang Y, et al. The epidemiology of sepsis in Chinese ICUs: a National Cross-Sectional Survey. Crit Care Med. 2020;48(3):e209–e18. doi:10.1097/CCM.0000000000004155
3. Rhee C, Dantes R, Epstein L, et al. Incidence and trends of sepsis in US hospitals using clinical vs claims data, 2009–2014. JAMA. 2017;318(13):1241–1249. doi:10.1001/jama.2017.13836
4. White LE, Chaudhary R, Moore LJ, et al. Surgical sepsis and organ crosstalk: the role of the kidney. J Surg Res. 2011;167(2):306–315. doi:10.1016/j.jss.2010.11.923
5. Bateman BT, Schmidt U, Berman MF, et al. Temporal trends in the epidemiology of severe postoperative sepsis after elective surgery: a large, nationwide sample. Anesthesiology. 2010;112:917–925. doi:10.1097/ALN.0b013e3181cea3d0
6. Milano PK, Desai SA, Eiting EA, et al. Sepsis bundle adherence is associated with improved survival in severe sepsis or septic shock. West J Emerg Med. 2018;19:774–781. doi:10.5811/westjem.2018.7.37651
7. van Zanten AR, Brinkman S, Arbous MS, et al. Guideline bundles adherence and mortality in severe sepsis and septic shock. Crit Care Med. 2014;42:1890–1898. doi:10.1097/CCM.0000000000000297
8. Brakenridge SC, Efron PA, Cox MC, et al. Current epidemiology of surgical sepsis: discordance between inpatient mortality and 1-year outcomes. Ann Surg. 2019;270(3):502–510. doi:10.1097/SLA.0000000000003458
9. Stortz JA, Murphy TJ, Raymond SL, et al. Evidence for persistent immune suppression in patients who develop chronic critical illness after sepsis. Shock. 2018;49(3):249–258. doi:10.1097/SHK.0000000000000981
10. Mira JC, Gentile LF, Mathias BJ, et al. Sepsis pathophysiology, chronic critical illness, and persistent inflammation-immunosuppression and catabolism syndrome. Crit Care Med. 2017;45(2):253–262. doi:10.1097/CCM.0000000000002074
11. Gardner AK, Ghita GL, Wang Z, et al. The development of chronic critical illness determines physical function, quality of life, and long-term survival among early survivors of sepsis in surgical ICUs. Crit Care Med. 2019;47(4):566–573. doi:10.1097/CCM.0000000000003655
12. Xiao W, Mindrinos MN, Seok J, et al. A genomic storm in critically injured humans. J Exp Med. 2011;208(13):2581–2590. doi:10.1084/jem.20111354
13. Yang N, Li B, Ye B, et al. The long-term quality of life in patients with persistent inflammation-immunosuppression and catabolism syndrome after severe acute pancreatitis: a retrospective cohort study. J Crit Care. 2017;42:101–106. doi:10.1016/j.jcrc.2017.07.013
14. Hu D, Ren J, Wang G, et al. Persistent inflammation-immunosuppression catabolism syndrome, a common manifestation of patients with enterocutaneous fistula in intensive care unit. J Trauma Acute Care Surg. 2014;76:725–729. doi:10.1097/TA.0b013e3182aafe6b
15. Rubin LG, Levin MJ, Ljungman P, et al. 2013 IDSA clinical practice guideline for vaccination of the immunocompromised host. Clin Infect Dis. 2014;58:309–318. doi:10.1093/cid/cit816
16. Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016;315(8):801–810. doi:10.1001/jama.2016.0287
17. Gentile LF, Cuenca AG, Efron PA, et al. Persistent inflammation and immunosuppression: a common syndrome and new horizon for surgical intensive care. J Trauma Acute Care Surg. 2012;72(6):1491–1501. doi:10.1097/TA.0b013e318256e000
18. Vanzant EL, Lopez CM, Ozrazgat-Baslanti T, et al. Persistent inflammation, immunosuppression, and catabolism syndrome after severe blunt trauma. J Trauma Acute Care Surg. 2014;76:
19. Brusselaers N, Lagergren J. The Charlson Comorbidity Index in registry-based research. Methods Inf Med. 2017;56:401–406. doi:10.3414/ME17-01-0051
20. Price CL, Knight SC. Methylglyoxal: possible link between hyperglycaemia and immune suppression? Trends Endocrinol Metab. 2009;20(7):312–317. doi:10.1016/j.tem.2009.03.010
21. Bozinovski S, Vlahos R, Anthony D, et al. COPD and squamous cell lung cancer: aberrant inflammation and immunity is the common link. Br J Pharmacol. 2016;173(4):635–648. doi:10.1111/bph.13198
22. Zhang Y, Bauersachs J, Langer HF. Immune mechanisms in heart failure. Eur J Heart Fail. 2017;19(11):1379–1389. doi:10.1002/ejhf.942
23. Sinapidis D, Kosmas V, Vittoros V, et al. Progression into sepsis: an individualized process varying by the interaction of comorbidities with the underlying infection. BMC Infect Dis. 2018;18(1):242. doi:10.1186/s12879-018-3156-z
24. Furman D, Campisi J, Verdin E. Chronic inflammation in the etiology of disease across the life span. Nat Med. 2019;25(12):1822–1832. doi:10.1038/s41591-019-0675-0
25. Berger MM, Reintam-Blaser A, Calder PC, et al. Monitoring nutrition in the ICU. Clin Nutr. 2019;38(2):584–593. doi:10.1016/j.clnu.2018.07.009
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

