Back to Journals » International Journal of General Medicine » Volume 15
Dyspepsia-Like Symptoms in Helicobacter pylori-Negative Chronic Gastritis are Associated with ASCA-, ANCA-, and Celiac Seropositivity but Not with Other Autoimmune Parameters: A Single-Centre, Retrospective Cross-Sectional Study
Authors Zádori N , Németh D, Frim L , Vörhendi N, Szakó L, Váncsa S , Hegyi P, Czimmer J
Received 9 July 2022
Accepted for publication 26 August 2022
Published 12 October 2022 Volume 2022:15 Pages 7789—7796
DOI https://doi.org/10.2147/IJGM.S380419
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Noémi Zádori,1,2 Dávid Németh,1 Levente Frim,1 Nóra Vörhendi,1,2 Lajos Szakó,1,2 Szilárd Váncsa,1– 3 Péter Hegyi,1– 3 József Czimmer1,4
1Institute for Translational Medicine, Medical School, University of Pécs, Pécs, Hungary; 2János Szentágothai Research Centre, University of Pécs, Pécs, Hungary; 3Centre for Translational Medicine, Semmelweis University, Budapest, Hungary; 4Division of Gastroenterology, First Department of Medicine, Medical School, University of Pécs, Pécs, Hungary
Correspondence: József Czimmer, First Department of Medicine, University of Pécs Medical School, Ifjúság street 13, Pécs, H-7624, Hungary, Email [email protected]
Introduction: Dyspeptic symptoms are frequent in the general population, with a high socioeconomic burden. Helicobacter pylori (H. pylori) might be a possible etiological factor; however, it is also common in H. pylori negative gastritis. Clarification of the underlying aetiology might be beneficial to set up the optimal treatment strategy for dyspepsia and chronic gastritis (CG) itself. We aimed to assess the prevalence of dyspeptic symptoms in patients with H. pylori negative CG and explore autoimmunity’s possible role.
Methods: This retrospective study included data from patients with H. pylori negative CG. Exclusion criteria were (1) acute gastritis; (2) reactive gastropathy; (3) subjects without any serology testing results; (4) H. pylori positivity; (5) presence of atrophy, intestinal metaplasia (IM), gastroesophageal reflux disease (GERD), ulcer, or cancer. The following endpoints were assessed (1) the rate of dyspepsia-like symptoms; (2) association between dyspepsia and autoimmune disease-related seromarker positivity (AISP); (3) frequency of other symptoms in CG and its association with AISP; (4) location of the inflammation and its association with AISP.
Results: From a total of 285 patients, 175 were included in this study. Among these patients, 95 experienced dyspeptic symptoms (54.29%) and were associated more with AISP (p = 0.012), especially with celiac seropositivity (p = 0.045), anti-neutrophil cytoplasmic antibody (ANCA) and anti-Saccharomyces cerevisiae antibodies (ASCA) positivity (p = 0.043). A significant association was not found with other tested autoimmune (AI)-related antibody positivity.
Conclusion: Positivity of seromarkers of autoimmune diseases in chronic gastritis may predispose to have dyspeptic symptoms and may be the causative factor behind some cases of uninvestigated dyspepsia. These data suggest that further prospective studies are needed to clarify whether screening for autoantibodies in patients with dyspepsia is cost-effective and helps the earlier diagnosis of autoimmune diseases.
Keywords: chronic gastritis, autoimmunity, auto-antibody, dyspepsia
Introduction
Dyspepsia is a complex condition, refers to a group of symptoms, which originate from the upper gastrointestinal region. The Rome IV criteria define dyspepsia as any combination of the four following symptoms: postprandial fullness, early satiety, epigastric pain, and epigastric burning sensation.1 Regarding the aetiology, organic and functional dyspepsia can be distinguished. When dyspeptic symptoms are not manifestations of organic pathologies, such as gastroesophageal reflux disease (GERD), peptic ulcer disease, or gastric tumour, it is classified as functional dyspepsia (FD).
Dyspeptic symptoms are frequent in the general population, with a prevalence of 20–40%,2–4 and it is the most common indication for upper gastrointestinal (GI) endoscopy.5 The diagnostic value of gastroscopy in diagnosing dyspepsia is controversial. Although it is a possible method to differentiate patients with organic dyspepsia from those with functional, referring the patients to endoscopy should be considered due to its invasiveness and low cost-effectivity. Furthermore, a large number of uninvestigated dyspepsia cases are functional.6,7
The exact pathogenesis of FD is unknown; however, visceral hypersensitivity, such as gastric hypersensitivity to distension and acids and abnormal gastric motility, might play a role in developing dyspeptic symptoms.8,9 According to the definition, functional disorders are characterised by the absence of any organic pathology explaining the symptoms. A notable exception can be the Helicobacter pylori (H. pylori) infection, which is included in the definition of FD according to the Rome III criteria.10 Moreover, extensive population-based studies indicate that H. pylori might be a possible etiological factor in the pathogenesis of FD.11,12
A recent study has shown that patients with FD had a high prevalence and severity of chronic gastritis (CG) without H. pylori infection.13 Nevertheless, H. pylori infection was thought to be the leading cause of chronic gastritis.14 The aetiology of CG in H. pylori-negative patients was unknown and its implications in the pathogenesis in FD. Data regarding the relationship between H. pylori negative chronic gastritis and specific dyspeptic symptoms are lacking. Therefore, clarification of the underlying aetiology might be beneficial to set up the optimal treatment strategy for dyspepsia, and the H. pylori negative CG itself.
Studies suggest that immune activation might play a role in the pathogenesis of FD.15,16 Innate immune activation in the mucosa in FD has been described,17,18 but the prevalence of AI disorders due to immune activation in FD is uncertain.
This study aimed to assess the occurrence and pattern of GI symptoms, the prevalence of dyspeptic symptoms in patients with H. pylori-negative CG, and explore the possible role of the established etiological factors behind CG autoimmunity in the expression of dyspeptic symptoms.
Materials and Methods
Patients histologically diagnosed with chronic gastritis who underwent immune-serological testing between January 2016 and January 2020 were enrolled. For diagnosing gastritis, multiple biopsies (minimum of five) were taken from definite sites of the stomach, predefined by the updated Sydney system.19 Additional biopsies were performed from the areas of every detected focal lesion if any presented. To avoid performance bias, diagnosis and treatment of enrolled patients were carried out by the same single-unit medical team (one pathologist specialised in GI pathology reviewed all the histological findings, and one gastroenterologist performed all the endoscopy). Another, no dyspepsia-related study was previously performed on this population.20
All patients having any of the followings: (1) acute gastritis; (2) reactive gastropathy;21 (3) subjects without any serology testing results; (4) H. pylori positivity; (5) GERD, ulcer, or cancer were excluded from this study. Diagnosis of H. pylori infection was established by endoscopy, serological testing, followed by a urea breath test. The diagnosis of acute gastritis, reactive gastropathy, GERD, ulcer or cancer was confirmed by histological findings. Regarding the well-known association between dyspepsia and H. pylori infection, H. pylori can be considered as a confounding factor. Therefore, patients with H. pylori infection were excluded from the analysis to reduce bias.
Possible eligible patients from all clinical records of the outpatient unit led by a single specialist investigator were identified from an electronic database. Data collection was performed focusing on baseline characteristics of the population, histological results (location of the inflammation); autoantibody positivity (celiac disease-, Sjögren’s syndrome-, systemic lupus erythematosus (SLE)-, AI hepatitis-, rheumatoid arthritis (RA)-, SSc (systemic sclerosis)-, polymyositis/dermatomyositis-, AI thyroiditis-, IBD-, vasculitis-, AIG-related antibodies); H. pylori infection status (histology, results of the urea breath test and/or serology), symptoms (key symptoms, presence of dyspepsia-like symptoms: postprandial fullness, early satiety, epigastric pain, and epigastric burning). Patients were also categorised according to their autoantibody positivity: autoantibody seropositive (AISP) and autoantibody seronegative (AISN) groups. Patients were categorised into AISP group in case of at least one antibody positivity.
Autoantibody positivity was assessed using the threshold of our laboratory in accordance with the European Autoimmunity Standardisation Initiative (EASI).22,23 According to their occurrence in these conditions, detected autoantibodies were divided into autoimmune disease groups (Supplementary File 1). Grouping of patients was performed as per our previous autoimmune seromarker positivity and CG-related study.20
The following primary endpoint was investigated: association between AI positivity and dyspepsia-like symptoms (according to the Rome IV criteria1). In the case of the presence of one or more of the following symptoms: postprandial fullness, early satiety, epigastric pain, and epigastric burning, patients were categorized into the dyspepsia group.
The following secondary endpoints were assessed (1) the frequency of symptoms in CG, assessed in each patient by the same gastroenterologist; (2) the association between AISP and the most frequently occurred symptoms; (3) the location of the inflammation in the stomach assessed in each patient during endoscopy by the same gastroenterologist and confirmed by histopathological results; (4) association between AISP and the affected region of the inflammation.
The assessment of all variables was done on the level of AI disease and according to AISP and AISN groups.
Approval for this study was retrieved officially from the president of the Clinical Centre and the director of the First Department of Medicine of the University of Pécs (Institutional Review Board; case number: KK/999-1/2020). This study complies with the ethical guidelines of the Declaration of Helsinki updated in 2013 as reflected in a priori approval by the Institutional Review Board.24
The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guideline25 was followed during the data collection and analysis and the current legal environment (Supplementary File 2). According to the GDPR, all participating patients received a numeric code to protect privacy and personal data. Informed consent was not required in this retrospective set, although the data of those patients who refused data handling for scientific causes were not included.
Statistical Analysis
SPSS 25.0 software was used for the analysis of the data. Descriptive statistics (mean, standard deviation, minimum, maximum), and univariate analyses were performed. A 2-sided Pearson Chi-square test was done to compare dichotomous variables. In case of significant differences, standardised residuals were also observed to reveal the exact results. In the case of continuous variables, an independent sample t-test was used. We observed the distribution on Q-Q-plot. A P-value of less than 0.05 was considered statistically significant.
Results
In the final analysis, 175 patients (52 men and 123 women) with H. pylori-negative chronic gastritis were included. The mean age of the study population was 61.6 years (±15.13 years), ranging from 21 to 89. As described in our previous study, fifty-five per cent (97/175) of the analyzed patients had positive immunoserology (AISP group).20
Clinical Symptoms
Most frequently occurred symptoms were the followings: retrosternal burning sensation in 17.14% (30/175 patients); bloating and/or diarrhoea in 9.14% (16/175); diffuse abdominal discomfort/pain not relating to meals in 8.57% (15/175); globus sensation in 4% (7/175); nausea in 4.57% (8/175) and vomitus in 2.29% (4/175). All details about the symptoms can be seen in Table 1.
 |
Table 1 Distribution of Frequently Occurred Symptoms and Location of the Inflammation Between AI Positive and Negative Groups |
Diffuse abdominal pain/discomfort in the AISP group was significantly more common than in the AISN group (9 vs 6 patients, respectively, p = 0.023). Globus pharyngeus was more common in group AISP than the AISN group (p < 0.001): 6 patients experienced globus sensation in the AISP group, while one patient in the AISN group.
We did not find any significant differences with the other symptoms between AISP and AISN groups in our analysis. Retrosternal burning occurred in 12 patients in the AISP group and 18 patients in the AISN group (p = 0.0713). Less common symptoms included nausea (4 AISP and 4 AISN patients, p = 1.000), vomiting (1 AISP and 3 AISN patients, p = 0.325), and bloating and/or diarrhoea (9 AISP and 7 AISN patients, p = 0.152) (Table 1).
Dyspepsia-Like Symptoms in Autoimmune Seropositivity
Dyspepsia-like symptoms were present in 54.29% of the patients (95/175) and were associated more with AISP (p = 0.012). Association was found regarding celiac disease-related antibody positivity and dyspepsia (p = 0.045), while ANCA and ASCA positivity were also associated with dyspepsia-like symptoms (p = 0.043). However, the analysis could not find a significant association between dyspepsia-like symptoms and other AI-related antibody positivity, like Sjögren’s syndrome, SLE, AI hepatitis, RA, SSc, polymyositis/dermatomyositis, and AI thyroiditis (p > 0.05). No significant association was found considering AIG-related antibody positivity and dyspepsia either (p = 0.677). Detailed results regarding the association between autoimmunity and dyspeptic symptoms are given in Table 2.
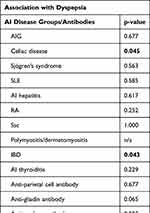 |
Table 2 Detailed Results Regarding the Association Between Autoimmunity and Dyspeptic Symptoms |
Location and Extent of the Inflammation
Most of the examined patients had pangastritis (59.43%); the inflammation affects the entire stomach in 57 (58.76%) patients of the AISP group and 47 (60.26%) patients of the AISN group. Lesions of gastritis were found in the antrum in 33 (34%) AISP and 23 AISN patients and were associated with autoimmune positivity (p = 0.042). Isolated corpus affection was related to autoimmunity as well (p = 0.023); inflammation of the corpus was found in 9 (9.28%) AISP and 6 (7.70%) AISN patients, respectively (Table 2.).
Discussion
This retrospective cross-sectional study, including data of 175 patients, aimed to investigate the possible relationship between autoimmunity and dyspeptic symptoms in patients with H. pylori negative chronic gastritis. One of our significant findings was that the prevalence of dyspepsia-like symptoms was 54.29%. Regarding the association between the symptoms and autoimmunity, dyspeptic symptoms, diffuse abdominal pain/discomfort, and globus pharyngeus seem to be more common in patients with AISP. A significant association was found between celiac disease-related antibody positivity, ASCA and ANCA positivity and dyspeptic symptoms. However, the analysis could not prove that other AI disease-related antibody positivity was more common in CG patients with FD.
It was previously shown in the literature that H. pylori infection might be associated with FD: the prevalence of H. pylori infection is more frequent in dyspeptic patients than in healthy controls.26,27 A meta-analysis of 12 randomized controlled studies concluded that eradication of H. pylori is associated with improvement of dyspeptic symptoms in patients with FD.28 Several studies suggested that H. pylori can alter gastric functions: it causes hypergastrinemia, hyperpepsinogenemia, and acid hypersecretion, which might play a role in the pathogenesis of FD.29
A high prevalence of dyspeptic symptoms was also reported in patients with H. pylori-negative CG.13 Although CG is a prevalent pathology found in upper GI endoscopy, the underlying aetiology often remains unknown;30 therefore, we looked for possible causative factors behind CG that may be associated with dyspeptic symptoms.
In our study, more than half of the patients with non-investigated chronic gastritis showed systemic autoantibody positivity, and it was associated with dyspeptic symptoms. Several articles in the literature mention the possible association between autoimmune diseases and dyspepsia. Dyspeptic symptoms are presented in 50–60% of the patients with AI disorders and may result from gastroparesis and antral distension.31,32 However, Koloski et al concluded that autoimmune diseases are risk factors for functional gastrointestinal disorders, such as FD, due to immune dysregulation.33
In line with our results, Jocelyn A Silvester et al showed that FD occurs in 27% of patients with coeliac disease, which is relieved in most cases following the treatment of a gluten-free diet,34 and A. Maertens et al reported a case about how dyspepsia led a diagnosis of Morbus Crohn.35 Furthermore, our study confirms the investigation of Lebwohl et al about the association between H. pylori-negative CG with celiac disease.36 Higher incidence of dyspeptic symptoms has also been observed in patients with Sjögren’s syndrome, SLE, RA, and AI thyroiditis;31,32,37–42 however, our study could not confirm these associations.
Gastrointestinal manifestation occurs in most patients with systemic autoimmune disorders,43–45 and these symptoms might be subclinical, non-specific, with considerable overlap among different conditions. Sometimes it can be the only presented sign of an underlying AI disease. The advent of serologic testing for immune-mediated GI disorders (eg, celiac disease, IBD) allows broader screening, helping differentiate organic disease from functional GI disorders.
To our knowledge, this is the first study, which investigated the possible organic etiological factors behind chronic H. pylori-negative gastritis in association with FD. As mentioned above, there were previous descriptions of the possible connection between certain autoimmune disorders and dyspepsia; however, a comprehensive study, excluding confounding factors to answer this question in a targeted manner, has not been performed previously. This work contains the investigation of the widest coverage of systemic AI disorders related antibody positivity and dyspepsia, and the study was conducted following a rigorous, pre-defined methodology. Furthermore, in the chronic gastritis patient population, where there is no identified etiological factor behind chronic inflammation, the cause of dyspepsia-like symptoms has not been investigated before.
However, our research had several limitations, which should be considered for a correct interpretation. The results are based on a single-centre, retrospective analysis, with a relatively low event rate in each antibody positivity, which might be the reason for insignificance in some cases. It is well known that the prevalence of FD is significantly higher in women, smokers, non-steroidal anti-inflammatory drug (NSAID) users,27 and in the ageing stomach;46 they should be considered confounding factors in our study. Moreover, chronic atrophic gastritis itself may contribute to the development of dyspeptic symptoms by influencing the level of gastric acid, pepsin, and ghrelin secretion;8,47 however, data regarding the relationship between atrophic gastritis and specific dyspeptic symptoms are lacking. The limited information on this topic and our research’s limitations could serve as a subject for conducting prospective clinical studies with a larger event rate.
Conclusion
In conclusion, autoimmune positivity in histologically established H. pylori-negative CG may predispose to dyspeptic symptoms and may be the causative factor behind uninvestigated FD. In this study, celiac disease-related antibody positivity, ASCA and ANCA positivity were associated with dyspeptic symptoms. However, our analysis could not prove any association between dyspepsia-like symptoms and Sjögren’s syndrome, SLE, AI hepatitis, RA, SSc, Polymyositis/dermatomyositis, AI thyroiditis, or even AIG.
Based on our data, screening for celiac disease or ASCA and ANCA-related AI disorders (IBD, vasculitis) in the presence of dyspeptic symptoms might be crucial. Furthermore, screening for these autoantibodies (ANCA-, ASCA-, celiac-disease-related antibodies) in patients with FD can be more cost-effective, considering the earlier diagnosis of these autoimmune diseases. However, our results should be interpreted with caution since the retrospective nature of this study. To establish a higher quality of evidence, further prospective studies are required to prove the association between AI disorders (especially GI-related AI disorders; eg, IBD, celiac disease and vasculitis) and dyspeptic symptoms.
Abbreviations
AI, autoimmune; AISN, autoimmune disease-related seromarker negativity; AISP, autoimmune disease-related seromarker positivity; ANCA, anti-neutrophil cytoplasmic antibody; ASCA, anti-Saccharomyces cerevisiae antibody; CG, chronic gastritis; FD, functional dyspepsia; GERD, gastro-esophageal reflux disease; GI, gastrointestinal; H. pylori, Helicobacter pylori; IBD, inflammatory bowel disorders; IM, intestinal metaplasia; NSAID, non-steroidal anti-inflammatory drug; RA, rheumatoid arthritis; SLE, systemic lupus erythematosus; Ssc, systemic sclerosis.
Ethics and Dissemination
Ethical approval: University of Pécs, Clinical Centre, Institutional Review Board; case number: KK/999-1/2020.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
There is no funding to report.
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Stanghellini V, Chan FK, Hasler WL, et al. Gastroduodenal Disorders. Gastroenterology. 2016;150(6):1380–1392. doi:10.1053/j.gastro.2016.02.011
2. Heading R. Prevalence of upper gastrointestinal symptoms in the general population: a systematic review. J Gastroenterol. 1999;231:3–8.
3. El‐Serag H, Talley N. The prevalence and clinical course of functional dyspepsia. Aliment Pharmacol Ther. 2004;19(6):643–654. doi:10.1111/j.1365-2036.2004.01897.x
4. Camilleri M, Dubois D, Coulie B, et al. Prevalence and socioeconomic impact of upper gastrointestinal disorders in the United States: results of the US Upper Gastrointestinal Study. Clin Gastroenterol Hepatol. 2005;3(6):543–552. doi:10.1016/S1542-3565(05)00153-9
5. Olokoba A, Olokoba L, Jimoh A, Salawu F, Danburam A, Ehalaiye B. Upper gastrointestinal tract endoscopy indications in northern Nigeria. JCPSP. 2009;19(5):327–328.
6. Ford AC, Marwaha A, Lim A, Moayyedi P. What is the prevalence of clinically significant endoscopic findings in subjects with dyspepsia? Systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2010;8(10):830–837. doi:10.1016/j.cgh.2010.05.031
7. Faintuch JJ, Silva FM, Navarro-Rodriguez T, et al. Endoscopic findings in uninvestigated dyspepsia. BMC Gastroenterol. 2014;14:19. doi:10.1186/1471-230X-14-19
8. Chung SH, Lee KJ, Kim JY, et al. Association of the extent of atrophic gastritis with specific dyspeptic symptoms. J Neurogastroenterol Motil. 2015;21(4):528–536. doi:10.5056/jnm15074
9. Tack J, Talley NJ, Camilleri M, et al. Functional gastroduodenal disorders. Gastroenterology. 2006;130(5):1466–1479. doi:10.1053/j.gastro.2005.11.059
10. Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology. 2006;130(5):1480–1491. doi:10.1053/j.gastro.2005.11.061
11. Suzuki H, Moayyedi P. Helicobacter pylori infection in functional dyspepsia. Nat Rev Gastroenterol Hepatol. 2013;10(3):168–174. doi:10.1038/nrgastro.2013.9
12. Bazzoli F, Palli D, Zagari R, et al. The Loiano‐Monghidoro population‐based study of Helicobacter pylori infection: prevalence by 13C‐urea breath test and associated factors. Aliment Pharmacol Ther. 2001;15(7):1001–1007. doi:10.1046/j.1365-2036.2001.00972.x
13. Peura DA, Haber MM, Hunt B, Atkinson S. Helicobacter pylori-negative gastritis in erosive esophagitis, nonerosive reflux disease or functional dyspepsia patients. J Clin Gastroenterol. 2010;44:3. doi:10.1097/MCG.0b013e3181ac9830
14. Goh KL, Chan WK, Shiota S, Yamaoka Y. Epidemiology of Helicobacter pylori infection and public health implications. Helicobacter. 2011;16:1–9. doi:10.1111/j.1523-5378.2011.00874.x
15. Liebregts T, Adam B, Bredack C, et al. Small bowel homing T cells are associated with symptoms and delayed gastric emptying in functional dyspepsia. Am J Gastroenterol. 2011;106(6):1089–1098. doi:10.1038/ajg.2010.512
16. Keely S, Walker MM, Marks E, Talley NJ. Immune dysregulation in the functional gastrointestinal disorders. Eur J Clin Invest. 2015;45(12):1350–1359. doi:10.1111/eci.12548
17. Walker M, Talley N, Prabhakar M, et al. Duodenal mastocytosis, eosinophilia and intraepithelial lymphocytosis as possible disease markers in the irritable bowel syndrome and functional dyspepsia. Aliment Pharmacol Ther. 2009;29(7):765–773. doi:10.1111/j.1365-2036.2009.03937.x
18. Walker MM, Aggarwal KR, Shim LS, et al. Duodenal eosinophilia and early satiety in functional dyspepsia: confirmation of a positive association in an A ustralian cohort. J Gastroenterol Hepatol. 2014;29(3):474–479. doi:10.1111/jgh.12419
19. Dixon MF, Genta RM, Yardley JH, Correa P. Classification and grading of gastritis: the updated Sydney system. Am J Surg Pathol. 1996;20(10):1161–1181. doi:10.1097/00000478-199610000-00001
20. Zádori N, Németh D, Szakó L, et al. Prevalence of autoimmune-phenomena behind chronic gastritis of unknown origin, and its role in poor histological outcome of the stomach: a single-centre, retrospective cross-sectional study. JGLD. 2022;31(2):168–175. doi:10.15403/jgld-4218
21. Chen W, Catherine E. Reactive (chemical) gastropathy; 2022. Available from: https://www.pathologyoutlines.com/topic/stomachchemical.html.
22. Bizzaro N, Bossuyt X, Haapala AM, Shoenfeld Y, Sack U. Accreditation in autoimmune diagnostic laboratories. A position paper of the European Autoimmunity Standardisation Initiative (EASI). Autoimmun Rev. 2017;16(1):81–86. doi:10.1016/j.autrev.2016.09.021
23. Damoiseaux J, Olschowka N, Shoenfeld Y. EASI – European Autoimmunity Standardisation Initiative: facing the challenges of diagnostics in autoimmunity. Clin Chem Lab Med. 2018;56(10):1620–1623. doi:10.1515/cclm-2017-0826
24. Goodyear MD, Krleza-Jeric K, Lemmens T. The declaration of Helsinki. BMJ. 2007;335:624–625. doi:10.1136/bmj.39339.610000.BE
25. Von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med. 2007;147(8):573–577. doi:10.7326/0003-4819-147-8-200710160-00010
26. Shah SS, Bhatia SJ, Mistry FP. Epidemiology of dyspepsia in the general population in Mumbai. Indian J Gastroenterol. 2001;20(3):103–106.
27. Ford AC, Marwaha A, Sood R, Moayyedi P. Global prevalence of, and risk factors for, uninvestigated dyspepsia: a meta-analysis. Gut. 2015;64(7):1049–1057. doi:10.1136/gutjnl-2014-307843
28. Zhao B, Zhao J, Cheng W-F, et al. Efficacy of helicobacter pylori eradication therapy on functional dyspepsia: a meta-analysis of randomized controlled studies with 12-month follow-up. J Clin Gastroenterol. 2014;48(3):241–247. doi:10.1097/MCG.0b013e31829f2e25
29. McColl K. Helicobacter pylori and acid secretion: where are we now? Eur J Gastroenterol Hepatol. 1997;9(4):333–335. doi:10.1097/00042737-199704000-00004
30. Bai Y, Li ZS, Zou DW, et al. Alarm features and age for predicting upper gastrointestinal malignancy in Chinese patients with dyspepsia with high background prevalence of Helicobacter pylori infection and upper gastrointestinal malignancy: an endoscopic database review of 102,665 patients from 1996 to 2006. Gut. 2010;59(6):722–728. doi:10.1136/gut.2009.192401
31. Geyl S, Jacques J, Parreau S, et al. La gastroparésie peut être à l’origine de symptômes digestifs hauts inexpliqués chez les patients souffrant d’un syndrome de Goujerot-Sjögren. [Gastroparesis may cause unexplained upper gastrointestinal symptoms in patients with Goujerot-Sjögren syndrome]. La Revue de Méd Interne. 2018;39(6):427–430. doi:10.1016/j.revmed.2018.02.007
32. Ohlsson B, Scheja A, Janciauskiene S, Mandl T. Functional bowel symptoms and GnRH antibodies: common findings in patients with primary Sjögren’s syndrome but not in systemic sclerosis. Scand J Rheumatol. 2009;38(5):391–393. doi:10.1080/03009740802709069
33. Koloski N, Jones M, Walker MM, et al. Population based study: atopy and autoimmune diseases are associated with functional dyspepsia and irritable bowel syndrome, independent of psychological distress. Aliment Pharmacol Ther. 2019;49(5):546–555. doi:10.1111/apt.15120
34. Silvester JA, Graff LA, Rigaux L, et al. Symptoms of functional intestinal disorders are common in patients with celiac disease following transition to a gluten-free diet. Dig Dis Sci. 2017;62(9):2449–2454. doi:10.1007/s10620-017-4666-z
35. Maertens A, Persyn D, Van Moerkercke W. How dyspepsia led to the diagnosis of Morbus Crohn. Acta Clin Belg. 2020;75(4):293–295. doi:10.1080/17843286.2019.1590497
36. Lebwohl B, Green PHR, Genta RM. The coeliac stomach: gastritis in patients with coeliac disease. Aliment Pharmacol Ther. 2015;42(2):180–187. doi:10.1111/apt.13249
37. Sjogren RW. Gastrointestinal features of scleroderma. Curr Opin Rheumatol. 1996;8(6):569–575. doi:10.1097/00002281-199611000-00012
38. García-Carrasco M, Mendoza-Pinto C, Autrán-Limón MA, et al. Prevalence of functional gastrointestinal disorders in adults with systemic lupus erythematosus. Lupus. 2018;27(5):788–793. doi:10.1177/0961203317747718
39. al-Hakeem MS, McMillen MA. Evaluation of abdominal pain in systemic lupus erythematosus. Am J Surg. 1998;176(3):291–294. doi:10.1016/s0002-9610(98)00155-x
40. Dyspepsia DA. Refractory to conventional measures in patients with rheumatoid arthritis: don’t forget helicobacter pylori. J Assoc Physicians India. 2018;66(12):107.
41. Gibberd FB. Dyspepsia in patients with rheumatoid arthritis. Acta Rheumatol Scand. 1966;12(2):112–121. doi:10.3109/rhe1.1966.12.issue-1-4.13
42. Ebert EC. The thyroid and the gut. J Clin Gastroenterol. 2010;44(6):402–406. doi:10.1097/MCG.0b013e3181d6bc3e
43. McFarlane IM, Bhamra MS, Kreps A, et al. Gastrointestinal manifestations of systemic sclerosis. Rheumatology. 2018;8(1). doi:10.4172/2161-1149.1000235
44. Li Z, Xu D, Wang Z, et al. Gastrointestinal system involvement in systemic lupus erythematosus. Lupus. 2017;26(11):1127–1138. doi:10.1177/0961203317707825
45. Ebert EC. Gastrointestinal and hepatic manifestations of Sjogren syndrome. J Clin Gastroenterol. 2012;46(1):25–30. doi:10.1097/MCG.0b013e3182329d9c
46. Walker MM, Talley NJ. Functional dyspepsia in the elderly. Curr Gastroenterol Rep. 2019;21(10):1–6. doi:10.1007/s11894-019-0722-5
47. Sipponen P. Update on the pathologic approach to the diagnosis of gastritis, gastric atrophy, and Helicobacter pylori and its sequelae. J Clin Gastroenterol. 2001;32(3):196–202. doi:10.1097/00004836-200103000-00003
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
