Back to Journals » Clinical Ophthalmology » Volume 16
Early Onset of Neovascular Glaucoma After Intra-Arterial Thrombolysis for Central Retinal Artery Occlusion: A Possible Complication?
Authors Furashova O , Thalwitzer J, Matthé E
Received 11 February 2022
Accepted for publication 11 April 2022
Published 22 April 2022 Volume 2022:16 Pages 1235—1244
DOI https://doi.org/10.2147/OPTH.S362019
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Olga Furashova,1 Joerg Thalwitzer,2 Egbert Matthé3
1Department of Ophthalmology, Klinikum Chemnitz gGmbH, Chemnitz, Germany; 2Department of Neuroradiology, Klinikum Chemnitz gGmbH, Chemnitz, Germany; 3Department of Ophthalmology, University Hospital Carl Gustav Carus, Technische Universität Dresden, Dresden, Germany
Correspondence: Olga Furashova, Department of Ophthalmology Klinikum Chemnitz gGmbH, Flemmingstrasse 2, Chemnitz, 09116, Germany, Tel +49 371 333 33230, Fax +49 371 333 33223, Email [email protected]
Purpose: To report on four cases of central retinal artery occlusion (CRAO) treated with intra-arterial thrombolysis with early onset neovascular glaucoma in the further disease course.
Patients and Methods: Retrospective analysis of the medical records of six consecutive patients treated with intra-arterial thrombolysis for CRAO of which four developed neovascular glaucoma.
Results: All six patients were diagnosed with acute CRAO and treated with intra-arterial thrombolysis 4.5– 6 hours after symptom onset. The patients had no significant carotid artery stenosis and unremarkable ophthalmic history. No visual improvement could be achieved after treatment. Four to seven weeks after CRAO onset, four of these patients developed severe painful neovascular glaucoma.
Conclusion: Early onset of aggressive neovascular glaucoma following intra-arterial thrombolysis for CRAO might be a complication of CRAO itself, still possible association with intra-arterial thrombolysis in our patients should be discussed.
Keywords: neovascular glaucoma, retinal artery occlusion, intra-arterial thrombolysis
Introduction
The causative relationship of neovascular complications after retinal artery occlusion (RAO) is still a matter of debate for ophthalmologists worldwide. In retinal vein occlusion, resultant neovascular disease of the eye is common due to increased production of the vascular endothelial growth factor (VEGF) in hypoxic retina cells.1 In RAO, it is suggested that infarcted retina is not able to produce any VEGF and the cases of neovascular glaucoma after RAO always involve an underlying chronic ischemic condition besides RAO itself. Hayreh et al analyzed the natural history of 64 central RAO (CRAO) eyes, where all 11 NVG cases developed due to severe carotid artery stenosis.2 The authors concluded that there is no causative relationship between CRAO and neovascular complications.
However, since then many reports have been published describing ocular neovascularization (ONV) signs after RAO without any evidence for underlying chronic ocular ischemic syndrome. Degoumois et al reported on five cases of NVG after CRAO with normal carotid artery and ophthalmic artery status.3 A large retrospective review of 214 CRAO eyes revealed a 10.9% incidence of iris neovascularization (NVI) with normal carotid artery status in half of all NVI cases.4 Furthermore, the authors observed a statistically significant difference in reperfusion rate and prevalence of diabetes in the group with NVI development.
Regarding the time of onset of ocular neovascularization following CRAO, the study reports vary from 2 weeks to 2 years after CRAO diagnosis, while the majority of cases occur between 8 and 12 weeks after CRAO.5,6
Although there have already been some large retrospective reviews and even prospective studies on the natural course of RAO and the development of its possible complications like ocular neovascularization, little is known about the influence of thrombolysis therapy in acute RAO on further course of the disease. We describe six cases of acute CRAO treated with intra-arterial thrombolysis, four of which developed early aggressive neovascular glaucoma requiring several surgeries. Although there have been multiple trials in the past testing the benefit of intra-arterial thrombolysis after CRAO,7–9 numbers of this magnitude have not been reported.
Methods
Rationale for Intra-Arterial Thrombolysis (IAT)
Currently, there is no accepted or approved therapy for non-arteritic central retinal artery occlusion. Intra-arterial thrombolysis has been discussed, analyzed and examined broadly7–9 and is being performed in hospitals equipped with the necessary technology and personnel, if the patient’s history and general health make him eligible. Since 2021, it is possible to offer this off-label treatment to our own patients in cooperation with the Department of Neuroradiology. Prior to procedure, patients are informed about the off-label character as well as possible risks and benefits of intra-arterial or intravenous thrombolysis. In case of agreement, intra-arterial thrombolysis is performed as described later.
Patient Selection
We conducted a retrospective monocentric analysis of medical records of six consecutive patients treated with intra-arterial thrombolysis (IAT) for acute nonarteritic central retinal artery occlusion between March 2021 and February 2022. Four of these six patients developed severe neovascular glaucoma (NVG), which required several surgeries.
The present study adhered to the tenets of the Declaration of Helsinki and has been approved by the Institutional Review Board of Technische Universität Dresden (Dresden, Germany). Informed patients’ consent was waived because of the retrospective design and because no study-related investigations were necessary. The investigation has been registered in ClinicalTrials.gov (ClinicalTrials.gov Identifier NCT03061526).
IAT Protocol
Guidelines of the German Neurological Society from treatment of ischemic stroke are the basis for any decisions, indications and contraindications. CRAO is treated as an ischemic stroke. The decision to perform or not perform thrombolysis – and whether to perform it intra-arterially or intravenously – is made by the Departments of Neurology and Neuroradiology according to the guidelines.
An interventional neuroradiologist (J.T.) performed selective digital subtraction angiography (DSA) under local anesthesia. Nonionic contrast medium (Ultravist 300) was used in all cases, and biplane angiograms were obtained. The microcatheter (Excelsior SL-10 45°; Rebar-18) was placed in the proximal segment of the ophthalmic artery, and up to 30 mg recombinant tissue plasminogen activator (rtPA) was slowly injected by hand. Additionally, heparin was administered intravenously during the procedure (2500–5000 IE). An ophthalmologist checked visual acuity and performed funduscopic examination to evaluate retinal changes during the IAT.
Ophthalmic Examination
Before any treatment was done, a comprehensive ophthalmic examination was performed including best-corrected visual acuity (BCVA), applanation tonometry, slit-lamp biomicroscopy, indirect binocular ophthalmoscopy, color fundus photography and SD-OCT imaging (Spectralis®, Heidelberg Engineering Inc., Heidelberg, Germany). SD-OCT examination was performed using Spectralis OCT. The macula was scanned with an acquisition speed of 40,000 A-scans per second using “fast macular volume” protocol, consisting of a 25-line horizontal raster scan covering 20° × 20° centered on the fovea with standard nine frames. The eye tracking system (ART Module, Heidelberg Engineering Inc.) was used to minimize motion artifacts. Because time is crucial when trying to perform lysis therapy, no fluorescein angiography (FA) was done prior to initiating the intra-arterial thrombolysis.
The diagnosis of CRAO was based on patient’s history with sudden painless loss of vision, which must have been recognized in daytime with both eyes open to ensure the time of vision loss was plausible. Furthermore, clinical findings including ischemic retinal whitening with cherry-red spot and reduced filling of retinal arteries on ophthalmoscopy as well as hyperreflective changes and swelling of inner retinal layers on SD-OCT were required. According to retinal changes on OCT described elsewhere (thickness, hyperreflectivity), CRAO was classified in incomplete, subtotal and total according to severity of retinal ischemia at the time of first presentation and once again after lysis.10
Calculation of Retinal Thickness, Relative Retinal Thickness, Grade of Retinal Artery Occlusion and Retinal Reflectivity
Based on the SD-OCT images taken following the above given protocol, data of retinal thickness and relative retinal thickness were obtained as described earlier.11,12 Retinal reflectivity and grade of retinal artery occlusion were measured and derived as described earlier.10 All the data were obtained before and 6 hours after lysis.
Follow-Up Regimen
After acute inpatient treatment with intra-arterial thrombolysis and further investigation on systemic cardiovascular risk factors had been done, the patients were sent to the outpatient ophthalmologist for further observation. There were no regular follow-up visits in the hospital.
Results
During the IAT, only one (case 2) of six patients reported mild subjective visual improvement, while no objective BCVA improvement could be seen. The other patients did not experience any visual improvement, unfortunately.
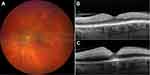
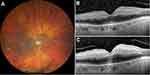
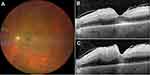
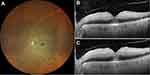
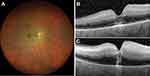
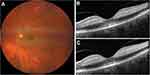
Demographic and clinical characteristics of the cases are summarized in Table 1. All patients had unremarkable previous ophthalmologic history with full visual acuity. Figures 1–6 demonstrate color fundus photographs and SD-OCT images prior to IAT and 6 hours after treatment in all cases. Initially, incomplete ischemia severity grade was observed in cases 1 and 6, while other four cases demonstrated more remarkable subtotal severity grade.
 |
Table 1 Demographic and Clinical Characteristics of the Included Patients |
Four patients (cases 1–4) presented with acute painful neovascular glaucoma 4 to 7 weeks after IAT with intraocular pressure (IOP) ranging from 41mmHg to 50mmHg despite local IOP-lowering medication. In all these cases, pronounced iris neovascularization with anterior chamber angle neovascularization could be observed. No complete panretinal photocoagulation (PRP) could be done in these cases because of corneal edema, insufficient mydriasis and/or cataract. The patients were treated with intravitreal bevacizumab and pars-plana-vitrectomy with dense endolaser photocoagulation. In two cases with residual visual function, subsequent glaucoma tube surgery was done to control the IOP. Two cases ended up with painless blindness with uncontrolled high IOP levels (cases 3 and 4).
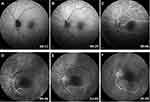
In three out of four NVG cases (cases 1, 2 and 4), FA was done in the later course of the disease, showing persistent delay in arm-retina-time. Figure 7 illustrates FA findings in two cases (cases 1 and 4), while the image quality of case 2 was unfortunately too poor for demonstration.
Past medical history of the patients included controlled diabetes in two cases with no signs of diabetic retinopathy in the fellow eye, two cases with previous ischemic stroke and three cases with pathologic carotid artery status.
Discussion
We describe six cases of intra-arterial thrombolysis treatment for acute central retinal artery occlusion, four of which developed severe neovascular glaucoma 4 to 7 weeks after treatment.
NVG remains one of the most challenging complications of retinal occlusive disorders. NVG can commonly occur as a result of an underlying chronic ischemic retinal condition, such as retinal vein occlusion, diabetic retinopathy or ocular ischemic syndrome. There are many controversies among ophthalmologists regarding possible NVG development after retinal artery occlusion. Hayreh postulates that infarcted retina – like in RAO – is not able to produce any vasoproliferative factors to promote ocular neovascularization.1,2,13,14 In his large observational cohorts, all cases of NVG following RAO developed as a result of chronic ocular ischemia due to the underlying systemic conditions (carotid artery stenosis, diabetes, etc) and were not causative to RAO.
Conversely, there have been several large retrospective studies and a prospective cohort study reporting on ocular neovascularization (ONV) and NVG following retinal artery occlusion. The rate of ONV varies between the studies ranging from 3% to 28%.15 Still, these reports have in common that neovascular glaucoma cases have been documented in patients without any other reasons for chronic ocular ischemia.3–5,16 Hayreh et al argue that ocular ischemia might be present despite normal findings on Doppler ultrasonography of internal carotid arteries (ICA) as ophthalmic artery occlusion might exist without significant occlusion of ipsilateral ICA.14
Degoumois et al reported recently on five cases of NVG 4–13 weeks after central retinal artery occlusion.3 Interestingly, the authors of this study ruled out chronic ocular ischemia not only using Doppler ultrasonography of internal carotid arteries but also ultrasound color Doppler imaging of the ophthalmic artery.
Hayreh underlines that even if the central retinal artery is completely occluded, there is usually still some degree of slow retinal perfusion seen on fluorescein angiography (FA).14 This residual retinal circulation in RAO is due to collateral circulation via cilio-retinal capillary anastomoses within the optic nerve head as well as pial and intraneural anastomoses of the central retinal artery itself proximal to the occlusion site. It might be possible that this residual retinal circulation still supports some retinal cells and saves them from being infarcted. Instead, they might become hypoxic und start expressing the vascular endothelial growth factor. Furthermore, according to the studies of Hayreh et al, thinner peripheral retina with partial oxygen supply and nutrition from the choroidal vascular bed might survive longer in RAO compared to the central retina, simply because the diffusion of oxygen from the choroid might reach inner retina easier because of thinner outer retina.1,2,13,14 This fact is supported by the study of Kim et al, who found central scotoma to be the most common visual field defect pattern in early central RAO.17 These results underline possible survival of peripheral retinal tissue under ischemic conditions in RAO. It might be possible that these survived areas become ischemic and start expressing VEGF, resulting in neovascular complications. It would as well explain why usually no retinal neovascularization but only anterior segment neovascularization are observed after retinal infarction. However, this very important question might be answered with the soon-to-start REVISION trial, which will in addition to benefits of lysis in terms of visual acuity look for the development of neovascularization after thrombolysis.
Jung et al showed in their retrospective analysis of CRAO that the most important factor for the development of ONV was the failure to gain reperfusion.4 None of the eyes with neovascular glaucoma after CRAO gained reperfusion during the first 3 months of follow-up time, while among the eyes without NVG, reperfusion rate was 94.7%.
We describe four cases of early onset aggressive neovascular glaucoma following intra-arterial thrombolysis in CRAO. In natural RAO course, ocular neovascularizations have been described between 2 weeks and 2 years after CRAO onset, with the majority of cases occurring between 8 and 12 weeks after CRAO.5,17 In our study, all four cases presented 4–7 weeks after the original event with severe ocular neovascularizations and painful uncontrolled IOP levels. This time frame has been reported also for untreated CRAO, still it is on the earlier side of the range, so that we believe there might be some causative relationship with the treatment.
To the best of our knowledge, there have been no reports in the literature on NVG as a possible complication of thrombolysis therapy. Tang et al described early onset ocular neovascularization 1 months after successful hyperbaric oxygenation (HBO) therapy in a CRAO patient.18 They postulated that HBO treatment might have resulted in a prolonged ischemic phase of the retina due to improvement in oxygen saturation. This ischemic condition leads to enhanced VEGF expression in the retina, thus potentially contributing to neovascular complication development.
In our cases, IAT might have dissolved the platelet-fibrin part of retinal emboli, still the cholesterol and calcified material as the most common retinal emboli compartments19 remained. This might result in partial re-opening of the retinal vessel, thus converting some parts of retinal tissue from infarcted into hypoxic promoting VEGF expression. Unfortunately, up to date, there is no safe and reliable in vivo method for investigating the composition of retinal emboli in CRAO.
The rate of NVG after lysis in our study is very high compared to other reports.7,20 Ahn et al described only 2 of 57 patients with elevated IOP levels after intra-arterial thrombolysis treatment for CRAO.7 This discrepancy might be due to different IAT procedure standards as well as time frames for treatment.
Unfortunately, we could not observe any objective visual improvement after intra-arterial thrombolysis in our cases. This might be due to potentially more embolic than thrombotic components of the embolus in our patients and already irreversible central retinal damage prior to therapy begin. SD-OCT images (Figures 1–6) demonstrate even increasing hyperreflectivity and thickness of inner retinal layers 6 hours following thrombolysis, underlying progressive central ischemic damage of the retina (also shown in Table 1). Still – like in the case of HBO described previously – intra-arterial thrombolysis in our patients might have led to partially enhanced perfusion of peripheral retina, thus supporting its hypoxic condition and probably VEGF expression.
Three out of four NVG cases had underlying internal carotid artery disease without significant stenosis, one patient had ulcerative ICA plaques. It might be possible that the underlying chronic ischemia might cause the development of ocular neovascularization, irrespective of CRAO. Still, because of very short time frame between the CRAO/its treatment and these aggressive neovascular glaucoma cases, we believe that there is an underlying causative relationship. In cases 1, 2 and 4 of this study, FA at 3 months showed still abnormal retinal perfusion with arm-retina-time of more than 30 seconds, supporting the results of Jung et al on NVG development in eyes without retinal reperfusion.
It is still arguable, whether these eyes would have developed NVG without intra-arterial thrombolysis, as this complication has been described in the natural course of CRAO. In the study of Jung et al, the mean time from CRAO onset to diagnosis of iris neovascularization was 3 months.4 Regarding that, iris neovascularization represents an early neovascular complication and some more time is needed for the development of painful neovascular glaucoma (like in our study), the cases presented in our study with aggressive NVG diagnosis 4–7 weeks after CRAO are suspicious to have causative relationship to intra-arterial thrombolysis. It is possible that in our cases, IAT led to partial reopening of the retinal vessel dissolving the thrombotic part of the embolus, thus leading to the survival of some retinal cells under hypoxic conditions and promoting VEGF expression.
Data Sharing Statement
The authors report that no further data besides what is included in the manuscript will be shared.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Hayreh SS, Podhajsky P. Ocular neovascularization with retinal vascular occlusion: II: occurrence in central and branch retinal artery occlusion. Arch Ophthalmol. 1982;100(10):1585–1596. doi:10.1001/archopht.1982.01030040563002
2. Hayreh SS, Podhajsky PA, Zimmermann MB. Retinal artery occlusion: associated systemic and ophthalmic abnormalities. Ophthalmology. 2009;116(10):1928–1936. doi:10.1016/j.ophtha.2009.03.006
3. Degoumois A, Miocque S, Denion E. Central retinal artery occlusion without underlying chronic ocular ischemic syndrome may lead to neovascular glaucoma. J Fr Ophtalmol. 2017;40(9):758–762. doi:10.1016/j.jfo.2017.05.005
4. Jung YH, Ahn SJ, Hong JH, et al. Incidence and clinical features of neovascularization of the iris following acute central retinal artery occlusion. Korean J Ophthalmol. 2016;30(5):352–359. doi:10.3341/kjo.2016.30.5.352
5. Rudkin AK, Lee AW, Chen CS. Ocular neovascularization following central retinal artery occlusion: prevalence and timing of onset. Eur J Ophthalmol. 2010;20(6):1042–1046. doi:10.1177/112067211002000603
6. Schaefer S, Lang GE. Iris neovascularization as a complication of central artery occlusion. Klin Monbl Augenheilkd. 2005;222:343–345. doi:10.1055/s-2005-858076
7. Ahn SJ, Kim JM, Hong JH, et al. Efficacy and safety of intraarterial thrombolysis in central retinal artery occlusion. Invest Ophthalmol Vis Sci. 2013;54:7746–7755. doi:10.1167/iovs.13-12952
8. Pielen A, Pantenburg S, Schmoor C, et al. EAGLE Study Group. Predictors of prognosis and treatment outcome in central retinal artery occlusion: local intra-arterial fibrinolysis vs. conservative treatment. Neuroradiology. 2015;57(10):1055–1062. PMID: 26349479. doi:10.1007/s00234-015-1588-3
9. Wolf A, Schumacher M, Neubauer AS, et al. European assessment group for lysis in the eye studien gruppe. vergleich der superselektiven intraarteriellen fibrinolyse mit konservativer therapie. einsatz bei patienten mit akutem nichtarteriitischem zentralarterienverschluss [comparison of superselective intraarterial fibrinolysis with conservative therapy. Use in patients with acute non-arteritic central retinal artery occlusion]. Ophthalmologe. 2010. 107(9):799–805. doi:10.1007/s00347-010-2247-z
10. Furashova O, Matthé E. Retinal changes in different grades of retinal artery occlusion: an optical coherence tomography study. Invest Ophthalmol Vis Sci. 2017;58(12):5209–5216. doi:10.1167/iovs.17-22411
11. Ochakovski GA, Wenzel DA, Spitzer MS, et al. Retinal oedema in central retinal artery occlusion develops as a function of time. Acta Ophthalmol. 2020;98(6):e680–e684. doi:10.1111/aos.14375
12. Wenzel DA, Kromer R, Poli S, et al. Optical coherence tomography-based determination of ischaemia onset - the temporal dynamics of retinal thickness increase in acute central retinal artery occlusion. Acta Ophthalmol. 2021;99(2):e247–e252. PMID: 32767551. doi:10.1111/aos.14563
13. Hayreh SS. Central retinal artery occlusion. Indian J Ophthalmol. 2018;66(12):1684–1694. doi:10.4103/ijo.IJO_1446_18
14. Hayreh SS. Prevalent misconceptions about acute retinal vascular occlusive disorders. Prog Retin Eye Res. 2005;24(4):493–519. doi:10.1016/j.preteyeres.2004.12.001
15. Sagong M, Kim J, Chang W. Intravitreal bevacizumab for the treatment of neovascular glaucoma associated with central retinal artery occlusion. Korean J Ophthalmol. 2009;23(3):215–218. doi:10.3341/kjo.2009.23.3.215
16. Mason JO, Patel SA, Feist RM, et al. Ocular neovascularization in eyes with a central retinal artery occlusion or a branch retinal artery occlusion. Clin Ophthalmol. 2015;9:995–1000. PMID: 26089631; PMCID: PMC4467756. doi:10.2147/OPTH.S82796
17. Kim HM, Park YJ, Park KH, Woo SJ. Visual field defects and changes in central retinal artery occlusion. PLoS One. 2019;14(1):e0209118. doi:10.1371/journal.pone.0209118
18. Tang PH, Engel K, Parke DW. Early onset of ocular neovascularization after hyperbaric oxygen therapy in a patient with central retinal artery occlusion. Ophthalmol Ther. 2016;5(2):263–269. PMID: 27613631; PMCID: PMC5125125. doi:10.1007/s40123-016-0064-4
19. Arruga J, Sanders MD. Ophthalmologic findings in 70 patients with evidence of retinal embolism. Ophthalmology. 1982;89(12):1336–1347. doi:10.1016/S0161-6420(82)34626-6
20. Schumacher M, Schmidt D, Jurklies B, et al. EAGLE-Study Group. Central retinal artery occlusion: local intra-arterial fibrinolysis versus conservative treatment, a multicenter randomized trial. Ophthalmology. 2010;117:1367–1375. doi:10.1016/j.ophtha.2010.03.061
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.