Back to Journals » Infection and Drug Resistance » Volume 15
Durability of SARS-CoV-2 Specific IgG Antibody Responses Following Two Doses of Match and Mixed COVID-19 Vaccines Regimens in Saudi Population
Authors Mubarak A , Almutairi S, Al-Dhabbah AD, Aldabas SY, Bhat R, Alqoufail MM, Abdel-Maksoud MA, Almanaa TN, Farrag MA, Alturaiki W
Received 7 April 2022
Accepted for publication 6 July 2022
Published 15 July 2022 Volume 2022:15 Pages 3791—3800
DOI https://doi.org/10.2147/IDR.S369769
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Ayman Mubarak,1 Saeedah Almutairi,1 Abulrahman D Al-Dhabbah,1 Shaha Y Aldabas,1 Rauf Bhat,1 Mahfoudh M Alqoufail,1 Mostafa A Abdel-Maksoud,1 Taghreed N Almanaa,1 Mohamed A Farrag,1 Wael Alturaiki2
1Department of Botany and Microbiology, College of Science, King Saud University, Riyadh, 11451, Saudi Arabia; 2Department of Medical Laboratory Sciences, College of Applied Medical Sciences, Majmaah University, Majmaah, 11952, Saudi Arabia
Correspondence: Wael Alturaiki, Email [email protected]
Background: SARS-CoV-2 pandemic continues to threaten the human population with millions of infections and deaths worldwide. Vaccination campaigns undertaken by several countries have resulted in a notable decrease in hospitalization and deaths. However, with the emergence of new virus variants, it is critical to determine the longevity and the protection efficiency provided by the current authorized vaccines.
Aim: The aims of this study are to provide data about the magnitude of immune responses in individuals fully vaccinated against COVID-19 in Riyadh province of Saudi Arabia. Also, to evaluate the continuity of specific IgG levels and compare the titers in individuals who have been received two doses of the matched and mixed vaccines, including Pfizer and AstraZeneca against SARS-CoV-2 during the period of three to six months. Moreover, we analyze the current state of immune response in terms of antibody responses in thepopulation postvaccination using homogenous or hetrogenous vaccine regimen.
Methods: A total of 141 healthy volunteers were recruited to our study; blood (n=63) and the saliva samples (n=78) and were collected from fully vaccinated individuals in Riyadh city. We employed a specific ELISA assay in plasma and saliva of fully vaccinated individuals.
Results: IgG levels varied with age groups with the highest concentration in the age group 19– 29 years, but the age group (≥ 50) had the lowest IgG concentration. The IgG levels in both serum and saliva were higher after three months and start to wane after six months. Individuals who received mixed types of vaccines had significantly better response than Pfizer vaccine alone.
Conclusion: The current study investigates the status of humoral responses in different age groups, in terms of antibody measurements. These data will help to evaluate the need for further COVID-19 vaccine doses and to what extent a two-dose regimen will protect vaccinated individuals.
Keywords: SARS-CoV-2, antibody titer, anti-spike IgG antibody, Pfizer vaccine, AstraZeneca vaccine
Introduction
The SARS-CoV-2 pandemic is still ongoing with more than 464 million infections and up to six million deaths.1 Shortly after the virus spread on a global scale, vaccine production and trials were started by several multinational pharma companies and the vaccine produced by Pfizer/BioNTech was the first to be authorized for emergency use. However, the duration of immunological protection at individual level still necessitates further investigation. Evaluating the durability of the immune response induced by the SARS-CoV-2 infection, particularly the humoral immune response, is critical for understanding the pathogenesis of SARS-CoV-2 and evaluating the protection efficiency of the current vaccines.2,3
Human antibody responses against SARS-CoV-24 are generated by viral proteins such as spike glycoprotein (S protein) and nucleocapsid protein, of which the former can induce neutralizing antibodies required for viral neutralization and eradication by inhibiting viral interaction with the host cells.5 SARS-CoV-2 penetrates host cells similarly to SARS-CoV-1 by binding to angiotensin-converting enzyme 2 (ACE2) using the S protein.6 ACE-2 is expressed on the surface of several cell types such as alveolar epithelial cells, small intestine epithelial cells, endothelial cells, and arterial smooth muscle cells.7,8 SARS-CoV-2 S protein is approximately 180 kDa in size and is composed of S1 and S2 subunits, of which the former contains the ACE2 receptor-binding domain (RBD, amino acid residues 331–524).9
The FDA approved the emergency use of SARS-CoV-2 mRNA vaccines with a two-dose schedule in December 2020; BNT162b2/Pfizer for instance.10,11 While phase 3 trials for both vaccines showed excellent effectiveness in preventing symptomatic SARS-CoV-2 infections after the second dose, new studies suggest that a single dose is enough to increase immunity to high levels in previously infected people.12–15 Since anti-SARS-CoV-2 seropositivity significantly exceeds reported COVID-19 cases in the population,16,17 it is crucial to see if vaccination response patterns change depending on SARS-CoV-2 exposure history. This is very important because the majority of infections are asymptomatic or mild, and the intensity of symptoms predicts the level of antibodies following natural infection.18,19
Our research group sought to determine the level of serum and saliva anti-S IgG titer among different age groups and assessed the persistent of antibodies following two doses of different COVID-19 vaccines.
Materials and Methods
Sample Collection
A total of 141 healthy volunteers were recruited to our study and were collected from fully vaccinated individuals in Riyadh city. The collection time of samples was after three and six months of vaccination. One group provided blood samples (n=63) and the other group provided saliva samples (n=78) and all were collected at the blood bank departments. The mean age was 32 years (range: 13–88 years). All of the subjects received two doses of COVID-19 vaccine either of Pfizer BNT162b2, AZD1222, or a mixture of vaccines, between February 17 and September 11, 2021. Of the enrolled participants, 72 subjects (51.1%) received Pfizer BNT162b2, 31 (21.9%) of AZD1222 and 38 (26.9%) immunized with mixture of Pfizer BNT162b2 and AZD1222.
Ethical Approval
The study was approved by the research ethics committee at King Saud University Medical City (22/0063/IRB) and followed the Declaration of Helsinki ethical standards. All subjects signed written informed consent before conducting the experiments. Also, parent or legal guardian of participants under 18 years of age provided informed consent.
Inclusion and Exclusion Criteria
The eligible participants for this study had experienced no recurrent infection or chronic diseases. Only individuals who had received two doses of COVID-19 vaccines either of the same type or mixed doses were included in our study. The exclusion criteria were pregnancy, immunocompromised people, diabetic, blood hypertension, organ graft, autoimmunity diseases and allergic, and chronic disease. All important information was collected from volunteered individuals who want to participate in the study; such as age, gender, date of the second dose of vaccine whether infected with SARS-CoV-2 or not during the pandemic according to a government application developed for citizens known as Tawakkalna.
Samples Processing
Blood was collected from the volunteers in an anticoagulant tube, later plasma was separated by centrifugation. The whole saliva samples were self-collected using saliva tubes provided in this study. In terms of saliva samples, viral inactivation with heat treatment at 56°C for 30 min was performed as described in Alkharaan et al.20 The processed samples were distributed into aliquots to avoid freeze-thaw recycle and were kept at −80°C for further analysis.
Measurement Anti-IgG SARS-CoV-2 Using ELISA Assay
To detect SARS-CoV-2 anti-S IgG antibodies, a specific SARS-CoV-2 IgG ELISA kit was used (BGI Europe A/S) according to the manufacturer’s instructions. The specificity and sensitivity of IgG antibody ELISA kit is 98.38% and 98.71%, respectively. Briefly, pre-coated 96-well with purified SARS-CoV-2 viral antigen ELISA plates were used. Positive and negative controls (to calculate the cutoff) and blanks were used in this assay. One hundred microliters of positive and negative controls were added to the assigned wells without dilution, and no liquid was added to the blank well. A 10 µL of the plasma and 20 µL of saliva were added to each well along with 100 µL of sample diluent buffer, and the plate was incubated at 37°C for 30 min. The plates were then washed 3–5 times using ELISA washer. A volume of 100 µL of anti-human IgG-HRP (conjugated antibody) was added to each well and incubated for 20 min at 37°C. The plates were washed 3–5 times, and 50 µL of substrates (A and B) were added to each well and incubated in the dark for 10 min at 37°C. Finally, 50 µL of the stop solution was added to each well. The optical density was determined at 450 nm. All samples were subtracted from the blank. The cutoff values (0.235) for anti-SARS-CoV-2 IgG antibody detection was calculated according to kit instructions (0.1 + mean absorbance (0.135) of the two negative controls).
Statistical Analysis
All data analysis was performed using GraphPad Prism statistical software. The response of IgG antibody was presented as standard error of mean (SEM) in unit/mL. Differences between independent groups were analyzed using Mann–Whitney U-test. The correlation coefficient between age and antibody titers was analyzed using Pearson's test. A p-value <0.05 was considered significant.
Results
Characteristics of the Participants
The level of antibodies in different gender and different vaccine was presented in Table 1, Females have slightly higher titer of IgG antibody than males (p=0.012) in plasma samples even in saliva, but there was no significant differences. Also, there are no differences between smoker and nonsmoker group but smoking has a slight effect on the induction of IgG level in plasma and saliva.
 |
Table 1 The Demographic Data of 141 Participants Vaccinated with Different COVID-19 Vaccines |
IgG Antibody Titer in Plasma and Saliva is Age Dependent
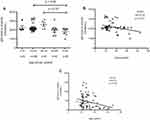
To investigate if any correlation between the ages (13 and 88 years) and the antibody levels exist, we measured the levels of antibody in plasma and saliva of different age groups. As shown in (Figure 1A), we observe a negative correlation between plasma antibody titer and age (n=63, r= −0.32, p=0.01). The result showed that different age groups have different level of IgG antibodies, 13–18 years, (2066 unit/mL), 19–29 years (2128 unit/mL), 30–39 years (2588 unit/mL, 40–50 years (2024 unit/mL) and ≥50 years (2064 unit/mL) (Figure 1B). The older group (≤50) shows the lowest titer of anti-S IgG antibodies in comparison with 30–39 years (p=0.02) and 19–29 years (p=0.02). The highest antibody titer was observed in the age group 19–39 years. As shown in (Figure 1C), in saliva, there was a similar pattern of significant correlation with age (n=78, r= −0.3, p=0.02).
The Levels of IgG Antibody Titer Waned Six Months Postvaccination
A significant increase was noticed after the second dose of SARS-COV-2 vaccine, followed by a gradual decrease in the level of IgG in plasma. It was also noticed (Figure 2A) in the participants that after three months, the titer of IgG in their plasma was higher than after six months following the immunization (2125 vs 1804 unit/mL, p=0.003). Also, IgG in saliva (Figure 2B) remarkably decreased after six months in comparison to three months postvaccination (45.44. vs 23.38; p=0.005).
Different Vaccine Types Lead to Variable IgG Titers
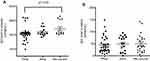
To assess the level of IgG in response to different vaccine types, several subjects were analyzed. As shown in Figure 3A, the mixture of vaccines had a significantly better response than the Pfizer vaccine alone (2424 vs 2080; p=0.02). Interestingly, the response observed in the AstraZeneca group is slightly lower (but not significant) than the mixed vaccine group (2213 vs 2424) but they were both more than the Pfizer group (2080). In case of saliva IgG (Figure 3B), mixed vaccines had slightly increased the level of IgG (51 units/mL) compared to single vaccine Pfizer (44 units/mL) and AstraZeneca (48.75 units/mL).
The IgG Levels Waned After Two Doses of Pfizer-BNT162b2 in Age Over 50
We have shown that the IgG levels waned after two doses of Pfizer-BNT162b2 in both plasma (Figure 4A, n=37, r= −0.35, p=0.03) and saliva (Figure 4B, n=35, r= −0.32, p=0.06), whereas the levels remained approximately constant after two doses of AZD1222 or mixed vaccine.
Discussion
Different vaccines to combat the deadly COVID-19 pandemic have been approved worldwide.21 These vaccines are produced by using a variety of platforms, including mRNA targeting particular SARS-CoV-2 antigens, viral-vector-based, or inactivated-virus-based vaccines, with intramuscular injection being the most common method of administration.21–23 To date, all vaccinations approved for use in the general public have demonstrated seroconversion.21 Since oral and nasal routes are the most favoured pathways for respiratory viruses, we investigated whether we can monitor antibody responses in individuals vaccinated against COVID-19 with the currently available vaccines. In the present study, we measured the magnitude of persistence of antibodies in plasma and saliva in individuals vaccinated with currently available anti-COVID-19 vaccines. The longevity of IgG antibody responses following two doses of SARS-CoV-2 vaccine regimens in both the plasma and saliva will decide the time point at which the booster dose should be administered to the vaccinated population.
We showed that antibody titer might be affected by gender. The number of female samples was very low, but we demonstrated that a higher anti-SARS-CoV-2 IgG is found in females than males (Table 1). The results are consistent with other study observation noted that females generate stronger humoral immunity and greater vaccine efficacy than males.24,25 Also, smoking may reduce the effectiveness of the vaccine. In our result, we noted that the smokers have lower antibody level than nonsmokers (Table 1). Other study showed the smokers were at risk of a reduced immune response to COVID-19 vaccines, although more research is needed before firm conclusions can be drawn.26
Our data shows that IgG titer response to SARS-CoV-2 antigen is generated both in systemic or mucosal compartments following the second dose of vaccine regimen. The antibody response against SARS-CoV-2 involves production of high concentrations of specific IgG in systemic circulation that then migrate to other mucosal sites but are detectable in very low amounts in saliva and other sites, even though the level of total IgA is high.27 In the current study, the IgG antibody response to SARS-CoV-2 was examined over a period of 180 days (six months) in both biofluids: plasma and saliva. The persistence of IgG was noticed until 120–160 days which then slowly declines within the time period of 180 days. Our group and other groups2,28 have noticed that saliva shows the presence of SARS-CoV-2 specific IgG. We have found there was no decline in the level of anti-spike IgG over the three-month period in plasma. Similar to plasma data, anti-IgG levels to SARS-CoV-2 antigens remains constant over the three-month period.
Our findings demonstrated that the level of anti-spike IgG was significantly declined in aged people after six months of having two doses of vaccine, suggesting that a third dose may be required to enhance their immune response. Other studies considered that decline is age dependent both in plasma and saliva.27,29,30 A study on healthcare workers support an inverse correlation between antibody response and age in those receiving BNT162b2 vaccine or the AZD1222 vaccine.24 Also, line with our findings, it has been reported that anti-SARS-CoV-2 antibody titer in older participants is significantly declined at six months after receiving two doses of BNT162b2 vaccine, and interestingly the smoking and age were the most important factors associated with lower antibody titers.31 Alkharran et al20 have shown that the IgG level remained constant in blood and saliva in most COVID-19 patients who had recovered from mild symptoms up to nine months and which can be considered as durable in relative to IgA antibody.
Previous studies of severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS) found antibodies in 80–100% of patients two weeks after symptoms onset.32–34 MERS and SARS antibody levels persist for two to three years following symptom onset.33,35 Previous reports have shown that patients infected with SARS-CoV-2 rarely produce specific antibodies (Abs) within the first seven days of symptoms. Also, more than 90% of SARS-CoV-2 patients acquire specific IgM and IgG antibodies within 10–11 days following the start of symptoms.36–38 Within 17 to 19 days after appearance of symptoms, 100% of patients tested positive for virus-specific IgG, whereas the proportion of patients with virus-specific IgM peaked at 94.1% 20 to 22 days later.39
During SARS-CoV-2 infection, the kinetics of the immune response, its magnitude, and its connection to disease severity have all been thoroughly studied.40 The immune response and the level of IgG titer is reduced in the asymptomatic,39 whereas the level of Ab titer is different between the patients regardless of the clinical course of SARS-CoV-2 infection, and approximately 5% of patients have undetectable antibody titers even with confirmed infection.41 Several long-term studies have found that most patients have detectable SARS-CoV-2 antibody responses up to 13 months after infection, suggesting that it may continue much longer than expected.42–45
Intriguingly, the persistence of salivary secretory antibodies after several months’ postvaccination could possibly be explained by the presence of circulatory determinants of viral protection and their presence in the oral mucosal cavity. However, data explaining this phenomenon in SARSCoV-2 infection or postvaccination is still very rare and further studies are required to decipher the mechanism.
For SARS-CoV-2 immunization, a novel technique known as the “mix-and-match” approach has developed, which may be required as evidence against vaccine supply disruptions and may also assist to limit the transmission in developing variations.46 Currently, several studies have revealed that mixing the Oxford AstraZeneca and the Pfizer BioNTech vaccines induces a more significant immune response than two doses of the same vaccine.47–50 Similarly, we observed that the mixed vaccine of Oxford AstraZeneca and the Pfizer BioNTech offers significantly better response and induced higher IgG levels than receiving two doses of Pfizer BioNTech (Figure 3), suggesting the importance of this approach to overcome vaccine shortage and induce an effective immune response against SARS-CoV-2 infection. We have shown that the IgG levels in the aged group waned after two doses of BNT162b2 (Figure 4), whereas levels remained approximately constant after two doses of AZD1222 or mixed vaccines. This could provide an important lead for future vaccination strategy keeping in view the availability factor too. Our data supports this in conformity with other studies.51,52
Although current vaccines have offered effective immune response against SARS-COV-2 infection, mutations occurring in the genome of SARS-CoV-2 have led to development of variants of concern that can reduce effectiveness of the available current vaccines.46 Furthermore, former studies on using heterologous vaccines have shown great success of vaccine performance through inducing both higher T-cell and humoral immune response.53 Moreover, to address the issue of vaccine shortage in developing countries, and evoke greater immune response in the recipients, the mix-and-match vaccine approach can serve as an effective tool in the vaccination campaign against COVID-19.46
Conclusions
The novelty of our study is that we included the individuals who had received mixed doses of vaccine and compared it to the individuals who had received two shots of individual vaccines. This provided an added benefit of the study to determine if the vaccine mix could provide better protection compared to individual vaccines in terms of salivary antibodies. Moreover,a this could provide additional evidence whether there exists any medium or long-term benefit of individual vaccines and more importantly, the vaccine mix. Our data indicates that the combi-vaccine mix offers more protection in terms of magnitude of secretory and systemic antibody responses. Thus, the secretory antibodies in saliva may offer primary protection against respiratory viruses. The same could be true for SARS-CoV-2 infection and postCOVID-19 vaccination. The response of antibody starts to decrease after six months and might become undetectable after 12 months, therefore a third dose is recommended to be taken to boost the immune response, especially in elderly and immunocompromised people.
Future Direction
Several aspects of humoral immune response, threshold titers of neutralizing antibodies required for protection, and the durability of immunity induced by natural infection or after COVID-19 vaccination are still being investigated, and future studies are expected to yield more specific conclusions about protection against SARS-CoV-2 infection. More research is needed to determine how long these immune responses can last and if booster doses are necessary. Variations of SARS-CoV-2 are now emerging as a significant factor in evaluating whether COVID-19 vaccines will be effective against these novel variants. As a result, a deeper knowledge of the nature and duration of immune responses following viral infections or after COVID-19 vaccination is essential for revealing immunological systems implicated in reinfection and vaccine protection.54
Institutional Review Board Statement
The study was approved by the research ethics committee at King Saud University Medical City (22/0063/IRB) and followed the Declaration of Helsinki ethical standards. All subjects signed a written informed consent before conducting the experiments. Also, parent or legal guardian of participants under 18 years of age provided informed consent.
Data Sharing Statement
Data are available upon request from the correspondence author.
Acknowledgments
We would like to acknowledgment King Saud University, Majmmah University and King Khaled Hospital for supporting the study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This research received no external funding.
Disclosure
The authors report no conflicts of interest in this work.
References
1. WHO. Coronavirus disease (COVID-19) pandemic. Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019.
2. Randad PR, Pisanic N, Kruczynski K, et al. COVID-19 serology at population scale: SARS-CoV-2-specific antibody responses in saliva. J Clin Microbiol. 2021;59(1):24–0112300.
3. Alturaiki W, Mubarak A, Al Jurayyan A, Hemida MG. The pivotal roles of the host immune response in the fine-tuning the infection and the development of the vaccines for SARS-CoV-2. Hum Vaccin Immunother. 2021;17(10):3297–3309. doi:10.1080/21645515.2021.1935172
4. Alosaimi B, Mubarak A, Hamed ME, et al. Complement anaphylatoxins and inflammatory cytokines as prognostic markers for COVID-19 severity and in-hospital mortality. Front Immunol. 2021;12:668725. doi:10.3389/fimmu.2021.668725
5. Poland GA, Ovsyannikova IG, Kennedy RB. SARS-CoV-2 immunity: review and applications to phase 3 vaccine candidates. Lancet. 2020;396(10262):1595–1606. doi:10.1016/S0140-6736(20)32137-1
6. Sia SF, Yan LM, Chin AWH, et al. Pathogenesis and transmission of SARS-CoV-2 in golden hamsters. Nature. 2020;583(7818):834–838. doi:10.1038/s41586-020-2342-5
7. Hamming I, Timens W, Bulthuis MLC, Lely AT, Navis GJ, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203(2):631–637. doi:10.1002/path.1570
8. Farrag MA, Amer HM, Bhat R, et al. SARS-CoV-2: an overview of virus genetics, transmission, and immunopathogenesis. Int J Environ Res Public Health. 2021;18(12):6312. doi:10.3390/ijerph18126312
9. Ou XY, Liu Y, Lei XB, et al. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat Commun. 2020;11(1). doi:10.1038/s41467-020-15562-9
10. Walsh EE, Frenck RW, Falsey AR, et al. Safety and immunogenicity of two RNA-based Covid-19 vaccine candidates. N Engl J Med. 2020;383(25):2439–2450. doi:10.1056/NEJMoa2027906
11. Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603–2615. doi:10.1056/NEJMoa2034577
12. Saadat S, Rikhtegaran Tehrani Z, Logue J, et al. Binding and neutralization antibody titers after a single vaccine dose in health care workers previously infected with SARS-CoV-2. JAMA. 2021;325(14):1467–1469. doi:10.1001/jama.2021.3341
13. Manisty C, Otter AD, Treibel TA, et al. Antibody response to first BNT162b2 dose in previously SARS-CoV-2-infected individuals. Lancet. 2021;397(10279):1057–1058. doi:10.1016/S0140-6736(21)00501-8
14. Krammer F, Srivastava K, Alshammary H, et al. Antibody responses in seropositive persons after a single dose of SARS-CoV-2 mRNA vaccine. N Engl J Med. 2021;384(14):1372–1374. doi:10.1056/NEJMc2101667
15. Bradley T, Grundberg E, Selvarangan R, et al. Antibody responses after a single dose of SARS-CoV-2 mRNA vaccine. N Engl J Med. 2021;384(20):1959–1961. doi:10.1056/NEJMc2102051
16. McDade TW, Schrock JM, D’Aquila R, et al. Symptoms of COVID-19 infection and magnitude of antibody response in a large community-based study. medRxiv. 2021. doi:10.1101/2021.02.04.21251170
17. McDade TW, McNally EM, Zelikovich AS, et al. High seroprevalence for SARS-CoV-2 among household members of essential workers detected using a dried blood spot assay. PLoS One. 2020;15(8):e0237833. doi:10.1371/journal.pone.0237833
18. Schrock JM, Ryan DT, Saber R, et al. Cohabitation with a known Coronavirus disease 2019 case is associated with greater antibody concentration and symptom severity in a community-based sample of seropositive adults. Open Forum Infect Dis. 2021;8(7). doi:10.1093/ofid/ofab244
19. Legros V, Denolly S, Vogrig M, et al. A longitudinal study of SARS-CoV-2-infected patients reveals a high correlation between neutralizing antibodies and COVID-19 severity. Cell Mol Immunol. 2021;18(2):318–327. doi:10.1038/s41423-020-00588-2
20. Alkharaan H, Bayati S, Hellstrom C, et al. Persisting salivary IgG against SARS-CoV-2 at 9 months after mild COVID-19: a complementary approach to population surveys. J Infect Dis. 2021;224(3):407–414. doi:10.1093/infdis/jiab256
21. García-Montero C, Fraile-Martínez O, Bravo C, et al. An updated review of SARS-CoV-2 vaccines and the importance of effective vaccination programs in pandemic times. Vaccines. 2021;9(5). doi:10.3390/vaccines9050433
22. Yen JS, Wang IK, Yen TH. COVID-19 vaccination and dialysis patients: why the variable response. QJM. 2021;114(7):440–444. doi:10.1093/qjmed/hcab171
23. Krammer F. SARS-CoV-2 vaccines in development. Nature. 2020;586(7830):516–527. doi:10.1038/s41586-020-2798-3
24. Angyal A, Longet S, Moore SC, et al. T-cell and antibody responses to first BNT162b2 vaccine dose in previously infected and SARS-CoV-2-naive UK health-care workers: a multicentre prospective cohort study. Lancet Microbe. 2022;3(1):e21–e31. doi:10.1016/S2666-5247(21)00275-5
25. Grzelak L, Velay A, Madec Y, et al. Sex differences in the evolution of neutralizing antibodies to severe acute respiratory syndrome coronavirus 2. J Infect Dis. 2021;224(6):983–988. doi:10.1093/infdis/jiab127
26. Nomura Y, Sawahata M, Nakamura Y, et al. Age and smoking predict antibody titres at 3 months after the second dose of the BNT162b2 COVID-19 vaccine. Vaccines-Basel. 2021;9(9):1042.
27. Dan JM, Mateus J, Kato Y, et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science. 2021;371(6529):eabf4063. doi:10.1126/science.abf4063
28. Faustini SE, Jossi SE, Perez-Toledo M, et al. Detection of antibodies to the SARS-CoV-2 spike glycoprotein in both serum and saliva enhances detection of infection. medRxiv. 2020. doi:10.1101/2020.06.16.20133025
29. Sterlin D, Mathian A, Miyara M, et al. IgA dominates the early neutralizing antibody response to SARS-CoV-2. Sci Transl Med. 2021;13:577. doi:10.1126/scitranslmed.abd2223
30. Cervia C, Nilsson J, Zurbuchen Y, et al. Systemic and mucosal antibody responses specific to SARS-CoV-2 during mild versus severe COVID-19. J Allerg Clin Immunol. 2021;147(2):545–557.e549. doi:10.1016/j.jaci.2020.10.040
31. Nomura Y, Sawahata M, Nakamura Y, et al. Attenuation of antibody titers from 3 to 6 months after the second dose of the BNT162b2 vaccine depends on sex, with age and smoking risk factors for lower antibody titers at 6 months. Vaccines. 2021;9(12):1500. doi:10.3390/vaccines9121500
32. Corman VM, Albarrak AM, Omrani AS, et al. Viral shedding and antibody response in 37 patients with middle east respiratory syndrome coronavirus infection. Clin Infect Dis. 2016;62(4):477–483. doi:10.1093/cid/civ951
33. Meyer B, Drosten C, Muller MA. Serological assays for emerging coronaviruses: challenges and pitfalls. Virus Res. 2014;194:175–183. doi:10.1016/j.virusres.2014.03.018
34. Hsueh PR, Huang LM, Chen PJ, Kao CL, Yang PC. Chronological evolution of IgM, IgA, IgG and neutralisation antibodies after infection with SARS-associated coronavirus. Clin Microbiol Infect. 2004;10(12):1062–1066. doi:10.1111/j.1469-0691.2004.01009.x
35. Wu LP, Wang NC, Chang YH, et al. Duration of antibody responses after severe acute respiratory syndrome. Emerg Infect Dis. 2007;13(10):1562–1564. doi:10.3201/eid1310.070576
36. Wang C, Li W, Drabek D, et al. A human monoclonal antibody blocking SARS-CoV-2 infection. Nat Commun. 2020;11(1):2251. doi:10.1038/s41467-020-16256-y
37. To KK, Tsang OT, Leung WS, et al. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infect Dis. 2020;20(5):565–574. doi:10.1016/S1473-3099(20)30196-1
38. Guo L, Ren L, Yang S, et al. Profiling early humoral response to diagnose novel coronavirus disease (COVID-19). Clin Infect Dis. 2020;71(15):778–785. doi:10.1093/cid/ciaa310
39. Long QX, Liu BZ, Deng HJ, et al. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat Med. 2020;26(6):845–848. doi:10.1038/s41591-020-0897-1
40. Jiang HW, Li Y, Zhang HN, et al. SARS-CoV-2 proteome microarray for global profiling of COVID-19 specific IgG and IgM responses. Nat Commun. 2020;11(1):3581. doi:10.1038/s41467-020-17488-8
41. Moscato G, Mazzetti P, Lucenteforte E, et al. Assessment of automated high-throughput serological assays for prediction of high-titer SARS-CoV-2 neutralizing antibody. J Clin Virology Plus. 2021;1(1):100016. doi:10.1016/j.jcvp.2021.100016
42. Yoo JH. What we do know and do not yet know about COVID-19 vaccines as of the beginning of the year 2021. J Korean Med Sci. 2021;36(6):e54–e54. doi:10.3346/jkms.2021.36.e54
43. Yao L, Wang GL, Shen Y, et al. Persistence of antibody and cellular immune responses in coronavirus disease 2019 patients over nine months after infection. J Infect Dis. 2021;224(4):586–594. doi:10.1093/infdis/jiab255
44. Gallais F, Gantner P, Bruel T, et al. Anti-SARS-CoV-2 antibodies persist for up to 13 months and reduce risk of reinfection. medRxiv. 2021. doi:10.1101/2021.05.07.21256823
45. Gaebler C, Wang Z, Lorenzi JCC, et al. Evolution of antibody immunity to SARS-CoV-2. Nature. 2021;591(7851):639–644. doi:10.1038/s41586-021-03207-w
46. Deming ME, Lyke KE. A ‘mix and match’ approach to SARS-CoV-2 vaccination. Nat Med. 2021;27(9):1510–1511. doi:10.1038/s41591-021-01463-x
47. Vogel G. Mixing Vaccines May Boost Immune Responses. American Association for the Advancement of Science; 2021.
48. Lewis D. Mix-and-match COVID vaccines: the case is growing, but questions remain. Nature. 2021;595(7867):344–345. doi:10.1038/d41586-021-01805-2
49. Borobia AM, Carcas AJ, Pérez-Olmeda M, et al. Immunogenicity and reactogenicity of BNT162b2 booster in ChAdOx1-S-primed participants (CombiVacS): a multicentre, open-label, randomised, controlled, Phase 2 trial. Lancet. 2021;398(10295):121–130. doi:10.1016/S0140-6736(21)01420-3
50. Hillus D, Schwarz T, Tober-Lau P, et al. Safety, reactogenicity, and immunogenicity of homologous and heterologous prime-boost immunisation with ChAdOx1-nCoV19 and BNT162b2: a prospective cohort study. medRxiv. 2021. doi:10.1101/2021.05.19.21257334
51. Schmidt T, Klemis V, Schub D, et al. Immunogenicity and reactogenicity of heterologous ChAdOx1 nCoV-19/mRNA vaccination. Nat Med. 2021;27(9):1530–1535. doi:10.1038/s41591-021-01464-w
52. Barros-Martins J, Hammerschmidt SI, Cossmann A, et al. Immune responses against SARS-CoV-2 variants after heterologous and homologous ChAdOx1 nCoV-19/BNT162b2 vaccination. Nat Med. 2021;27(9):1525–1529. doi:10.1038/s41591-021-01449-9
53. Kardani K, Bolhassani A, Shahbazi S. Prime-boost vaccine strategy against viral infections: mechanisms and benefits. Vaccine. 2016;34(4):413–423. doi:10.1016/j.vaccine.2015.11.062
54. Altawalah H. Antibody responses to natural SARS-CoV-2 infection or after COVID-19 vaccination. Vaccines. 2021;9(8):910. doi:10.3390/vaccines9080910
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.