Back to Journals » Clinical Ophthalmology » Volume 17
Early Lifetime Substance Use and Development of Visual Impairment: Analysis of the National Survey on Drug Use and Health Data
Authors Hussain ZS , Khan A, Loya A , Shah K , Woreta FA , Riaz KM
Received 28 December 2022
Accepted for publication 8 March 2023
Published 14 March 2023 Volume 2023:17 Pages 849—860
DOI https://doi.org/10.2147/OPTH.S401167
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Zain S Hussain,1,2 Asher Khan,2,3 Asad Loya,4 Kaushal Shah,5 Fasika A Woreta,6 Kamran M Riaz2
1University of Medicine and Health Sciences, Basseterre, Saint Kitts and Nevis; 2Department of Ophthalmology, Dean McGee Eye Institute, Oklahoma City, OK, USA; 3College of Medicine, University of Oklahoma, Oklahoma City, OK, USA; 4Department of Ophthalmology, Baylor College of Medicine, Houston, TX, USA; 5Department of Psychiatry and Behavioral Sciences, Oklahoma State University, Tulsa, OK, USA; 6Department of Ophthalmology, Johns Hopkins Wilmer Eye Institute, Baltimore, MD, USA
Correspondence: Kamran M Riaz, Dean McGee Eye Institute/University of Oklahoma, 608 Stanton L Young Blvd, Suite 313, Oklahoma City, OK, 73104, USA, Tel +1-405-271-1095, Fax +1-405-271-3680, Email [email protected]
Purpose: To investigate the association between early lifetime substance use on the development of severe visual acuity impairment or blindness on a national level.
Methods: National Survey of Drug Use and Health data was used to identify cases of substance use before 21* years of age, within the past year, and cases of self-reported blindness or visual impairment. Univariable and multivariable binary logistic regression with time-dependency was performed to evaluate odds of visual impairment influenced by 16 substances separated into three classes: prescription, non-prescription, and illicit drugs. Adjusted variables of interest included gender, marital status, race, level of education, total family income, poverty level, population density, and history of chronic disease.
Results: 55,824 total responses were analyzed with 2577 (4.6%) cases of self-reported blindness or significant visual impairment. All early-use substance categories, including prescription, non-prescription, and illegal substances, were significantly associated with self-reported VI (OR 2.068, CI 1.451– 2.949, p< 0.001; OR 1.352, CI 1.227– 1.489, p< 0.001); OR 1.211, CI 1.086– 1.352, p< 0.001), respectively). Non-prescription substances displayed parallel significances amongst all constituents (alcohol, cigarettes, inhalants, and marijuana) (OR=1.227, CI 1.12– 1.344, p< 0.001; OR 1.363, CI 1.243– 1.495, p< 0.001; OR 1.418, CI 1.134– 1.774; OR 1.388, CI 1.27– 1.518, p< 0.001, respectively). Univariable and multivariable analysis revealed several significant demographical and clinical adjustors.
Conclusion: Early lifetime use of all three classes of substances is associated with enhanced odds of subsequent visual impairment or blindness. Several readily available and commonly used substances have a greater risk. These findings may help clinicians and public health agencies in mitigation ventures including education, prevention, and rehabilitation efforts.
Keywords: substance use, ophthalmology, vision loss
Plain Language Summary
For the past three to four decades, multiple substance abuse epidemics, including the Opioid Use Epidemic and Methamphetamine Use Epidemic, have plagued the United States. A plethora of studies directly implicate the involvement of substance use, including cocaine, marijuana, and heroin, with visual impairment. This investigation of the National Survey of Drug Use and Health associates early lifetime substance abuse with the eventual onset of self-reported visual impairment or blindness. Notably, we observed that early lifetime use of all three classes of substances (prescription, non-prescription, and illegal) can contribute to later lifetime visual impairment. These findings are potentially important to improve future education, prevention, and rehabilitation efforts.
Introduction
Prior investigations of the NSDUH (National Survey on Drug Use and Health) acted as a provision for active investigations by the Centers for Disease Control and Prevention (CDC) into addressing high-risk substance use among American youth.1–3 The focus on youth is critical as the majority of adults diagnosed with substance use disorders initiated their addiction(s) during their teen and young adult years.4 Recent estimates of substance use for illicit, injectable, and misused prescription drugs approach 15% among high school students in the United States.3,5
Ocular pathology associated with substance use is well-described in the literature, ranging from mild vision loss to permanent, irreversible visual impairment.6 High-risk substances, such as cocaine, amphetamines, and opioids, have been linked to vision-threatening complications such as glaucoma, endophthalmitis, and retinal vascular events.6–9 Commonly used substances, such as alcohol and marijuana, are also associated with morbidity, including ocular surface disease.10–12
The NSDUH,13 operated by an agency under the Department of Human and Health Services Administration (HHS), represents a nationally representative database of substance use and behavioral/medical health-related registries within the United States.14 Numerous federal agencies use the NSDUH to provide important national updates on substance use and its sequelae as they relate to key societal and medical issues.15 This includes identifying at-risk demographics/geographical areas for efficient utilization of federal resources,16 monitoring developments within the National Drug Control Strategy,17,18 and understanding current immediate and longitudinal substance use trends nationally.14
Recent studies have investigated the NSDUH within the ophthalmic context. Andoh et al recently determined 20% increased odds of visual impairment in individuals with a history of criminal justice involvement (CJI).5 Furthermore, Han et al highlighted a strong prevalence of visual impairment associated with psychoactive substances, including alcohol, nicotine dependence, prescription opioids, and tranquilizers within the past year amongst middle-aged and older adults.19 Considering the aforementioned ocular pathology, there is a need to highlight longitudinal trends regarding future vision loss or blindness within a relatively high-risk generation.
To the best of our knowledge, a comprehensive analysis of early lifetime substance use and its association with visual impairment has not been elucidated. In the context of increasing incidence and prevalence of both vision loss and substance use disorders in the American population,20–22 this retrospective cohort analysis represents the first study to evaluate all NSDUH-contained time-dependent substance use variables and their association(s) with eventual blindness or significant visual impairment despite refraction correction.
Materials and Methods
The institutional review board (IRB) at the University of Oklahoma deemed the study exempt from review. As the NSDUH data are freely available and accessible on a public database, these patient de-identified data were gathered through governmental agencies with the informed consent of the survey respondents at the time of original data collection.23 For the purposes of our study, additional informed consent was not possible due to the already confirmed anonymity of the data. This study followed all guidelines outlined in the Declaration of Helsinki.
NSDUH cross-sectional estimates were curated utilizing a formalized multistage area probability sampling strategy of non-institutionalized US civilians of at least 12 years of age.9 Confidentiality and cultural/in-group bias in the context of sensitive questions were maintained and reduced, respectively, via computerized interviewing systems. Self-reported VI, despite refractive correction was established by yes or no responses to “Are you blind or do you have serious difficulty seeing, even when wearing glasses?” Case responses categorized as “bad data”, “don’t know”, “refused”, or “blank” (no answer) were excluded from the analysis (N=312).
To understand the association between early exposure of substance use and visual pathology, 16 time-dependent substance use variables were extracted from the NSDUH, which convey illicit consumer use of substances at least once before 21 years of age. Specifically, the substances analyzed were cigarettes, alcohol, marijuana, cocaine, crack, heroin, hallucinogens, lysergic acid (LSD), phencyclidine (PCP), ecstasy, inhalants, methamphetamine, misused pain relievers, tranquilizers, non-traditional stimulants, and sedatives. During post-hoc analysis, an additional variable broadly categorizing substances into three classes (prescription use, non-prescription use, and use of illegal substances) was developed to provide a clinically meaningful generalized prediction of the class-effect of early illicit substance use on eventual self-reported VI. As observed in prior studies,19 clarity regarding continued longitudinal substance use later in life is demonstrated by evaluating substance misuse rates within the past year. Furthermore, proper assessment of longitudinal use impact on vision loss is performed by evaluating odds of self-reported VI secondary to respondents who reported substance misuse before 21 years of age and misuse within the past year. For substances misused within the past year, the NSDUH defines misuse as “Use in any way not directed by a doctor, including use without a prescription of one’s own medication; use in great amounts, more often, or longer than told to take a drug; or use in any other way not directed by a doctor”.
Furthermore, the NSDUH does not label “misuse” or “abuse” for past-year use of crack cocaine, cocaine, nor heroin, as these substances are currently not legally consumed for any reason.
Demographical and clinical characteristics were extracted, including gender, marital status, age, race/ethnicity, level of education, total family income, poverty level, Core Based Statistical Areas (CBSA) for population density, and lifetime history of chronic disease (eg, hypertension, diabetes, heart disease, chronic obstructive pulmonary disease (COPD), asthma, cirrhosis, chronic kidney disease, chronic viral hepatitis B & C, COPD, cancer, HIV/AIDS). Due to well-documented associations with visual morbidity, the presence of systemic disease, specifically diabetes, heart disease, and hypertension, was each separately assessed with multivariable modeling in their association with self-reported VI. Univariable analyses were conducted with each substance or category as separate primary independent predictors of self-reported VI. Within the multi-variable analysis, all demographical and clinical characteristics of individuals in the database were adjusted to comprehensively 1) document psychosocial associations amongst substance users and 2) evaluate the NSDUH database to determine which patient characteristics may be high-risk or low-risk for associative self-reported VI.
Application of univariable binary logistic regression on all candidate substances was first performed. If univariable significance was achieved at p<0.05, then selected substances were sequentially qualified with multivariable regression. To compensate for family-wise error rates associated with multiple hypothesis testing, the Bonferroni correction was applied. Furthermore, model complexity and multi-collinearity were reduced via the formation of clinically correlative principal components with separate univariable and multivariable logistic regression models. Principal components considered all 16 substances, which were grouped according to the similarity of substance mechanism or function. Statistical analyses were performed utilizing IBM SPSS Statistics for Windows, Version 28 (IBM Corp., Armonk, NY, USA). All P values were two-tailed and 95% confidence intervals were reported. Statistical significance was achieved at p<0.05.
Results
Descriptive Analysis
Baseline demographic data are shown in Table 1. A total of 55,824 cases were considered for analysis with N=2577 (4.6%) cases self-reporting visual impairment despite refractive correction. Approximately 52.3% (N=29,200) of respondents were female, and 57.3% (N=31,980) identified as non-Hispanic White. Categorical age brackets were positively skewed, with most respondents between 18–25 years (N=14140, 25.3%). 27.9% of respondents (N=15588) were afflicted by at least one lifetime chronic disease. Additional information regarding marital status, education, family income, poverty level, and population density demographics are provided in this table.
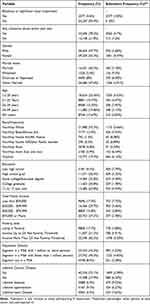 |
Table 1 Baseline Cohort and Subcohort* Demographical Characteristics |
Table 2 displays substance use data broadly divided into three categories: prescription use, non-prescription use, and illegal use before 21 years of age. For the category of prescription use, pain relievers comprised N=208 (0.4%) cases, with tranquilizers (N=172, 0.3%) and sedatives (N=27, 0.0%) also reported. Regarding non-prescription use within the 2019 NSDUH cohort, N=33464 (59.9%) reported first using alcohol before 21 years of age, followed by use of cigarettes (N=32,631, 58.5%) and marijuana (N=20903, 37.4%). Finally, when analyzing illegal drug use, hallucinogens (N=5937, 10.6%), inhalants (N=4567, 8.2%), cocaine (N=3658, 6.6%), LSD (N=3598, 6.5%), ecstasy (N=2597, 4.7%), methamphetamine (N=1449, 2.6%), crack (N=600, 1.1%), PCP (N=528, 0.9%), heroin (N=400, 0.7%), and non-traditional stimulants (N=198, 0.4%) were disclosed before 21 years of age. Furthermore, while the majority of respondents who did not use any substance before 21 years of age (58.2%) did not misuse any substance within the past year (58.2%), 94.4% of all respondents who did use substances before 21 years of age also reported abusing any substance within the past year.
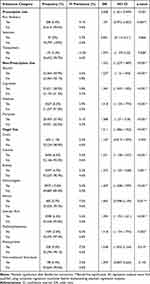 |
Table 2 Substance-Based Frequencies and Regression Outcomes for Vision Loss |
Logistic Regression Analysis
All early-use substance categories, including prescription, non-prescription, and illegal substances, were significantly associated with self-reported VI (OR 2.068, CI 1.451–2.949, p<0.001; OR 1.352, CI 1.227–1.489, p<0.001); OR 1.211, CI 1.086–1.352, p<0.001), respectively). Non-prescription substances was the only category displaying parallel significances amongst all individual constituents (alcohol, cigarettes, inhalants, and marijuana) (OR=1.227, CI 1.12–1.344, p<0.001; OR 1.363, CI 1.243–1.495, p<0.001; OR 1.418, CI 1.134–1.774; OR 1.388, CI 1.27–1.518, p<0.001, respectively). While individual substance use outcomes were not significantly associated with eventual self-reported VI, we noted that heroin (OR=1.465, CI 0.998–2.149, p=0.051) and pain relievers (OR=1.59, CI 0.972–2.603, p=0.065) showcased parallel trends; however, these groups did not reach statistical significance. Table 2 presents additional substance-stratified descriptive analyses and multivariate logistic regression outcomes for self-reported VI.
Univariable and multivariate analysis (Table 3) revealed several significant demographical and clinical adjustors, including age, population density, gender, high school education, marital status, race, poverty status, family income, and history of chronic disease. Among early-life substance users, those aged 26–34 years and 35–49 years at the time of interviewing demonstrated increased odds of self-reported VI as compared to those between 12 and 17 years of age (OR 1.347, CI 1.057–1.715, p=0.016; OR 1.514, CI 1.182–1.939, p=0.001, respectively).
 |
Table 3 Multivariable Logistic Regression for Substance Use and Clinical Adjustors |
Substance use yielding borderline or no significance with eventual self-reported VI or blindness were crack (OR=1.167, CI 0.813–1.674), heroin (OR=1.465, CI 0.998–2.149), pain relievers (OR=1.590, CI 0.972–2.603), non-traditional stimulants (OR=1.509, CI 0.867–2.626), tranquilizers (OR=1.193, CI 1.197–3.25), and sedatives (OR=0.841, CI 0.112–6.310).
Within the cohort of respondents who reported at least one incident of substance use both before 21 years of age and within the past year (N=43,656), prevalence of self-reported VI increased by 0.12% (4.72%). Furthermore, within this cohort, hypertension (N=4683, 10.7%), diabetes (N=2646, 6.1%), and any prior heart disease (N=2696, 6.2%) significantly influenced the relationship between substance use before 21 and self-reported VI (all p<0.001).
Discussion
Vision loss and blindness consistently top the list of greatest fears among Americans, surpassing serious ailments such as cancer, stroke, heart disease, and even death.24,25 Recent population-based estimates of visual acuity loss or blindness totaled 7.08 million persons, significantly superseding historical national estimates by 68.7%.6 Simultaneously, rates of substance use disorders among Americans, including the opioid crisis and methamphetamine abuse,26,27 are accelerating at an ever-increasing pace. Substance use disorders are well-documented to provide a heightened propensity for the development of a variety of psychiatric disorders, lower socioeconomic status, substantial lifetime trauma, and low accessibility to mental health services.28,29 Prior studies have also characterized the influence of substance use on ophthalmic pathology through anatomical stratification within the eye and orbit.6 Within ophthalmology, epidemiological analyses have previously highlighted strong associations between self-reported VI and illicit drug use among service members and veterans.22 While studies of the NSDUH within the ophthalmic literature are few in number,5,19 this national investigation validates an enormous body of literature regarding the harmful effects of substance use, while simultaneously recommending a heightened clinical awareness among all eye care providers for visual morbidity consequent to a national endemic of substance abuse.
Previous studies have noted significant risks of visual morbidity, especially when considering illegal substances. For example, one study reported an increased risk of open-angle glaucoma in cocaine and amphetamine users.7 Similarly, intravenous drug use has been reported to cause endogenous endophthalmitis, talc retinopathy, and septic emboli.30 When considering legal substances, both history of increased alcohol consumption and current heavy drinking (defined as at least 4 drinks per day) correlated with the presence of dry eye signs and symptoms.31,32 Furthermore, illicit drug use may not necessarily directly affect visual acuity; other visual domains of interest, including contrast sensitivity, prolonged glare recovery, and reduction in color discrimination, are evidenced to be in association with drug use.49
In the present study, we noted a significant risk of self-reported VI associated with early life use of both illegal and legal substances, the latter including both readily-available and prescription-required substances. First, we observed that 58.2% of respondents who did not use any substance before 21 years of age endorsed substance use within the past year of taking the survey. Furthermore, 94.4% of all respondents who used substances before 21 years of age also reported substance use within the past year of taking the survey. This observation is likely clinically important, as respondents are much more likely to continue drug abuse into adulthood if drug abuse occurred before 21 years of age. This observation is also statistically important as there is verification of significantly greater specificity of drug abuse later in life when drug abuse occurred earlier in life.
To inform clinicians and policymakers most meaningfully, the encompassed 16 substances were divided into three broad categories: prescription, non-prescription, and illegal substances. We noted that early substance use among all three categories led to significantly increased odds of self-reported VI or blindness later in life (Table 2). More specifically, 9 of 16 studied substances were independently associated with significant self-reported VI after early lifetime substance use. Furthermore, past-year substance misuse was observed in 94.4% of respondents who reported substance use before 21 years of age. This indicates an association of early lifetime substance use with past year substance misuse and further validates a cumulative lifetime impact of longitudinal substance use with vision.
Understanding the cumulative impact of early-onset substance use on a societal and economic basis, especially during teenage years and young adulthood, is a profound public health concern.17,33 Health care costs, along with strains in the workplace, households, and local communities, have historically and systematically been impacted. Logistically, non-prescription substances create a concern, given the high rates of ease of access among young adults. In this study, alcohol, cigarettes, inhalants, marijuana, and the all-inclusive “non-prescription group” individually and collectively demonstrated a strong relationship with the eventual onset of self-reported VI (all p<0.001). These findings alone require considerable discussion; however, these “gateway” or “light” substances could act as a provision for later involvement in more nefarious drugs, which synergistically impact ocular function.34
Additionally, this investigation highlights an extensive array of prescription-only and illegal substances (Table 2) that also demonstrate the potential for predicting self-reported VI. A non-significantly stronger association with self-reported VI is evident for prescription use (OR 2.069, p<0.001) as compared to non-prescription use (OR 1.352, p<0.001). Coupled with significantly fewer cases of prescription use before 21 years of age, these findings altogether would demonstrate a greater specificity for eventual self-reported VI with prescription use in tandem with greater sensitivity with non-prescription use. By extension, this rationale would firmly support the “gateway” theory concerning the adolescent transition of categorical substance use from non-prescription to prescription medications.35–39 Important regard for alternative mechanisms is evidenced by adjustors, including age, sex, marital status, poverty status, population density, family income, and lifetime chronic disease, reaching statistical significance. These factors underpin the intricacies mediating the relationship between vision loss and substance use. Ultimately, it is not merely substance use itself, but a myriad of psychosocial confounders, including access and quality of care, socioeconomic status, or familial support, that results in the onset of self-reported VI later in life (Table 3).
Young adolescents are susceptible to impulsivity, peer pressure or peer approval, and pleasurable drug experiences that ultimately contribute to psychological drug addiction and the use of more dangerous substances.1,3,33,34 In terms of accessibility, adolescents can potentially access these medications from prescriptions of family members within their respective households or second-hand from social gatherings.33 Contributing factors to this dynamic include social isolation and incompetency of safe management strategies for controlled medications within the household.33,34
Han et al showcased preliminary evidence of substance use exercising a widened prevalence within the past year among middle-aged and older adults suffering from self-reported VI or blindness.19 The authors suggest middle-aged and elderly adults may utilize these substances, either prescribed or obtained otherwise, to alleviate mood dysfunction secondary to vision loss. In contrast, our analysis highlights a time-dependent influence of early substance use on the development of self-reported VI or blindness later in life. Furthermore, when considering CDC-directed investigations concluding that early substance use strongly predicts a continuation of substance use into adulthood,17 then an alternative explanation can be considered. Instead of vision loss provoking the use of psychoactive substances amongst older adults, it is also likely that an early onset of substance use results in ocular, systemic, and psychiatric morbidity, which may primarily or secondarily explain continued substance use in older populations.35–39 Several studies that report the chronic, degenerative ocular effects of prescription, non-prescription, and illicit substances would additionally support our findings.6–13 However, it is possible that both explanations may apply on an individual basis: while some patients may have an early history of substance use associated with vision loss in later years, there may be also a significant number of patients who initiated substance use in middle-adulthood in response to complex medical conditions causing chronic pain and mood disorders.40–46
Regardless of the exact cause-effect time relationship between substance use and self-reported VI, eye care providers should practice a heightened level of awareness for identifying substance use disorders before chronic consumption of a given substance cumulatively impacts vision to a severe degree. In the clinical setting, eye care providers should strongly consider giving additional attention, especially when treating younger patients, to accurately document and review medical and substance use history, given long-term visual implications. This is notably important for patients residing in high-risk areas. For some patients, eye care providers may be the only medical provider encountered, whether it be for routine, elective, or emergent care; thus, eye care providers among all subspecialties should be aware of their gatekeeper role in the comprehensive well-being and health maintenance of high-risk patient populations. Subsequent education, establishing care with primary care doctors, and psychiatric referral for addiction rehabilitation, if necessary, should promptly follow. As a corollary, practitioners may need to play a critical role in substance use rehabilitation efforts. For example, recent visuoperceptive prediction models have highlighted the importance of visual skills remediation before the implementation of higher-order cognitive and emotional psychotherapy for patients diagnosed with severe alcohol use disorder.40 Thus, eye care providers may not only have to treat drug-induced ocular pathology but also participate in multidisciplinary efforts for successful long-term rehabilitation of this patient population.47,48
Strengths of this study include a considerably large cohort and a nationally representative database. Additionally, the comprehensive nature of the NSDUH allows for an ability to capture information on commonly misused prescription and non-prescription substances. NSDUH data utilization carries an inherent risk of social desirability and recall biases.24 Compensation for recall bias is developed within the interview style. For example, interview questions related to misuse of certain substances incorporate generic and brand names to aid in respondent recollection and accuracy (benzodiazepine tranquilizer, alprazolam, Xanax, etc). Furthermore, time-dependent NSDUH analyses, such as this study, ascertains legitimacy in documenting trends or associations over time by virtue of rates of under- or over-reporting remaining relatively constant over time. This is likely due to the social undesirability of substance use remaining constant over time. NSDUH-derived studies are usually cross-sectional in nature; however, causality can be inferred. This study demonstrates inferred causality in its use of time-dependent variables, namely substance use before a certain age, thereby creating a legitimate framework for establishing temporal associations. Future investigations may be able to improve on the limitations of this study.
Conclusions
In summary, this study demonstrated enhanced odds of self-reported visual impairment or blindness in relation to all three classes of substance use (prescription use, non-prescription use, and illegal use) before 21 years of age. A number of readily-available and commonly used substances have a greater risk for visual impairment. Eye care providers should be aware of their role in the diagnosis and management of this high-risk patient population. These findings may further help clinicians and public health agencies in mitigation efforts including education, prevention, and rehabilitation efforts.
Abbreviation
VI, visual impairment or blindness despite refractive correction.
Ethics Statement
The Institutional Review Board (IRB) at the University of Oklahoma reviewed the study, exempted it from further IRB review, and approved the research performed in this study. Informed consent was obtained from the study participants at the time of the US national agencies collecting the data. The guidelines outlined in the Declaration of Helsinki were followed. All data accessed complied with relevant data protection and privacy regulations.
Acknowledgments
This paper was presented at the 2022 Association for Research in Vision and Ophthalmology (ARVO) meeting as a poster presentation with interim findings. The poster’s abstract was published in “Poster Abstracts” in Investigative Ophthalmology & Visual Science (IOVS) June 2022 (Volume 63, No. 7) issue: https://iovs.arvojournals.org/article.aspx?articleid=2781774.
Disclosure
Dr. Riaz reports speaking fees from CorneaGen and Bausch and Lomb, and consulting fees from Ambrx, Inc and ImmunoGen outside the submitted work. None of the other authors report any other disclosures in this work.
References
1. National Survey on Drug Use and Health. Frequently asked questions. Available from: https://nsduhweb.rti.org/respweb/faq.html.
2. Underwood JM, Brener N, Thornton J, et al. Overview and methods for the youth risk behavior surveillance system — United States, 2019. MMWR Suppl. 2020;69(Suppl 1):S1–S10. doi:10.15585/mmwr.su6901a1
3. Substance Abuse and Mental Health Services Administration (US); Office of the Surgeon General (US). Facing addiction in America: the surgeon general’s report on alcohol, drugs, and health. Washington (DC): US Department of Health and Human Services; 2016. Available from: https://addiction.surgeongeneral.gov/sites/default/files/surgeon-generals-report.pdf.
4. National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention Division of Adolescent and School Health. Youth risk behavior survey; 2019. Available from: https://www.cdc.gov/healthyyouth/data/yrbs/pdf/YRBSDataSummaryTrendsReport2019-508.pdf.
5. Andoh J, Mir TA, Teng C, Wang EA, Nwanyanwu KH. Assessment of vision impairment among individuals with history of criminal justice involvement. Invest Ophthalmol Vis Sci. 2021;62(8):3493.
6. Peragallo J, Biousse V, Newman NJ. Ocular manifestations of drug and alcohol abuse. Curr Opin Ophthalmol. 2013;24(6):566–573. doi:10.1097/ICU.0b013e3283654db2
7. French DD, Margo CE, Harman LE. Substance use disorder and the risk of open-angle glaucoma. J Glaucoma. 2011;20(7):452–457. doi:10.1097/IJG.0b013e3181f7b134
8. Mir TA, Papudesu C, Fang W, Hinkle DM. Incidence of drug use-related endogenous endophthalmitis hospitalizations in the United States, 2003 to 2016. JAMA Ophthalmol. 2021;139(1):18–26. doi:10.1001/jamaophthalmol.2020.4741
9. Orum MH, Kalenderoglu A. Acute opioid use may cause choroidal thinning and retinal nerve fiber layer increase. J Addict Dis. 2021;39(3):322–330. doi:10.1080/10550887.2021.1874816
10. Kim RW, Juzych MS, Eliott D. Ocular manifestations of injection drug use. Infect Dis Clin North Am. 2002;16(3):607–622. doi:10.1016/S0891-5520(02)00013-2
11. Kim JH, Kim JH, Nam WH, et al. Oral alcohol administration disturbs tear film and ocular surface. Ophthalmology. 2012;119(5):965–971. doi:10.1016/j.ophtha.2011.11.015
12. Karimi S, Arabi A, Shahraki T. Alcohol and the eye. J Ophthalmic Vis Res. 2021;16(2):260–270. doi:10.18502/jovr.v16i2.9089
13. Nguyen AX, Wu AY. Cannabis and the cornea. Ocul Immunol Inflamm. 2021;29(5):1023–1028. doi:10.1080/09273948.2020.1726969
14. Centers for Medicare & Medicare Services. National survey on drug use and health (NSDUH); 2019. Available from: https://www.cms.gov/About-CMS/Agency-Information/OMH/resource-center/hcps-and-researchers/data-tools/sgm-clearinghouse/nsduh.
15. U.S. Department of Health & Human Services. Substance abuse and mental health services administration (SAMHSA); 2019. Available from: https://www.samhsa.gov/.
16. Substance Abuse and Mental Health Services Administration (SAMHSA). NSDUH data use; 2019. Available from: https://nsduhweb.rti.org/respweb/datause.html.
17. U.S. Department of Health & Human Services. FY 2021 annual performance plan and report - goal 2 objective 3; 2021. Available from: https://www.hhs.gov/about/budget/fy2021/performance/performance-plan-goal-2-objective-3/index.html.
18. Office of National Drug Control Policy. National drug control strategy; 2016. Available from: https://obamawhitehouse.archives.gov/ondcp/policy-and-research/ndcs#:~:text=The%20National%20Drug%20Control%20Strategy.
19. Han BH, Leddy JF, Lopez FA, Palamar JJ. Prevalence of psychoactive substance use among middle-aged and older adults with visual impairment in the US. JAMA Ophthalmol. 2022;140(1):94–95. doi:10.1001/jamaophthalmol.2021.4667
20. Key substance use and mental health indicators in the United States: results from the 2015 national survey on drug use and health. SAMHSA.gov; 2015. Available from: https://www.samhsa.gov/data/sites/default/files/NSDUH-FFR1-2015/NSDUH-FFR1-2015/NSDUH-FFR1-2015.pdf.
21. Kelly JF, Myers MG. Adolescents’ participation in alcoholics anonymous and narcotics anonymous: review, implications and future directions. J Psychoactive Drugs. 2007;39(3):259–269. doi:10.1080/02791072.2007.10400612
22. McDaniel JT, Jenkins WD, Albright DL, Null D, McIntosh S, McDaniel MR. Illicit drug use and self-reported vision loss among military service members or veterans. BMJ Mil Health. 2020;25:bmjmilitary-2020–001518.
23. SAMHDA. National survey on drug use and health 2020 (NSDUH-2020-DS0001); 2020. Available from: https://www.datafiles.samhsa.gov/dataset/national-survey-drug-use-and-health-2020-nsduh-2020-ds0001.
24. American Academy of Ophthalmology. Survey reveals most Americans know a lot less about eye health than they think they do: here’s why that’s a problem; 2020. Available from: https://www.aao.org/newsroom/news-releases/detail/survey-reveals-most-americans-know-less-eye-health.
25. Kumar V, Abbas AK, Fausto N, et al. Robbins and Cotran Pathologic Basis of Diseases.
26. CDC. Understanding the epidemic: CDC’s response to the opioid overdose epidemic; 2022. Available from: https://www.cdc.gov/opioids/basics/epidemic.html.
27. Jones CM, Compton WM, Mustaquim D. Patterns and characteristics of methamphetamine use among adults — United States, 2015–2018. MMWR Morb Mortal Wkly Rep. 2020;69(12):317–323. doi:10.15585/mmwr.mm6912a1
28. Palamar JJ, Davies S, Ompad DC, Cleland CM, Weitzman M. Powder cocaine and crack use in the United States: an examination of risk for arrest and socioeconomic disparities in use. Drug Alcohol Depend. 2015;149:108–116. doi:10.1016/j.drugalcdep.2015.01.029
29. Butler AJ, Rehm J, Fischer B. Health outcomes associated with crack-cocaine use: systematic review and meta-analyses. Drug Alcohol Depend. 2017;180:401–416. doi:10.1016/j.drugalcdep.2017.08.036
30. Luong PM, Tsui E, Batra NN, Zegans ME. Endogenous endophthalmitis and other ocular manifestations of injection drug use. Curr Opin Ophthalmol. 2019;30(6):506–512. doi:10.1097/ICU.0000000000000606
31. Moss SE, Klein R, Klein BE. Prevalence of and risk factors for dry eye syndrome. Arch Ophthalmol. 2000;118(9):1264–1268. doi:10.1001/archopht.118.9.1264
32. Cumurcu T, Gunduz A, Cumurcu BE, Gul IG, Akpolat N, Karlidag R. The changes in tear film parameters and impression cytology in heavily drinking men. Cornea. 2013;32(3):237–241. doi:10.1097/ICO.0b013e31825239d1
33. Engster SA, Bogen DL, Molina BSG. Adolescent and parent management of controlled prescription medications. Subst Use Misuse. 2019;54(14):2264–2274. doi:10.1080/10826084.2019.1645176
34. Mayo Clinic. Drug addiction (substance use disorder) - symptoms and causes. Mayo Clinic; 2022. Available from: https://www.mayoclinic.org/diseases-conditions/drug-addiction/symptoms-causes/syc-20365112.
35. Kandel ER, Kandel DB, Molecular A. Basis for nicotine as a gateway drug. N Engl J Med. 2014;371(10). doi:10.1056/NEJMsa1405092
36. Degenhardt L, Dierker L, Chiu WT, et al. Evaluating the drug use “gateway” theory using cross-national data: consistency and associations of the order of initiation of drug use among participants in the WHO World Mental Health Surveys. Drug Alcohol Depend. 2010;108(1–2):84–97. doi:10.1016/j.drugalcdep.2009.12.001
37. Martin CS. Timing of alcohol and other drug use. Alcohol Res Health. 2008;31(2):96–99.
38. Barry AE, King J, Sears C, Harville C, Bondoc I, Joseph K. Prioritizing alcohol prevention: establishing alcohol as the gateway drug and linking age of first drink with illicit drug use. J Sch Health. 2016;86(1):31–38. doi:10.1111/josh.12351
39. Secades-Villa R, Garcia-Rodríguez O, Jin CJ, Wang S, Blanco C. Probability and predictors of the cannabis gateway effect: a national study. Int J Drug Policy. 2015;26(2):135–142. doi:10.1016/j.drugpo.2014.07.011
40. Sinha R. Chronic stress, drug use, and vulnerability to addiction. Ann N Y Acad Sci. 2008;1141:105. doi:10.1196/annals.1441.030
41. Litt MD, Kadden RM, Tennen H, Dunn HK. Momentary coping and marijuana use in treated adults: exploring mechanisms of treatment. J Consult Clin Psychol. 2021;89(4):264–276. doi:10.1037/ccp0000633
42. Litt MD, Kadden RM, Kabela-Cormier E. Individualized assessment and treatment program for alcohol dependence: results of an initial study to train coping skills. Addiction. 2009;104(11):1837–1838. doi:10.1111/j.1360-0443.2009.02693.x
43. Roos CR, Maisto SA, Witkiewitz K. Coping mediates the effects of cognitive-behavioral therapy for alcohol use disorder among out-patient clients in Project MATCH when dependence severity is high. Addiction. 2017;112(9):1547–1557. doi:10.1111/add.13841
44. Gray KM, Squeglia LM. Research review: what have we learned about adolescent substance use? J Child Psychol Psychiatry. 2018;59(6):618–627. doi:10.1111/jcpp.12783
45. Feinstein EC, Richter L, Foster SE. Addressing the critical health problem of adolescent substance use through health care, research, and public policy. J Adolesc Health. 2012;50(5):431–436. doi:10.1016/j.jadohealth.2011.12.033
46. Harris SK, Louis-Jacques J, Knight JR. Screening and brief intervention for alcohol and other abuse. Adolesc Med State Art Rev. 2014;25(1):126–156.
47. Han RC, Jefferis JM, Taylor JP, Archibald NK, Clarke MP. A novel, multidisciplinary clinic for complex visual problems in older people. Eye. 2012;26(12):1536–1541. doi:10.1038/eye.2012.205
48. Jefferis JM, Mosimann UP, Clarke MP. Cataract and cognitive impairment: a review of the literature. Br J Ophthalmol. 2011;95(1):17–23. doi:10.1136/bjo.2009.165902
49. Lalanne L, Ferrand-Devouge E, Kirchherr S, et al. Impaired contrast sensitivity at low spatial frequency in cannabis users with early onset. Eur Neuropsychopharmacol. 2017;27(12):1289–1297. doi:10.1016/j.euroneuro.2017.09.006
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
