Back to Journals » Journal of Asthma and Allergy » Volume 16
Dupilumab Efficacy in Patients with Uncontrolled Moderate-to-Severe Type 2 Asthma Regardless of Perennial Aeroallergen Sensitization
Authors Corren J, Jackson DJ, Casale TB, Borish L, Rabe KF , Busse WW , Maspero JF, Jackson DJ, Daizadeh N, Altincatal A, Radwan A, Khodzhayev A, Djandji M, Jacob-Nara JA, Rowe PJ, Deniz Y
Received 9 August 2022
Accepted for publication 30 January 2023
Published 7 March 2023 Volume 2023:16 Pages 249—260
DOI https://doi.org/10.2147/JAA.S385645
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Amrita Dosanjh
Jonathan Corren,1 David J Jackson,2,3 Thomas B Casale,4 Larry Borish,5,6 Klaus F Rabe,7,8 William W Busse,9 Jorge F Maspero,10 Daniel J Jackson,11 Nadia Daizadeh,12 Arman Altincatal,12 Amr Radwan,13 Angela Khodzhayev,13 Michel Djandji,12 Juby A Jacob-Nara,12,14 Paul J Rowe,14 Yamo Deniz13
1David Geffen School of Medicine at UCLA, Los Angeles, CA, USA; 2King’s College London, London, UK; 3Guy’s and St Thomas’ NHS Foundation Trust, London, UK; 4Division of Allergy and Immunology, University of South Florida, Tampa, FL, USA; 5Asthma and Allergic Disease Center, University of Virginia Health System, Charlottesville, VA, USA; 6Carter Immunology Center, University of Virginia Health System, Charlottesville, VA, USA; 7LungenClinic Grosshansdorf (Member of the German Center for Lung Research [DZL]), Airway Research Center North (ARCN), Grosshansdorf, Germany; 8Christian-Albrechts University (Member of the German Center for Lung Research [DZL]), Airway Research Center North (ARCN), Kiel, Germany; 9UW Allergy, Pulmonary and Critical Care Medicine, University of Wisconsin School of Medicine and Public Health, Madison, WI, USA; 10Fundación CIDEA, Buenos Aires, Argentina; 11University of Wisconsin School of Medicine and Public Health, Madison, WI, USA; 12Sanofi, Cambridge, MA, USA; 13Regeneron Pharmaceuticals, Inc., Tarrytown, NY, USA; 14Sanofi, Bridgewater, NJ, USA
Correspondence: Jonathan Corren, David Geffen School of Medicine at UCLA, 10780 Santa Monica Blvd., Suite 280, Los Angeles, CA, 90025, USA, Email [email protected]
Purpose: Dupilumab, a fully human monoclonal antibody, blocks the shared receptor component for interleukins-4/-13, key and central drivers of type 2 (T2) inflammation in multiple diseases. In phase 3 QUEST (NCT02414854), dupilumab vs placebo significantly reduced asthma exacerbation rates (AER) and improved pre-bronchodilator forced expiratory volume in 1 second (FEV1) in patients with uncontrolled, moderate-to-severe asthma, with greater effects in patients with elevated T2 biomarkers (≥ 150 eosinophils/μL or fractional exhaled nitric oxide [FeNO] ≥ 25 parts per billion). Overall safety was consistent with the known dupilumab safety profile. This post hoc analysis assessed dupilumab efficacy in QUEST patients with T2 asthma with evidence of an allergic phenotype (baseline serum IgE ≥ 30 IU/mL and aeroallergen-specific IgE ≥ 0.35 IU/mL) by number of aeroallergen sensitizations: 1, 2, 3, or ≥ 4. Non-sensitized patients (serum total IgE < 30 IU/mL without evidence of allergic phenotype) were also assessed.
Patients and Methods: Endpoints were annualized AER, change from baseline in pre-bronchodilator FEV1 and asthma control (5-item Asthma Control Questionnaire [ACQ-5]), and FeNO and serum total IgE levels over the 52-week treatment period.
Results: In all subgroups by number of allergens sensitized, dupilumab vs placebo reduced AER by 35– 67% and improved both pre-bronchodilator FEV1 at Week 12 (least squares mean differences: 0.10– 0.26 L across subgroups) and ACQ-5 score at Week 52 (– 0.26 to – 0.43). Dupilumab significantly reduced FeNO and total IgE levels at Week 52 compared with placebo. Similar results were observed in non-sensitized patients.
Conclusion: Dupilumab improved clinical outcomes and reduced biomarker levels in patients with uncontrolled, moderate-to-severe T2 asthma irrespective of allergen sensitization status or number.
Clinical Trial Registration: ClinicalTrials.gov Identifier: NCT02414854.
Keywords: dupilumab, allergic asthma, type 2 asthma, perennial aeroallergen
Introduction
Patients with type 2 inflammation represent a large proportion of the overall population of patients with asthma. Many of those with type 2 asthma also have evidence of an allergic phenotype, which is characterized by an increased expression of specific IgE to 1 or more aeroallergens.1,2 Re-exposure to allergens and their binding to IgE results in the release of inflammatory mediators that attract T helper type 2 cells. These, in turn, secrete cytokines, including interleukin (IL)-4, IL-13, and IL-5, all of which are thought to contribute to the airway inflammation associated with allergic asthma.2 These cytokines are also key drivers in type 2 inflammatory pathways and, in recent years, a number of biologics have been developed to target them and inhibit their signaling.3 One of these, dupilumab is a fully human VelocImmune®-derived monoclonal antibody that blocks the shared receptor component of IL-4 and IL-13, thus inhibiting their signaling, and has demonstrated efficacy in a number of type 2 inflammatory diseases.4–7
In the phase 3 LIBERTY ASTHMA QUEST study (NCT02414854), add-on dupilumab 200 mg and 300 mg every 2 weeks (q2w) vs matched placebo significantly reduced severe asthma exacerbations and improved pre-bronchodilator forced expiratory volume in 1 second (FEV1) in patients with uncontrolled, moderate-to-severe asthma. Overall safety was consistent with the known dupilumab safety profile.8 Treatment effects were greater in patients with elevated type 2 biomarkers at baseline (blood eosinophils ≥150 cells/μL or fractional exhaled nitric oxide [FeNO] ≥25 parts per billion [ppb]). Dupilumab has also been shown to be efficacious in the subgroup of patients in QUEST with evidence of an allergic phenotype, and suppresses type 2 inflammatory biomarkers, highlighting the key role of IL-4 and IL-13 in airway inflammation.9
Previous work demonstrated that dupilumab reduced severe asthma exacerbation rates and improved pre-bronchodilator FEV1 and asthma control across subgroups of patients with moderate-to-severe asthma with different baseline levels of serum total IgE.10 However, it is unknown whether the number of perennial aeroallergen sensitizations affects the efficacy of dupilumab in patients with moderate-to-severe asthma. This post hoc analysis of QUEST aimed to address this knowledge gap by assessing the efficacy of dupilumab in the population of enrolled patients who had a type 2 inflammatory phenotype, defined as baseline levels of ≥150 eosinophils/µL or FeNO ≥25 ppb, and who showed evidence of an allergic phenotype with sensitization to 1, 2, 3, or ≥4 common perennial aeroallergens at baseline. Efficacy was also assessed in the subpopulation of patients with type 2 asthma who were not sensitized to any perennial aeroallergen at baseline.
Materials and Methods
Study Design and Patients
CONSORT diagrams and full details of the study design, eligibility criteria, and methodology of LIBERTY ASTHMA QUEST have been reported previously.8,11 This study was a phase 3, multinational, randomized, double-blind, placebo-controlled, parallel-group design conducted in patients with uncontrolled, moderate-to-severe asthma despite receiving continuous treatment with medium-to-high doses of inhaled corticosteroids (ICS) plus 1 or 2 additional asthma medications (eg, long-acting beta agonist, leukotriene receptor antagonist, theophylline, etc.) Patients requiring oral steroids as controller medication or biologics were excluded from the study. Patients were enrolled in the study without a required minimum level of any type 2 biomarker (blood eosinophils, serum total IgE, or FeNO). Following a 4-week screening period (±1 week), eligible patients ≥12 years of age were randomized in a 2:2:1:1 ratio to receive 52 weeks of add-on treatment with subcutaneous injections of dupilumab 200 mg (after a 400 mg loading dose), or 300 mg (after a 600 mg loading dose) q2w, or matched-volume placebo.
The study was sponsored by Sanofi and Regeneron Pharmaceuticals, Inc.; data were collected by the study investigators and analyzed by the sponsors. QUEST was conducted in accordance with the Declaration of Helsinki, the International Conference on Harmonisation Good Clinical Practice guideline, and applicable regulatory requirements. An independent data and safety monitoring committee conducted blinded monitoring of patient safety data. The Institutional Review Board of the study was the Copernicus Group, which oversaw trial conduct and documentation according to ethics committees at each trial center (Supplementary Section 1). All patients (or their legal guardians) provided written informed consent before participating in the trial.
Populations Analyzed
The primary population of interest in this post hoc analysis of QUEST comprised patients with elevated type 2 biomarkers at baseline (ie, blood eosinophils ≥150 cells/µL or FeNO ≥25 ppb). Patients with a type 2 phenotype were classified according to whether they met the criteria for allergic asthma at QUEST baseline. Evidence of allergic asthma was defined as a total serum IgE ≥30 IU/mL and ≥1 perennial aeroallergen-specific IgE ≥0.35 IU/mL at baseline, as described previously.9 Sensitization to the following common perennial aeroallergens was assessed: molds (Aspergillus fumigatus, Alternaria tenuis/alternata, Cladosporium herbarum), dust mites (Dermatophagoides farinae, Dermatophagoides pteronyssinus), cat and dog dander, and German and Oriental cockroaches. Patients with evidence of allergic asthma were further categorized into subgroups according to the number of perennial aeroallergens to which they were sensitized: 1, 2, 3, or ≥4 allergens.
The population of patients in QUEST who showed evidence of a type 2 signature at baseline but had no evidence of an allergic phenotype at baseline (ie, total serum IgE <30 IU/mL and no sensitivity to any of the perennial aeroallergens tested [specific IgE <0.35 IU/mL]) was also assessed.
Endpoints
Endpoints assessed included the annualized rate of severe exacerbation events during the 52-week treatment period. A severe asthma exacerbation was defined as a deterioration of asthma requiring treatment with systemic glucocorticoids for ≥3 days or hospitalization/emergency room visit requiring systemic glucocorticoids.12 Changes from baseline over the 52-week treatment period were measured in pre-bronchodilator FEV1 and in asthma control, as assessed using the 5-item Asthma Control Questionnaire (ACQ-5; negative score denotes improvement [scale 0–6, with higher scores indicating lower asthma control]).13 The effect of treatment on levels of biomarkers (FeNO and serum total IgE) over the 52-week treatment period was also assessed.
Statistical Analysis
Adjusted annualized rates of severe exacerbation events were analyzed using a negative binomial regression model, which included the total number of events observed from randomization up to Week 52 or last study contact date (whichever came earlier) as the response variable. Treatment group, age, region (pooled country), baseline eosinophil strata, baseline ICS dose level, and number of severe exacerbation events within 1 year prior to the study were included as covariates, log-transformed standardized observation duration as an offset variable. The interaction between number of allergen sensitizations and dupilumab effect on exacerbation rates was tested using a negative binomial model, which included the same response variable and covariates listed above, with subgroup (if different from the aforementioned covariates) and treatment-by-subgroup interaction as additional covariates.
Change from baseline in pre-bronchodilator FEV1 was analyzed using a linear mixed-effect model with repeated measures (MMRM), which used change from baseline in pre-bronchodilator FEV1 values up to Week 52 as the response variable. Covariates were treatment, age, sex, baseline height, region (pooled country), baseline eosinophil strata, baseline ICS dose level, visit, treatment-by-visit interaction, baseline pre-bronchodilator FEV1 value, and baseline-by-visit interaction. Change from baseline in ACQ-5 score over time was also analyzed using a linear MMRM, with change from baseline in ACQ-5 score up to Week 52 as the response variable. Covariates were treatment, age, region (pooled country), baseline eosinophil strata, baseline ICS dose level, visit, treatment-by-visit interaction, baseline ACQ-5, and baseline-by-visit interaction. The interaction between number of perennial aeroallergen sensitizations and dupilumab effect on pre-bronchodilator FEV1 at Week 12 and ACQ-5 score at Week 24 was tested using a linear MMRM. The response variable was change from baseline in pre-bronchodilator FEV1 values up to Week 12 or ACQ-5 values up to Week 24. Covariates were the same as those listed above and subgroup (if different from the aforementioned covariates), subgroup-by-treatment interaction, and subgroup-by-treatment-by-visit interaction were included as additional covariates. The least squares (LS) means are predicted population margins (ie, marginal means) which were obtained from the adjusted multivariate regression models. LS means difference is the difference between the two predicted group/treatment LS means.
The effect of treatment on biomarkers was assessed using a rank-transformed analysis of covariance model adjusted for baseline FeNO or serum total IgE, age, sex, region (pooled country), baseline eosinophil strata, and baseline ICS dose level comparing dupilumab vs matched placebo. Interaction between the number of perennial aeroallergen sensitizations and dupilumab effect on biomarker levels was tested by including the subgroups defined by number of perennial aeroallergen-specific IgE ≥0.35 IU/mL and subgroup-by-treatment interaction as covariates in the aforementioned model. P values denoting statistical significance were included for statistical testing performed on the change from baseline in FeNO or serum total IgE for combined dupilumab vs combined matched placebo at each timepoint.
Results
Study Patients
Of 1902 patients (the intention-to-treat [ITT] population) randomized to QUEST, 885 (47%) had a type 2 phenotype and were considered to have allergic asthma as evidenced by a total serum IgE ≥30 IU/mL and sensitivity to at least 1 of the perennial aeroallergens assessed (ie, allergen-specific IgE levels of ≥0.35 IU/mL). Of these, 254 (29%) had sensitivity to 1 allergen, 219 (25%) to 2 allergens, 173 (20%) to 3 allergens, and 239 (27%) to 4 or more allergens; 114 (6% of the overall ITT population) patients with a type 2 phenotype did not present evidence of an allergic phenotype based on total serum IgE levels <30 IU/mL and non-sensitivity to each of the panels of perennial aeroallergens assessed.
The baseline demographics and disease characteristics of patients with a type 2 phenotype are shown in Table 1 by combined treatment group and number of allergen sensitizations (1, 2, 3, ≥4, and non-sensitized). Patients with a type 2 phenotype who were sensitized were more likely to have ongoing atopic comorbidities and higher total serum IgE at baseline vs those who were non-sensitized. Non-sensitized patients with a type 2 phenotype were more likely to be older and female.
Annualized Rate of Severe Asthma Exacerbations
Dupilumab vs placebo significantly reduced severe exacerbation rates in patients with type 2 asthma and aeroallergen sensitization across most subgroups of number of allergens. Reductions in exacerbation rates (relative risk, 95% confidence interval [CI]; P value) were 40% (0.597, 0.386–0.923; P = 0.0205), 67% (0.332, 0.209–0.526; P < 0.0001), 49% (0.515, 0.292–0.907; P = 0.0220), and 35% (0.651, 0.394–1.073; P = 0.0921) in the groups of patients sensitized to 1, 2, 3, and ≥4 allergens, respectively (Figure 1). The number of allergen sensitizations did not impact dupilumab efficacy in reducing exacerbations (P = 0.2737 for the interaction). Patients with a type 2 phenotype who were non-sensitized also benefited significantly from dupilumab, with a 65% reduction in exacerbations compared with placebo treatment (0.351, 95% CI 0.176–0.700; P = 0.0033) (Figure 1).
Pre-Bronchodilator FEV1 Over Time
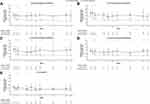
Patients with a type 2 phenotype, irrespective of whether they were sensitized to 1 or more perennial aeroallergens, had improvements in pre-bronchodilator FEV1 observed over time with dupilumab vs placebo (Figure 2A–D). Improvements in pre-bronchodilator FEV1 were observed as early as the first assessment at Week 2, and these were maintained for the duration of the 52-week treatment period. While patients sensitized to 2 or 3 perennial aeroallergens showed the highest numerical improvements in pre-bronchodilator FEV1 vs placebo throughout most of the study period, the effect of dupilumab on lung function was not significantly different across subgroups by number of aeroallergen sensitizations (P = 0.2730 for the interaction at Week 12). The LS mean differences vs matched placebo (95% CI; P value) in pre-bronchodilator FEV1 improvement at Week 12 in patients with 1, 2, 3, and ≥ 4 allergen sensitizations were 0.12 L (0.03–0.22; P = 0.0124), 0.26 L (0.13–0.38; P < 0.0001), 0.14 L (0.01–0.28; P = 0.0383), and 0.10 L (–0.01 to 0.21; P = 0.0825), respectively. By Week 52, LS mean differences (95% CI; P value) in pre-bronchodilator FEV1 improvement in patients with 1, 2, 3, and ≥4 allergen sensitizations were 0.19 L (0.09–0.30; P = 0.0004), 0.23 L (0.09–0.36; P = 0.0013), 0.17 L (0.03–0.31; P =0.0195), and 0.14 L (0.02–0.26; P = 0.0219), respectively.
Numerical improvements, statistically significant at some timepoints, in pre-bronchodilator FEV1 with dupilumab vs placebo were also observed in patients with type 2 asthma without evidence of an allergic phenotype (Figure 2E). At Week 12, the LS mean difference vs matched placebo (95% CI; P value) in this subgroup was 0.12 L (–0.02 to 0.26; P = 0.0865). In patients with type 2 asthma who were non-sensitized, the LS mean difference vs matched placebo (95% CI; P value) in change from baseline in pre-bronchodilator FEV1 at Week 52 was 0.16 L (0.004–0.32; P = 0.0451).
Asthma Control (ACQ-5 Scores)
Numerical improvements in asthma control, as measured by ACQ-5 score, were observed with dupilumab vs placebo as early as the first assessment at Week 2 and throughout the study period in patients with a type 2 phenotype irrespective of the number of allergen sensitizations (Figure 3A–D). Such improvements in asthma control were statistically significant in patients sensitized to 1 and 2 perennial aeroallergens but did not reach statistical significance (except at a few timepoints) in patients sensitized to 3 and ≥4 perennial aeroallergens, among whom greater increases in ACQ-5 score in the placebo arm were noticed compared with the 1 and 2 perennial aeroallergen sensitization subgroups. Despite this, dupilumab’s effect on asthma control was not impacted by the number of perennial aeroallergen sensitizations (P = 0.3027 for the interaction at Week 24). At Week 52, the LS mean difference vs matched placebo (95% CI; P value) in change from baseline ACQ-5 score in patients with 1, 2, 3, and ≥4 perennial aeroallergen sensitizations was –0.43 (–0.70 to –0.16; P = 0.0018), –0.41 (–0.71 to –0.12; P = 0.0067), –0.27 (–0.58 to 0.03; P = 0.0786), and –0.26 (–0.53 to 0.01; P = 0.0585), respectively. The greater improvements observed with dupilumab vs placebo at Week 52 in patients sensitized to only 1 or 2 perennial aeroallergens, compared with those sensitized to more perennial aeroallergens, were driven by relatively higher LS mean (Standard error) changes evident in the placebo arms of groups sensitized to 3 or ≥4 perennial aeroallergens (ie, –1.05 [0.11] and –1.16 [0.12] in placebo recipients with 1 and 2 perennial aeroallergen sensitizations, compared with –1.22 [0.12] and –1.49 [0.12] in placebo recipients with 3 and ≥4 perennial aeroallergen sensitizations).
Rapid and, in most cases, statistically significant improvements sustained during the treatment period were also observed with dupilumab vs placebo in patients with type 2 phenotype and without evidence of an allergic phenotype (Figure 3E). At Week 52, the LS mean difference vs matching placebo (95% CI; P value) in change from baseline in ACQ-5 score was –0.68 (–1.16 to –0.19; P = 0.0071).
Biomarkers
FeNO
Dupilumab vs placebo significantly reduced FeNO levels during treatment in patients with a type 2 phenotype irrespective of whether they were sensitized to 1, 2, 3, or ≥4 perennial aeroallergens (Figure 4A–D). Median baseline levels of FeNO in these subgroups ranged from 27.5 ppb to 38.5 ppb across sensitization subgroups and treatment arms (Table 1). At Week 52, the median percentage change from baseline in FeNO levels in dupilumab vs placebo arms by number of perennial aeroallergen sensitizations was –43.6% vs 8.6% (absolute median change [95% CI]: –11.0 [–15.0 to –8.0] ppb vs 2.0 [–3.0 to 5.0] ppb, P < 0.0001) in patients sensitized to 1 allergen; –44.0% vs –21.8% (–11.5 [–19.0 to –8.0] ppb vs –7.5 [–13.0 to –5.0] ppb, P = 0.1074) in patients sensitized to 2 allergens; –49.1% vs 4.3% (–13.5 [–18.0 to –8.0] ppb vs 1.0 [–4.0 to 9.0] ppb, P = 0.0006) in patients sensitized to 3 allergens; and –47.2% vs –18.8% (–13.0 [–19.0 to –10.0] ppb vs –3.0 [–8.0 to 0.0] ppb, P = 0.0003) in patients sensitized to ≥4 allergens. The number of allergen sensitizations did not impact dupilumab effect on FeNO levels (P = 0.0782 for the interaction at Week 52).
Dupilumab also reduced FeNO levels over time compared with placebo in patients with type 2 phenotype without evidence of an allergic phenotype (Figure 4E). At Week 52, the median percentage change from baseline in FeNO levels was –40.7% (absolute median change [95% CI]: –6.5 [–12.0 to –4.0] ppb) in the dupilumab arm vs 2.8% (0.5 [–3.0 to 3.0] ppb) in the placebo arm (P = 0.0244).
Serum Total IgE
Dupilumab vs placebo significantly reduced serum total IgE levels during treatment in patients with a type 2 phenotype, irrespective of the number of perennial aeroallergen sensitizations (Figure 5A–D). Median baseline levels of total serum IgE were higher with an increasing number of perennial aeroallergen sensitizations, with values ranging from 208.0–243.0 IU/mL in patients sensitized to 1 perennial aeroallergen to 730.0–834.5 IU/mL in those sensitized to ≥4 perennial aeroallergens for dupilumab and placebo treatment arms (Table 1).
At Week 52, the median percentage change from baseline in serum total IgE for dupilumab vs placebo in patients sensitized to 1 perennial aeroallergen was –70.2% vs 1.9% (absolute median change [95% CI]: –136.5 [–184.0 to –114.0] IU/mL vs 3.0 [–25.0 to 28.0] IU/mL; P < 0.0001). These values were –73.4% vs –8.5% (–224.0 [–283.0 to –159.0] IU/mL vs –16.0 [–44.0 to 13.0] IU/mL; P < 0.0001) in patients sensitized to 2 perennial aeroallergens; –72.8% vs –15.6% (–267.0 [–342.0 to –183.0] IU/mL vs –50.0 [–80.0 to –8.0] IU/mL; P < 0.0001) in patients sensitized to 3 perennial aeroallergens; and –72.0% vs –1.9% (–523.0 [–633.0 to –382.0] IU/mL vs –5.5 [–118.0 to 12.0] IU/mL; P < 0.0001) in patients sensitized to ≥4 perennial aeroallergens. Therefore, dupilumab vs placebo significantly reduced levels of total IgE at the end of the treatment; this effect was independent on the number of allergens (P = 0.1812 for the interaction at Week 52). Significant reductions in serum total IgE levels were also observed with dupilumab vs placebo in patients with type 2 phenotype without evidence of an allergic phenotype (Figure 5E). At Week 52, the percentage change in serum total IgE levels in the dupilumab vs placebo arm was –56.0% vs 17.3% (absolute median change [95% CI]: –9.0 [–11.0 to –7.0] IU/mL vs 1.0 [0.0–5.0]; P = 0.0006).
Discussion
In this post hoc analysis of QUEST, dupilumab significantly reduced severe exacerbation rates while improving lung function (pre-bronchodilator FEV1) and asthma control (ACQ-5 score) in patients with type 2 asthma (≥150 eosinophils/µL or FeNO ≥25 ppb) and evidence of an allergic asthma phenotype at baseline, as demonstrated by total serum IgE ≥30 IU/mL and sensitivity to at least 1 of a panel of common perennial aeroallergens (allergen-specific IgE levels ≥0.35 IU/mL). The improvements in lung function and asthma control were achieved rapidly in all subgroups by number of perennial aeroallergens sensitized, and these were then sustained throughout the 52-week treatment period, which is consistent with the findings in other dupilumab studies.8,9,14–16 Dupilumab vs placebo also improved clinical outcomes in patients with a type 2 inflammatory phenotype who had serum total IgE <30 IU/mL and were not sensitized to any perennial aeroallergen.
Although dupilumab improved clinical outcomes regardless of the number of perennial aeroallergen sensitizations, we noticed that exacerbation rates were numerically relatively lower in both the placebo and dupilumab groups of patients sensitized to more perennial aeroallergens (3 and, in particular, ≥4 allergen sensitizations), which may suggest a decrease in exacerbation susceptibility for these patients.
Consistent with its mechanism of action and in keeping with results from other dupilumab studies, dupilumab also suppressed the levels of FeNO and serum total IgE in patients with type 2 asthma and evidence of an allergic phenotype.9,16 Furthermore, the levels of these biomarkers were also reduced in the subgroup of patients without evidence of an allergic phenotype.
Other biologics, such as omalizumab,17 benralizumab,18 and mepolizumab,19,20 have been evaluated for the treatment of allergic asthma, and their effects were shown to be independent of serum total IgE concentrations and number of sensitized allergens. In this analysis of patients with type 2 asthma, dupilumab demonstrated efficacy across all subgroups of patients with total serum IgE ≥30 IU/mL and 1, 2, 3, or ≥4 perennial aeroallergen sensitizations at baseline, and with no interaction between number of sensitized aeroallergens and dupilumab benefits. The fact that efficacy was also observed in patients with type 2 asthma who did not have an allergic asthma phenotype at baseline (ie, total serum IgE <30 IU/mL and no sensitivity to any of the perennial aeroallergens tested), suggests that a type 2 phenotype is a greater predictor of dupilumab efficacy than an allergic phenotype.
Strengths of this analysis include the large sample size of QUEST, and the fact that patients were included in QUEST without any requirement for minimum serum total IgE levels. In terms of limitations, this is a post hoc analysis in subgroups that were not pre-specified. The study was not powered to detect differences among patients with controlled, moderate-to-severe asthma by number of perennial aeroallergen sensitizations, or between patients with and without evidence of allergic phenotype. Additionally, over-the-counter antihistamine medication use was allowed in QUEST. Patients with sensitization to a higher number of aeroallergens might have been more likely to take anti-allergy medications that are available without a prescription, which could impact the interpretation of some observations in this analysis. Finally, seasonal allergens were not accounted for in this analysis, so one cannot exclude that some patients in the non-sensitized subgroup might have been sensitized to 1 or more seasonal allergens. Further discussion of limitations can be found in previous work.9
Conclusion
Dupilumab reduced severe asthma exacerbations and improved lung function and asthma control in patients with type 2 (≥150 eosinophils/µL or FeNO ≥25 ppb), uncontrolled, moderate-to-severe asthma, regardless of their sensitization status and number of perennial aeroallergen sensitizations. Dupilumab also significantly reduced levels of FeNO and serum total IgE.
Abbreviations
ACQ-5, 5-item Asthma Control Questionnaire; CI, confidence interval; FeNO, fractional exhaled nitric oxide; FEV1, forced expiratory volume in 1 second; ICS, inhaled corticosteroids; IL, interleukin; ITT, intention-to-treat; MMRM, mixed-effect model with repeated measures; ppb, parts per billion; q2w, every 2 weeks; SD, standard deviation; SE, standard error.
Data Sharing Statement
Qualified researchers may request access to patient level data and related study documents including the clinical study report, study protocol with any amendments, blank case report form, statistical analysis plan, and dataset specifications. Patient level data will be anonymized, and study documents will be redacted to protect the privacy of our trial participants. Further details on Sanofi’s data sharing criteria, eligible studies, and process for requesting access can be found at: https://www.vivli.org
Acknowledgments
Nora Crikelair of Regeneron Pharmaceuticals, Inc. Colin Mitchell of Sanofi. Research sponsored by Sanofi and Regeneron Pharmaceuticals, Inc. ClinicalTrials.gov Identifier: NCT02414854. Medical writing/editorial assistance was provided by Erin McClure Carroll, PhD, of Excerpta Medica, and was funded by Sanofi and Regeneron Pharmaceuticals, Inc., according to the Good Publication Practice guideline.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all of these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
Dr Corren reports research grants and serving as a consultant for AstraZeneca, Genentech, Novartis, Regeneron Pharmaceuticals, Inc., and Sanofi; and speaker fees from AstraZeneca, Genentech, and Novartis. Dr Jackson reports advisory board fees from AstraZeneca, Boehringer Ingelheim, GSK, Novartis, Sanofi, and Teva. Dr Casale reports research support from American Lung Association, Genentech, NIH, Novartis, PCORI, and Sanofi; serving as a consultant for AstraZeneca, Boehringer Ingelheim, Genentech, Novartis, and Regeneron Pharmaceuticals, Inc.; and serving on the speakers’ bureau for Genentech. Dr Borish is an advisory board member of Genentech, Regeneron Pharmaceuticals, Inc., and Sanofi Genzyme; and reports research funding from Astra Zeneca, GSK, NIH, and Regeneron Pharmaceuticals, Inc. Dr Rabe reports speaker fees and is a consultant for AstraZeneca, Berlin Chemie, Boehringer Ingelheim, Chiesi Pharmaceutical, GSK, Novartis, Roche Pharma, Sanofi & Regeneron, and Teva. Dr Busse is a consultant and reports speaker fees from AstraZeneca, Genentech, GSK, Novartis, Sanofi & Regeneron, and Teva. Dr Maspero is a consultant for AstraZeneca and Sanofi; reports speaker fees from GSK, Menarini, Novartis, Teva, and Uriach; and reports research grants from Novartis. Dr Jackson is a consultant for AstraZeneca, Genentech, GSK, Pfizer, Regeneron Pharmaceuticals, Inc., Sanofi, and Vifor Pharma; and is a member of the data and safety monitoring board of Pfizer. He also reports grants from NIH/NIAID and NIH/NHLBI. Dr Daizadeh is a former employee of Sanofi and may hold stock and/or stock options in the company. Mr Altincatal, Dr Djandji, Dr Jacob-Nara, and Dr Rowe are employees of Sanofi and may hold stock and/or stock options in the company. Dr Radwan, Dr Khodzhayev, and Dr Deniz are employees and shareholders of Regeneron Pharmaceuticals, Inc. The authors report no other conflicts of interest in this work.
References
1. Kuruvilla ME, Lee FE, Lee GB. Understanding asthma phenotypes, endotypes, and mechanisms of disease. Clin Rev Allergy Immunol. 2019;56(2):219–233. doi:10.1007/s12016-018-8712-1
2. Hall S, Agrawal DK. Key mediators in the immunopathogenesis of allergic asthma. Int Immunopharmacol. 2014;23(1):316–329. doi:10.1016/j.intimp.2014.05.034
3. Godar M, Blanchetot C, de Haard H, Lambrecht BN, Brusselle G. Personalized medicine with biologics for severe type 2 asthma: current status and future prospects. MAbs. 2018;10(1):34–45. doi:10.1080/19420862.2017.1392425
4. Macdonald LE, Karow M, Stevens S, et al. Precise and in situ genetic humanization of 6 Mb of mouse immunoglobulin genes. Proc Natl Acad Sci U S A. 2014;111(14):5147–5152. doi:10.1073/pnas.1323896111
5. Murphy AJ, Macdonald LE, Stevens S, et al. Mice with megabase humanization of their immunoglobulin genes generate antibodies as efficiently as normal mice. Proc Natl Acad Sci U S A. 2014;111(14):5153–5158. doi:10.1073/pnas.1324022111
6. Gandhi NA, Pirozzi G, Graham NMH. Commonality of the IL-4/IL-13 pathway in atopic diseases. Expert Rev Clin Immunol. 2017;13(5):425–437. doi:10.1080/1744666X.2017.1298443
7. Le Floc’h A, Allinne J, Nagashima K, et al. Dual blockade of IL-4 and IL-13 with dupilumab, an IL-4Rα antibody, is required to broadly inhibit type 2 inflammation. Allergy. 2020;75(5):1188–1204. doi:10.1111/all.14151
8. Castro M, Corren J, Pavord ID, et al. Dupilumab efficacy and safety in moderate-to-severe uncontrolled asthma. N Engl J Med. 2018;378(26):2486–2496. doi:10.1056/NEJMoa1804092
9. Corren J, Castro M, O’Riordan T, et al. Dupilumab efficacy in patients with uncontrolled, moderate-to-severe allergic asthma. J Allergy Clin Immunol Pract. 2020;8(2):516–526. doi:10.1016/j.jaip.2019.08.050
10. Carr W, Jackson DJ, Corren J, et al. Dupilumab efficacy in patients with uncontrolled, moderate-to-severe asthma by immunoglobin E levels at baseline. Eur Respir J. 2019;54(suppl 63):PA536.
11. Busse WW, Maspero JF, Rabe KF, et al. Liberty asthma QUEST: phase 3 randomized, double-blind, placebo-controlled, parallel-group study to evaluate dupilumab efficacy/safety in patients with uncontrolled, moderate-to-severe asthma. Adv Ther. 2018;35(5):737–748. doi:10.1007/s12325-018-0702-4
12. Reddel HK, Taylor DR, Bateman ED, et al. An official American Thoracic Society/European Respiratory Society statement: asthma control and exacerbations: standardizing endpoints for clinical asthma trials and clinical practice. Am J Respir Crit Care Med. 2009;180(1):59–99. doi:10.1164/rccm.200801-060ST
13. Juniper EF, O’Byrne PM, Guyatt GH, Ferrie PJ, King DR. Development and validation of a questionnaire to measure asthma control. Eur Respir J. 1999;14:902–907. doi:10.1034/j.1399-3003.1999.14d29.x
14. Wenzel S, Castro M, Corren J, et al. Dupilumab efficacy and safety in adults with uncontrolled persistent asthma despite use of medium-to-high-dose inhaled corticosteroids plus a long-acting β2 agonist: a randomised double-blind placebo-controlled pivotal phase 2b dose-ranging trial. Lancet. 2016;388(10039):31–44. doi:10.1016/S0140-6736(16)30307-5
15. Rabe KF, Nair P, Brusselle G, et al. Efficacy and safety of dupilumab in glucocorticoid-dependent severe asthma. N Engl J Med. 2018;378(26):2475–2485. doi:10.1056/NEJMoa1804093
16. Busse WW, Maspero JF, Lu Y, et al. Efficacy of dupilumab on clinical outcomes in patients with asthma and perennial allergic rhinitis. Ann Allergy Asthma Immunol. 2020;125(5):565–576.e1. doi:10.1016/j.anai.2020.05.026
17. Soong W, Yoo B, Pazwash H, Holweg CTJ, Casale TB. Omalizumab response in patients with asthma by number and type of allergen. Ann Allergy Asthma Immunol. 2021;127(2):223–231. doi:10.1016/j.anai.2021.04.002
18. Chipps BE, Newbold P, Hirsch I, Trudo F, Goldman M. Benralizumab efficacy by atopy status and serum immunoglobulin E for patients with severe, uncontrolled asthma. Ann Allergy Asthma Immunol. 2018;120(5):504–511.e4. doi:10.1016/j.anai.2018.01.030
19. Ortega H, Chupp G, Bardin P, et al. The role of mepolizumab in atopic and nonatopic severe asthma with persistent eosinophilia. Eur Respir J. 2014;44(1):239–241. doi:10.1183/09031936.00220413
20. Pelaia C, Crimi C, Pelaia G, et al. Real-life evaluation of mepolizumab efficacy in patients with severe eosinophilic asthma, according to atopic trait and allergic phenotype. Clin Exp Allergy. 2020;50(7):780–788. doi:10.1111/cea.13613
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.