Back to Journals » OncoTargets and Therapy » Volume 13
Dual Targeting of the Epidermal Growth Factor Receptor Using Combination of Nimotuzumab and Erlotinib in Advanced Non-Small-Cell Lung Cancer with Leptomeningeal Metastases: A Report of Three Cases
Authors Xu H, Zhou L, Lu Y, Su X, Cheng P, Li D, Gao H, Li H , Yuan W, Zhang L, Zhang T
Received 9 September 2019
Accepted for publication 16 December 2019
Published 21 January 2020 Volume 2020:13 Pages 647—656
DOI https://doi.org/10.2147/OTT.S230399
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Federico Perche
Hongyu Xu, 1 Lin Zhou, 2 You Lu, 2 Xiaomei Su, 1 Peng Cheng, 1 Dong Li, 1 Hui Gao, 1 Hua Li, 1 Weiwei Yuan, 1 Ling Zhang, 1 Tao Zhang 1
1Department of Oncology, The General Hospital of Western Theater Command, Chengdu, People’s Republic of China; 2Department of Thoracic Oncology, West China Hospital, Sichuan University, Chengdu, People’s Republic of China
Correspondence: Tao Zhang
Department of Oncology, The General Hospital of Western Theater Command, No 270, Rongdu Road, Jin Niu District, Chengdu, Sichuan 610083, People’s Republic of China
Email [email protected]
Abstract: Leptomeningeal metastases (LM) occur in 3– 5% of patients with advanced non-small-cell lung cancer (NSCLC) and are associated with a dismal prognosis. We report three cases of NSCLC with LM who were treated with the combination of nimotuzumab and erlotinib. Magnetic Resonance Imaging (MRI) evaluation during follow-up showed significant improvement in cancer symptoms and decreased tumor size in all three patients. Grade 3 and 4 toxicities were rarely seen. Based on apparent efficacy of the regimen and fewer side effects, we suggest that nimotuzumab in combination with erlotinib may be a promising option for the treatment of NSCLC with LM.
Keywords: leptomeningeal metastases, non-small cell lung cancer, nimotuzumab, erlotinib
Introduction
Leptomeningeal metastases (LM) occur in 3–5% of patients with non-small-cell lung caner (NSCLC) and are a detrimental complication associated with poor prognosis.1,2 Patients with LM are usually unable to accept systematic chemotherapy and present devastating headache. Whole brain radiotherapy (WBRT) remains controversial, some authors claim that it predicts favorable survival, but others proclaim its severe side effects accelerate patients’ death.3,4 Therefore, optimal therapeutic approaches are a big challenge. The epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor (TKI) is proved to be effective for patients with EGFR sensitive mutations in NSCLC in recent years.1,5,6 Some authors have reported that the incidence of harboring EGFR sensitive mutations for patients with LM is 58.1%.7 Erlotinib is more widely used to treat patients with central nerve system (CNS) metastases including LM because of its better penetration of brain-blood barriers (BBB) than gefitinib and icotinib.8,9 Nevertheless, the concentration in cerebrospinal fluid (CSF) is much lower than that in blood.10 Consequently, there are emerging reports indicating high-dose EGFR-TKI could increase the concentration in CSF for EGFR=mutated patients with LM, especially in patients’ CNS progression after taking standard-dose EGFR-TKI.11,12 High-dosage erlotinib did improve the control rate of LM, but it was associated with a serious rash reaction and/or diarrhea, and thus it was not recommended.13,14 Osimertinib, the third-generation TKI, has stronger central activity than the first generation. However, there is not much data on LM. Furthermore, after drug resistance to osimertinib, the limited countermeasures and high costs led doctors to hesitate to use osimertinib as a first-line treatment for EGFR-sensitive mutation.15 Scholars in Taiwan have found that the combination of cetuximab and afatinib is effective in controlling lung cancer with LM. Cetuximab combined with erlotinib has achieved good results in solid tumors.16–18 Nimotuzumab as another EGFR monoclonal antibody has also been reported in the application of lung cancer with LM.19 Macias et al20 found the disease control rate (DCR) of nimotuzumab combined with whole brain radiotherapy can reach to 91.6%. However, the application of nimotuzumab in LM has been rarely reported. Therefore, the aim of study was to research dual targeting of the epidermal growth factor receptor using combination of nimotuzumab and erlotinib in advanced NSCLC with LM to provide ideas for clinical practice.
Case 1
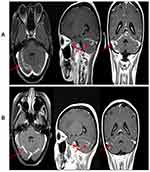
A 45-year-old female, was diagnosed with lung stage IV adenocarcinoma in 2014, with primary tumors of 2 cm in the upper left lobe, liver, bone and left eye metastases. Demographic profiles of this case are listed in Table 1. She started to take gefitinib (250 mg/cetuximab) orally with unknown EGFR mutation status. Cerebellum and brain stem metastases were observed 2 years later. WBRT (40 Gy/20 f) was used and gefitinib was still taken. S1 radiotherapy was performed in October 2015 because of serious pain in her waist. In October 2016 the patient developed unbearable headaches, nausea, vomiting, and diplopia in the left eye. The cranial contrast-enhanced magnetic resonance imaging (MRI) showed hydrocephalus with associated meningeal enhancement (Figure 1A). Lumbar puncture examination of CSF revealed a small number of nuclear cells but no tumor cells. NGS results of the CSF sample is EGFR 19del without T790M. Then, erlotinib (150 mg/day) orally and intravenous infusion nimotuzumab (200 mg/m2) once a week were performed. During the first 7 days, a corresponding improvement in the symptoms of headache and vomiting was found. A repeated cranial contrast-enhanced MRI at 1 month of posttreatment follow-up showed a decrease in size, range and degree of LM loci in her brain stem and cerebellum (Figure 1B). After 6 weeks, nimotuzumab was discontinued while erlotinib was maintained. After 2 months, her symptoms were resolved and she was discharged from hospital and continued to take erlotinib (150 mg/day) orally.
 |
Table 1 Patient Baseline Characteristics and Treatment Regimens |
Case 2
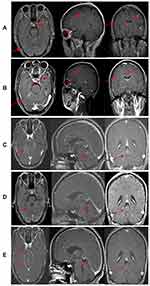
In February 2015, a 44-year-old female was diagnosed with adenocarcinoma of the upper left lobe of lung with bone metastasis. DNA sequencing of her tumor specimen obtained before treatment revealed a EGFR mutation (exon 19 deletion). Thus she was treated with gefitinib (250 mg/day) orally. In January 2017, she had disease progression with enlargement of skin nodules in the left parietal thus surgery was required. After surgery, contrast-enhanced MRI of her head demonstrated evidence of progressive intracranial metastasis (Figure 2A). A tissue sample test showed there was T790M by ARMS. However, the patient refused to take osimertinib due to economic problems. WBRT (40 Gy/20 f) was used and the patient started to take erlotinib. After 4 months, the metastases in her brain had gone (Figure 2B). In February 2019, she presented with double vision, facial twitching, and loss of hearing. A contrast-enhanced MRI of the brain showed progressive intracranial metastases with new leptomeningeal involvement (Figure 2C). The patient started to take erlotinib (150 mg/day) orally plus intravenous infusion nimotuzumab (200 mg/m2) once a week for 6 weeks. After 2 weeks, a repeated cranial contrast-enhanced MRI showed a remarkable shrinkage of LM (Figure 2D). One month later, an obvious improvement was shown on another repeated cranial contrast-enhanced MRI of her head (Figure 2E). The patient was discharged from hospital and continued to take erlotinib (150 mg/day) orally.
Case 3
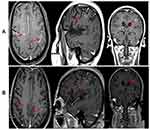
A 78-year-old female, PET-CT showed some lumps in the right upper lobe of lung and some loci in thoracic and lumbar vertebra with abnormally high carcinoembryonic antigen (CEA). Since she declined bronchoscopy and percutaneous lung puncture, she was highly suspected as lung cancer with thoracic and lumbar vertebra metastases. DNA sequencing of her blood specimen obtained revealed a EGFR mutation (exon 19 deletion), in addition, her demographic profile is listed in Table 1. Therefore, she initiated standard dosing of erlotinib (150 mg) and achieved partial response for 1 year. In May 2015, a radiotherapy in T4 (34 Gy/17 f) was initiated and erlotinib was still being taken. In December 2015, her clinical course was complicated by involuntary jitter of limbs, and word-finding difficulty. A brain contrast-enhanced MRI showed likelihood of leptomeningeal and parenchyma metastases in her brain (Figure 3A). DNA sequencing of her blood specimen obtained revealed a EGFR mutation (exon 19 deletion) without T790M. The patient then began treatment with the combination of erlotinib (150 mg/day) orally plus intravenous infusion nimotuzumab (200 mg/m2) weekly for 6 weeks. After 1 week, the patient’s condition continued to deteriorate. Considering these observations, WBRT was performed with patient consent in the hope of damaging BBB function to increase the level of CSF erlotinib. One month later (Figure 3B), the patient noted a concomitant improvement in her symptoms of tremor. Her lower limbs strength and memory improved, and she was able to return to work and continued to take erlotinib (150 mg/day) orally.
Discussion
Historically, median survival from the diagnosis of LM was 46 weeks if left untreated or treated for 23 months.21 Chemotherapy and radiation therapy are the main methods to treat NSCLC patients with LM.19 The three patients in this study were all previously treated with chemotherapy and radiation therapy, however, their condition continued to deteriorate.
It has been reported that EGFR-TKIs were effective for treating patients with LM harboring EGFR-sensitive mutation.12,22–26 Gefitinib and erlotinib are small-molecule, first-generation EGFR-TKIs. Studies have shown that the percentage of erlotinib penetrating the CSF is 2–3%.10 Porta et al27 retrospectively identified the efficacy of erlotinib in 17 brain metastases patients with EGFR mutations, with a median progression-free survival (PFS) of 11.7 months. A Phase II prospective study showed that the objective response rate (ORR), median PFS and overall survival (OS) of brain metastasis patients with EGFR mutations treated with erlotinib were 83%, 6.6 months, and 15.9 months, respectively.28 However, conventional doses of erlotinib have a moderate effect on the control of brain metastasis, and some scholars have proposed high-dose pulse administration.11 A phase II study showed that the ORR was 74% and median PFS was 10 months with brain metastasis patients taking pulse-continuous dose erlotinib.29 However, high-dosage of erlotinib will cause more serious side effects and economic effects, so it is not widely used in clinical practice. In our cases, two of the patients were treated with gefitinib at an earlier stage. Compared with gefitinib, the serum concentrations of erlotinib were relatively high.30 We speculated that erlotinib, instead of high-dose of gefitinib, may be also effective for the treatment of LM. In these cases, we used erlotinib (150 mg/d) for the treatment of patients with EGFR mutant or gefitinib-refractory NSCLC with LM and good efficacy with minor side effects were achieved.
For the second generation of EGFR-TKIs, afatinib is the main representative, there were no significant differences between it and the first generation EGFR-TKIs in the study results of LUX-lung 7.31 In another trial, after the progression of chemotherapy and first-generation EGFR-TKI treatment in 100 patients with LM, the median time to progression with afatinib was 3.6 months, and the results were similar to those patients without LM.32 In our case, we did not switch to afatinib after gefitinib failed.
In the clinical setting, mutations during therapy lead to acquired EGFR-TKI resistance.33 For example, T790M substitution in EGFR exon 20 has been reported in approximately 60% of cases with acquired resistance to EGFR-TKIs.34 Under this context, osimertinib, the third generation of EGFR-TKIs, emerged. In the AURA 3 subgroup analysis, compared with chemotherapy, the median PFS of 144 patients with T790M and brain metastasis treated with osimertinib prolonged for 4 months (8.5 M vs 4.2 M), ORR 54% and CR 12%.35 However, in the FLAURA trial, brain metastases occurred in 21% of the 556 patients, the median PFS of patients treated with osimertinib was 15.2 months (15.2 vs 9.6 months)36 compared with first-generation EGFR-TKIs. Resistance progression of first-generation EGFR occurred in all three cases in this study. Furthermore, resistance progression in this study was local progress. According to the latest NCCN guidelines, patients with local progression can follow the original regimen with local treatment.37 However, osimertinib is only authorized in patients with EGFR T790M, so we did not choose to switch to osimertinib immediately after resistance without EGFR T790M.
AZD 3759 is an oral TKI developed for brain metastasis, the ratio of unbound brain to plasma concentration was as high as 0.65.38 In the BLOOM study, intracranial ORR and extracranial ORR of 38 patients with EGFR mutation and NSCLC metastasis to brain/perichondrial were 63% and 50% respectively.39 But further clinical studies are needed to confirm its efficacy and safety. Tesevatinib is an oral reversible TKI. The Phase 2 study is being conducted in patients who have progressed with brain metastases and/or symptomatic leptomeningeal metastases while on prior therapy with other EGFR inhibitors. Four of those five patients, CNS lesions were controlled. One enrolled patient with no prior treatment who presented with brain metastases showed a robust partial response in brain metastases in an MRI taken on Study Day 29 and showed a partial response in both brain metastases and peripheral disease at Study Day 57.40 Preliminary trials have shown some intracranial and extracranial efficacy, but the evidence is still insufficient.
Radiotherapy could further aggravate the edema of normal brain tissue and aggravate intracranial hypertension and is not the preferred choice for LM. However, it can be tried as a complementary method. Radiotherapy can selectively open the BBB of the target area and protect the BBB of normal tissue from damage.41–43 Therefore, the concentration of TKI and macromolecular monoclonal antibody in the cerebrospinal fluid is significantly increased, thereby improving the efficiency of anti-tumor. As shown in case 1 and case 3, the shrinkage of the LM lesions was observed after the additional radiotherapy when the condition of the patients deteriorated after the combination therapy. The curative effect of a single drug on LM is limited and appropriate combination drugs can achieve a synergistic effect, such as radiotherapy. In addition, first-line meta-analysis summarized 15 clinical trials and concluded that radiotherapy combined with EGFR-TKI for the treatment of intracranial, median PFS was superior to TKI alone or radiotherapy alone, and significantly improved OS and PFS of intracranial and extracranial, but the rash also increased.44 Stereotactic radiosurgery (SRS) combined with TKI has also achieved considerable efficacy. A retrospective study analyzed 222 patients with EGFR-mutated NSCLC, and concluded that OS of SRS early treatment was up to 64 months, while OS of erlotinib was only 26 months.45 In our case, all patients received whole-brain radiotherapy, and meningeal metastasis occurred again after whole-brain radiotherapy, so re-radiotherapy was no longer suitable.
In a retrospective trial, 109 cases with EGFR-mutated NSCLC with brain metastases were treated with EGFR-TKI combined with bevacizumab while the other cases used EGFR-TKI only. After treatment, the median PFS in the study was 14.4 vs 9.9 months and the intracranial PFS was 14.0 vs 8.2 months, which initially showed some efficacy.46 However, whether the fact that bevacizumab can cause intracranial hemorrhage or not remains unclear and bevacizumab is only used in non-squamous NSCLC. Thus, more RCT clinical data are still needed. Because of the safety of bevacizumab and the high PS score of the patients, we did not choose erlotinib combined with bevacizumab to control LM. PD-1 inhibitor combined with EGFR-TKI is not ideal for treatment, so it is not recommended.47,48 There are few reports on dual targeting therapy for central metastasis of lung cancer. In 2014, Lin et al, in the journal Lung Cancer, proposed that afatinib combined with cetuximab had good activity and tolerance in advanced non-small-cell lung cancer with leptomeningeal metastases.49 It has been reported that double-targeting (afatinib combined with cetuximab) can overcome acquired resistance to gefitinib in NSCLC with EFFR T790M mutation.50,51 Those evidences strongly support our use of dual-targeted therapy. Previous studies showed nivolumab had a good effect in glioma.52,53 However, the normal blood-brain barrier (BBB) will prevent nivolumab (MW: 150 000) from transmitting to brain lesions. The integrity of BBB of tumor patients or patients treated with radiation is impaired. Nivolumab may cross the BBB of these patients.54 In addition, erlotinib, as small-molecule and first-generation EGFR-TKIs, has a higher rate (2–3%) of penetrating the CSF.10 The treatment of brain/meningeal metastases of non-small cell lung cancer with erlotinib is widely accepted. However, high-dosage of erlotinib will cause more serious side effects and economic effects. Therefore, we attempted to use erlotinib which had the highest concentration of cerebrospinal fluid and nivolumab to overcome drug resistance when the meningeal lesions were more serious.
Nimotuzumab (h-R3) is a humanized IgG1 monoclonal antibody generated against the extracellular domain of EGFR purified from human placenta.55 Its activity is similar to that of cetuximab. Several studies have shown that nimotuzumab is effective for NSCLC.19,55 The expression rate of EGFR is 40–80% which provides a theoretical basis for the treatment of lung cancer with nimotuzumab in NSCLC. A study from Japan has shown nimotuzumab improved the antitumor effect of radiation in certain human NSCLC cell lines in vitro and nude mice in vivo.55 The superiority of combining EGFR targeting with the tyrosine kinase inhibitor gefitinib and monoclonal antibody cetuximab has been approved.56,57 Patients with NSCLC treated with nimotuzumab combined with methotrexate (MTX) had prolonged OS over 1 year with minimal adverse effects.19 Qi et al58 found that PFS was significantly prolonged by 1 month in the group treated with nimotuzumab compared with those without. Additionally, in a phase Ib/II study, combination treatment of afatinib and nimotuzumab demonstrated an acceptable safety profile and encouraged antitumor activity in advanced NSCLC patients with acquired resistance to gefitinib or erlotinib.59 However, Kim et al60 found that the dual inhibition of EGFR with nimotuzumab plus gefitinib was not associated with better outcomes than gefitinib alone in patients with advanced NSCLC. Over expression of the EGFR is associated with 90% of NSCLC patients, we believe the collaborative effect of nimotuzumab plus erlotinib in NSCLC patients with LM may achieve good response. Therefore, we speculated that, with the highest concentration of cerebrospinal fluid, erlotinib combining with nimotuzumab may overcome the resistance of the first generation of EGFR-TKI. For the first time, we choose erlotinib (150 mg/day) plus nimotuzumab (200 mg/m2) weekly to treat patients with EGFR mutant or gefitinib-refractory NSCLC with LM, and good efficacy was achieved. When patients were treated with nimotuzumab combined with erlotinib, their symptoms of headache, vomiting, double vision, hearing loss, tremor of limbs, strength and memory improved. Our case indicates the potential of dual targeted therapy against EGFR in treating LMC in EGFR-positive NSCLC patients who still progressed after high-dose TKI.
Conclusion
In conclusion, combination therapy of erlotinib with nimotuzumab was a promising treatment for patients with NSCLC with LM because it can provide good tolerance and incredible efficacy. However, further study with a larger number of samples is warranted.
Ethics Approval and Consent to Participate
The Ethics Committee of the General Hospital of Western Theater Command approved the study (No. 2019ky57). All participants signed an approved informed-consent form. We also obtained consent to publication of their medical data.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Lee Y, Han JY, Kim HT, et al. Impact of EGFR tyrosine kinase inhibitors versus chemotherapy on the development of leptomeningeal metastasis in never smokers with advanced adenocarcinoma of the lung. J Neurooncol. 2013;115:95–101. doi:10.1007/s11060-013-1199-y
2. Riess JW, Nagpal S, Iv M, et al. Prolonged survival of patients with non-small-cell lung cancer with leptomeningeal carcinomatosis in the modern treatment era. Clin Lung Cancer. 2014;15:202–206. doi:10.1016/j.cllc.2013.12.009
3. Lee SJ, Lee JI, Nam DH, et al. Leptomeningeal carcinomatosis in non-small-cell lung cancer patients: impact on survival and correlated prognostic factors. J Thorac Oncol. 2013;8:185–191. doi:10.1097/JTO.0b013e3182773f21
4. Morris PG, Reiner AS, Szenberg OR, et al. Leptomeningeal metastasis from non-small cell lung cancer: survival and the impact of whole brain radiotherapy. J Thorac Oncol. 2012;7:382–385. doi:10.1097/JTO.0b013e3182398e4f
5. Heon S, Yeap BY, Lindeman NI, et al. The impact of initial gefitinib or erlotinib versus chemotherapy on central nervous system progression in advanced non-small cell lung cancer with EGFR mutations. Clin Cancer Res. 2012;18:4406–4414. doi:10.1158/1078-0432.CCR-12-0357
6. Kuiper JL, Hendriks LE, van der Wekken AJ, et al. Treatment and survival of patients with EGFR-mutated non-small cell lung cancer and leptomeningeal metastasis: a retrospective cohort analysis. Lung Cancer. 2015;89:255–261. doi:10.1016/j.lungcan.2015.05.023
7. Li YS, Jiang BY, Yang JJ, et al. Leptomeningeal metastases in patients with NSCLC with EGFR mutations. J Thorac Oncol. 2016;11:1962–1969. doi:10.1016/j.jtho.2016.06.029
8. Lee E, Keam B, Kim DW, et al. Erlotinib versus gefitinib for control of leptomeningeal carcinomatosis in non-small-cell lung cancer. J Thorac Oncol. 2013;8:1069–1074. doi:10.1097/JTO.0b013e318294c8e8
9. Gong L, Xiong M, Huang Z, et al. Icotinib might be effective for the treatment of leptomeningeal carcinomatosis in non-small cell lung cancer with sensitive EGFR mutations. Lung Cancer. 2015;89:268–273. doi:10.1016/j.lungcan.2015.06.001
10. Togashi Y, Masago K, Masuda S, et al. Cerebrospinal fluid concentration of gefitinib and erlotinib in patients with non-small cell lung cancer. Cancer Chemother Pharmacol. 2012;70:399–405. doi:10.1007/s00280-012-1929-4
11. Kawamura T, Hata A, Takeshita J, et al. High-dose erlotinib for refractory leptomeningeal metastases after failure of standard-dose EGFR-TKIs. Cancer Chemother Pharmacol. 2015;75:1261–1266. doi:10.1007/s00280-015-2759-y
12. Dhruva N, Socinski MA. Carcinomatous meningitis in non-small-cell lung cancer: response to high-dose erlotinib. J Clin Oncol. 2009;27:e31–2. doi:10.1200/JCO.2008.21.0963
13. Park K, Yu CJ, Kim SW, et al. First-line erlotinib therapy until and beyond response evaluation criteria in solid tumors progression in asian patients with epidermal growth factor receptor mutation-positive non-small-cell lung cancer: the ASPIRATION study. JAMA Oncol. 2016;2:305–312. doi:10.1001/jamaoncol.2015.4921
14. Yang Z, Hackshaw A, Feng Q, et al. Comparison of gefitinib, erlotinib and afatinib in non-small cell lung cancer: a meta-analysis. Int J Cancer. 2017;140:2805–2819. doi:10.1002/ijc.v140.12
15. Berz D, Raymond VM, Garst JH, et al. Non-invasive urine testing of EGFR activating mutation and T790M resistance mutation in non-small cell lung cancer. Exp Hematol Oncol. 2015;5:24. doi:10.1186/s40164-016-0052-3
16. Weickhardt AJ, Price TJ, Chong G, et al. Dual targeting of the epidermal growth factor receptor using the combination of cetuximab and erlotinib: preclinical evaluation and results of the phase II DUX study in chemotherapy-refractory, advanced colorectal cancer. J Clin Oncol. 2012;30:1505–1512. doi:10.1200/JCO.2011.38.6599
17. Guarino MJ, Schneider CJ, Hosford MA, et al. Dual inhibition of the epidermal growth factor receptor pathway with cetuximab and erlotinib: a Phase I study in patients with advanced solid malignancies. Oncologist. 2009;14:119–124. doi:10.1634/theoncologist.2008-0124
18. Huang S, Armstrong EA, Benavente S, et al. Dual-agent molecular targeting of the epidermal growth factor receptor (EGFR): combining anti-EGFR antibody with tyrosine kinase inhibitor. Cancer Res. 2004;64:5355–5362. doi:10.1158/0008-5472.CAN-04-0562
19. Ju Y, Sun S, Wang J, et al. Prolonged overall survival of patients with leptomeningeal carcinomatosis from nonsmall cell lung cancer. J Cancer Res Ther. 2016;12:126–129. doi:10.4103/0973-1482.191638
20. Macias A, Neninger E, Santiesteban E, et al. 505 POSTER preliminary results of a phase II clinical trial of the anti EGFR monoclonal antibody Nimotuzumab in combination with whole brain radiation therapy in patients diagnosed with advanced non-small cell lung cancer tumors unresectable brain metastases. Eur J Cancer Suppl. 2008;6:160–161. doi:10.1016/S1359-6349(08)72439-X
21. Wasserstrom WR, Glass JP, Posner JB. Diagnosis and treatment of leptomeningeal metastases from solid tumors: experience with 90 patients. Cancer. 1982;49:759–772. doi:10.1002/(ISSN)1097-0142
22. Jackman DM, Holmes AJ, Lindeman N, et al. Response and resistance in a non-small-cell lung cancer patient with an epidermal growth factor receptor mutation and leptomeningeal metastases treated with high-dose gefitinib. J Clin Oncol. 2006;24:4517–4520. doi:10.1200/JCO.2006.06.6126
23. Katayama T, Shimizu J, Suda K, et al. Efficacy of erlotinib for brain and leptomeningeal metastases in patients with lung adenocarcinoma who showed initial good response to gefitinib. J Thorac Oncol. 2009;4:1415–1419. doi:10.1097/JTO.0b013e3181b62572
24. Clarke JL, Pao W, Wu N, et al. High dose weekly erlotinib achieves therapeutic concentrations in CSF and is effective in leptomeningeal metastases from epidermal growth factor receptor mutant lung cancer. J Neurooncol. 2010;99:283–286. doi:10.1007/s11060-010-0128-6
25. Chang JE, Robins HI, Mehta MP. Therapeutic advances in the treatment of brain metastases. Clin Adv Hematol Oncol. 2007;5:54–64.
26. Thatcher N, Chang A, Parikh P, et al. Gefitinib plus best supportive care in previously treated patients with refractory advanced non-small-cell lung cancer: results from a randomised, placebo-controlled, multicentre study (Iressa Survival Evaluation in Lung Cancer). Lancet. 2005;366:1527–1537. doi:10.1016/S0140-6736(05)67625-8
27. Porta R, Sanchez-Torres J, Paz-Ares L, et al. Brain metastases from lung cancer responding to erlotinib: the importance of EGFR mutation. Eur Respir J. 2011;37:624–631. doi:10.1183/09031936.00195609
28. Park S, Kim H, Lee D, et al. Efficacy of epidermal growth factor receptor tyrosine kinase inhibitors for brain metastasis in non-small cell lung cancer patients harboring either exon 19 or 21 mutation. Lung Cancer. 2012;77:556–560. doi:10.1016/j.lungcan.2012.05.092
29. Arbour KC, Kris MG, Riely GJ, et al. Twice weekly pulse and daily continuous‐dose erlotinib as initial treatment for patients with epidermal growth factor receptor–mutant lung cancers and brain metastases. Cancer. 2018;124:105–109. doi:10.1002/cncr.v124.1
30. Yi HG, Kim HJ, Kim YJ, et al. Epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs) are effective for leptomeningeal metastasis from non-small cell lung cancer patients with sensitive EGFR mutation or other predictive factors of good response for EGFR TKI. Lung Cancer. 2009;65:80–84. doi:10.1016/j.lungcan.2008.10.016
31. Paz-Ares L, Tan E-H, O’byrne K, et al. Afatinib versus gefitinib in patients with EGFR mutation-positive advanced non-small-cell lung cancer: overall survival data from the phase IIb LUX-Lung 7 trial. Ann Oncology. 2017;28:270–277. doi:10.1093/annonc/mdw611
32. Hoffknecht P, Tufman A, Wehler T, et al. Efficacy of the irreversible ErbB family blocker afatinib in epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor (TKI)–pretreated non–small-cell lung cancer patients with brain metastases or leptomeningeal disease. J Thor Oncol. 2015;10:156–163. doi:10.1097/JTO.0000000000000380
33. Sharma SV, Bell DW, Settleman J, et al. Epidermal growth factor receptor mutations in lung cancer. Nat Rev Cancer. 2007;7:169–181. doi:10.1038/nrc2088
34. Pao W, Miller VA, Politi KA, et al. Acquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domain. PLoS Med. 2005;2:e73. doi:10.1371/journal.pmed.0020073
35. Mok TS, Wu Y-L, Ahn M-J, et al. Osimertinib or platinum–pemetrexed in EGFR T790M–positive lung cancer. N Engl J Med. 2017;376:629–640. doi:10.1056/NEJMoa1612674
36. Soria J-C, Ohe Y, Vansteenkiste J, et al. Osimertinib in untreated EGFR-mutated advanced non–small-cell lung cancer. N Engl J Med. 2018;378:113–125. doi:10.1056/NEJMoa1713137
37. Wood DE. National Comprehensive Cancer Network (NCCN) clinical practice guidelines for lung cancer screening. Thorac Surg Clin. 2015;25:185–197. doi:10.1016/j.thorsurg.2014.12.003
38. Sukrithan V, Deng L, Barbaro A, et al. Emerging drugs for EGFR-mutated non-small cell lung cancer. Expert Opin Emerg Drugs. 2019;24:5–16. doi:10.1080/14728214.2018.1558203
39. Ahn MJ, Kim DW, Cho BC, et al. Activity and safety of AZD3759 in EGFR-mutant non-small-cell lung cancer with CNS metastases (BLOOM): a Phase 1, open-label, dose-escalation and dose-expansion study. Lancet Respir Med. 2017;5:891. doi:10.1016/S2213-2600(17)30378-8
40. Berz D, Subrananiam D, Tonra J, et al. P2. 03b-016 tesevatinib in NSCLC patients with EGFR activating mutations and brain Metastases (BM) or Leptomeningeal Metastases (LM): topic: brain meta. J Thor Oncol. 2017;12:S942–S3. doi:10.1016/j.jtho.2016.11.1297
41. Morganti JM, Jopson TD, Liu S, et al. Cranial irradiation alters the brain’s microenvironment and permits CCR2+ macrophage infiltration. PLoS ONE. 2014;9:e93650. doi:10.1371/journal.pone.0093650
42. Cao Y, Tsien CI, Shen Z, et al. Use of magnetic resonance imaging to assess blood-brain/blood-glioma barrier opening during conformal radiotherapy. J Clin Oncol. 2005;23:4127–4136. doi:10.1200/JCO.2005.07.144
43. Chacko AM, Li C, Pryma DA, et al. Targeted delivery of antibody-based therapeutic and imaging agents to CNS tumors: crossing the blood-brain barrier divide. Expert Opin Drug Deliv. 2013;10:907–926. doi:10.1517/17425247.2013.808184
44. Jiang T, Min W, Li Y, et al. Radiotherapy plus EGFR TKIs in non‐small cell lung cancer patients with brain metastases: an update meta‐analysis. Cancer Med. 2016;5:1055–1065. doi:10.1002/cam4.673
45. Magnuson WJ, Lester-Coll NH, Wu AJ, et al. Management of brain metastases in tyrosine kinase inhibitor–naïve epidermal growth factor receptor–mutant non–small-cell lung cancer: a retrospective multi-institutional analysis. J Clin Oncol. 2017;35:1070–1077. doi:10.1200/JCO.2016.69.7144
46. Jiang T, Zhou C. 189O EGFR-TKIs plus bevacizumab demonstrated survival benefit than EGFR-TKIs alone in EGFR-mutant NSCLC patients with multiple brain metastases. Ann Oncology. 2019;30:mdz068. doi:10.1093/annonc/mdz068
47. Lee CK, Man J, Lord S, et al. Checkpoint inhibitors in metastatic EGFR-mutated non–small cell lung cancer—a meta-analysis. J Thor Oncol. 2017;12:403–407. doi:10.1016/j.jtho.2016.10.007
48. Gainor JF, Shaw AT, Sequist LV, et al. EGFR mutations and ALK rearrangements are associated with low response rates to PD-1 pathway blockade in non–small cell lung cancer: a retrospective analysis. Clin Cancer Res. 2016;22:4585–4593. doi:10.1158/1078-0432.CCR-15-3101
49. Lin C-H, Lin M-T, Kuo Y-W, et al. Afatinib combined with cetuximab for lung adenocarcinoma with leptomeningeal carcinomatosis. Lung Cancer. 2014;85:479–480. doi:10.1016/j.lungcan.2014.06.002
50. Jing LI, Xin-Hu WU, Liu ZB, et al. Overcoming acquired resistance to gefitinib in NSCLC with EGFR T790M mutation using combination of afatinib and cetuximab. Tumor. 2013.
51. Gomes JR, Cruz MRS. Combination of afatinib with cetuximab in patients with EGFR-mutant non-small-cell lung cancer resistant to EGFR inhibitors. Onco Targets Ther. 2015;8:1137. doi:10.2147/OTT
52. Ramos TC, Figueredo J, Catala M, et al. Treatment of high-grade glioma patients with the humanized anti-epidermal growth factor receptor (EGFR) antibody h-R3: report from a phase I/II trial. Cancer Biol Ther. 2006;5:375–379. doi:10.4161/cbt.5.4.2522
53. Ju Y, Wang J, Sun S, et al. Nimotuzumab treatment and outcome analysis in patients with leptomeningeal metastasis from nonsmall cell lung cancer. J Cancer Res Ther. 2016;12:C181–C5. doi:10.4103/0973-1482.200596
54. Diserbo M, Agin A, Lamproglou I, et al. Blood-brain barrier permeability after gamma whole-body irradiation: an in vivo microdialysis study. Can J Physiol Pharmacol. 2002;80:670–678. doi:10.1139/y02-070
55. Akashi Y, Okamoto I, Iwasa T, et al. Enhancement of the antitumor activity of ionising radiation by nimotuzumab, a humanised monoclonal antibody to the epidermal growth factor receptor, in non-small cell lung cancer cell lines of differing epidermal growth factor receptor status. Br J Cancer. 2008;98:749–755. doi:10.1038/sj.bjc.6604222
56. Matar P, Rojo F, Cassia R, et al. Combined epidermal growth factor receptor targeting with the tyrosine kinase inhibitor gefitinib (ZD1839) and the monoclonal antibody cetuximab (IMC-C225): superiority over single-agent receptor targeting. Clin Cancer Res. 2004;10:6487–6501. doi:10.1158/1078-0432.CCR-04-0870
57. Janjigian YY, Smit EF, Groen HJ, et al. Dual inhibition of EGFR with afatinib and cetuximab in kinase inhibitor-resistant EGFR-mutant lung cancer with and without T790M mutations. Cancer Discov. 2014;4:1036–1045. doi:10.1158/2159-8290.CD-14-0326
58. Qi D, Cui Y, Wang Q, et al. A clinical trial on docetaxel and carboplatin therapy with or without nimotuzumab for the treatment of advanced nonsmall cell lung cancer. J Cancer Res Ther. 2015;11(Suppl 1):C32–7. doi:10.4103/0973-1482.163836
59. Lee JY, Sun JM, Lim SH, et al. A Phase Ib/II study of Afatinib in combination with nimotuzumab in non-small cell lung cancer patients with acquired resistance to gefitinib or erlotinib. Clin Cancer Res. 2016;22:2139–2145. doi:10.1158/1078-0432.CCR-15-1653
60. Kim HR, Jang JS, Sun JM, et al. A randomized, phase II study of gefitinib alone versus nimotuzumab plus gefitinib after platinum-based chemotherapy in advanced non-small cell lung cancer (KCSG LU12-01). Oncotarget. 2017;8:15943–15951. doi:10.18632/oncotarget.13056
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.