Back to Journals » Medical Devices: Evidence and Research » Volume 8
Dual-sided electrosurgery handpiece for simultaneous tissue cutting and coagulation: first report on a conceptual design validated by an animal experiment
Authors Tawfik H, Fouad Y , Hafez R
Received 23 March 2015
Accepted for publication 29 April 2015
Published 13 August 2015 Volume 2015:8 Pages 351—357
DOI https://doi.org/10.2147/MDER.S85262
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Hatem A Tawfik,1 Yousef A Fouad,2 Rashad Hafez3
1Department of Ophthalmology, Oculoplastics Service, Ain Shams University, 2Faculty of Medicine, Ain Shams University, 3Eye Subspecialty Centre, Cairo, Egypt
Objective: To introduce and evaluate the safety of a novel dual-sided electrosurgery handpiece design for simultaneous tissue cutting and coagulation.
Methods: We designed a prototype double-sided handpiece allowing automatic switching between two electrodes with a simple handpiece flip. The concept of the system as a surgical instrument was assessed by an animal experiment.
Results: The skin of 15 Wistar albino white rats could be successfully incised and coagulated using both ends of the handpiece, thereby confirming the prospects and clinical applications of the system.
Conclusion: The dual-sided electrosurgery handpiece is a simple and safe alternative to the traditional electrosurgery pencil, allowing the simultaneous use of two electrodes without the hassle of frequent electrode replacement.
Keywords: radiosurgery, ablative surgery, laser resurfacing, electrocautery, electrosurgery
Introduction
The authors have designed a dual-sided electrosurgery handpiece where a cylindrical electrode used for cutting and a spherical electrode used for coagulation are combined at the opposite ends of the same handpiece allowing alternative use of the two electrodes with a simple handpiece flip. The device could be a simple and safe alternative to the traditional electrosurgery pencil allowing the simultaneous use of two electrodes without the hassle of frequent electrode replacement.
Direct heating of tissues as a therapeutic tool has been used for thousands of years; however, it was not until 1900 and only by chance that Joseph Rivere accidentally proved D’Arsonval’s theorem that electric energy could be transformed into heat energy which can be used to coagulate tissues.1–3 More than 2 decades later, the American plant physiologist William T Bovie introduced his electrosurgical unit (ESU) to Dr Harvey Cushing, a neurosurgeon from Boston, Massachusetts, who used the instrument in 1926 to remove a highly vascular brain myeloma, and the era of electrosurgery was officially born.1,3
The two fundamental tissue effects of electrosurgery, ie, cutting and coagulation, involve Joule heating of the conductive tissue by high-frequency electric current that leads either to vaporization and ionization of the water content in tissues (cutting), which is achieved with a sinusoidal wave that results in extreme spikes of temperature rise, or thermal denaturation without tissue vaporization (coagulation), which is achieved with a series of discontinuous wave packets where periods of quiescence interrupt the sinusoidal wave cycles and allow the tissues to relatively cool down a little creating a coagulum (Figure 1).3,4
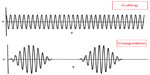  | Figure 1 Illustration showing the difference between the cutting mode which is composed of a pure continuous sine wave and the coagulation mode which is composed of intermittent pulses of high voltage current. |
Electrode geometry is a major factor influencing the final impact that an ESU has on tissues. Electrodes come in a variety of shapes and designs, each built for a specific purpose, but changing the electrodes during surgery every time the situation demands may be time-consuming; therefore, we designed a double-sided handpiece to accommodate two electrodes simultaneously where a spherical electrode used for coagulation could be attached to one end of the handpiece, while a cylindrical electrode used for cutting could be simultaneously attached to the other end obviating the need for frequent change of electrode tips.
Materials and methods
Study design
This study was conducted with the approval of the Ain Shams University Ethics Committee, and the study protocol was also approved by the University Animal Ethics Committee. Our handpiece design conform to the Association of Surgical Technologists recommended standards of practice for use of electrosurgery.5 Fifteen Wistar albino white rats of similar age and size were maintained and used in accordance with the Association for Research in Vision and Ophthalmology statement for the use of animals in ophthalmic and visual research. In addition, our handpiece design fully conformed to the Association of Surgical Technologists recommended standards of practice for use of electrosurgery.5
Instruments
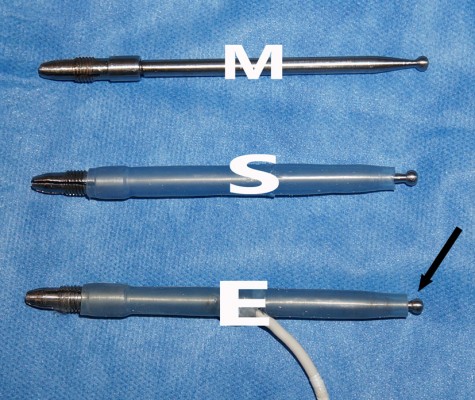
We designed a prototype dual-sided electrosurgery handpiece allowing automatic switching between two electrodes with a simple handpiece flip. The device was assembled locally in our hospital laboratory by making minor conceptual changes to the traditional design of the two-button electrosurgery pencil that is widely available in every operating theater. Instead of the electric current reaching one side of the pencil with the active electrode attached to the other end, and the rest of the probe sealed or coated, the source of electric current was shifted to the center of the handpiece. The entire shaft was covered with a fire and waterproof electrical insulator leaving only two uncoated ends with empty sockets for electrode placement, thus making both of them simultaneously active. Alternatively, one socket is left “open” for electrode fitting, while the other end is prefitted with a ball electrode for coagulation. Figures 2 and 3 show the outline of the handpiece in various stages of assemblage and its final design. The design is relatively simple, could be constructed inexpensively, and the handpiece is autoclavable.
Animals and the surgical procedure
Fifteen Wistar albino white rats were used in the study (250–270 g). The animals were bought and kept in the medical research center in Ain Shams University. They were kept in separate cages in rooms with controlled light and temperature, and were fed standard chow and water ad libitum.
Intraoperatively, the animals were anesthetized by an intraperitoneal injection of 10 mg/kg xylazine (Rompun Vet; Bayer AB, Solna, Sweden) and 40 mg/kg ketamine hydrochloride (Ketalar; Pfizer, Inc., New York, NY, USA) 5 minutes before the procedure, and monitored by a veterinary technician. Rats were positioned on a thermistor-controlled heating pad in the prone position and a rectal probe was inserted. Surgical procedure was performed under strictly sterile conditions.
The handpiece was connected to an MC-9 electrosurgery unit (Macan, Milton, DE, USA), at a minimal power setting of 0.5 U in coagulation mode. The cutting tip was used first to create a linear full-thickness incision followed by the coagulation tip which was used with no change of settings on the electrosurgery unit to create two circular coagulation scars (Figure 4 A and B). Wounds were deliberately left open to evaluate healing by secondary intention.
The rats were divided into three sets. In each set of five animals, a specific length of the electrosurgery handpiece was used:
Set 1: A 12 cm shaft was used.
Set 2: A 16 cm shaft was used.
Set 3: A 20 cm shaft was used.
In the early postoperative period, the rats routinely received 3 mL of saline intraperitoneally to compensate for the blood loss during the surgical procedure which was minimal because of the use of cautery. The rats were kept in ventilated cages and maintained on normal diet and water for 2 weeks, and the wounds were dressed daily by 10% povidone-iodine. After 2 weeks, the wounds were evaluated and documented photographically; then the animals were sacrificed and the visible scars were excised and submitted for histopathological examination.
Results
A normal healing process including epithelialization and wound contraction was observed in most animals at 2 weeks, and no evidence of ischemia, ulceration, or infection was noted in any of the wounds; however, one of the animals from set 1 and another from set 2 had partial dehiscence of the linear wound.
Set 1: We did not encounter direct or capacitive coupling in any animal (unintended tissue injury through the spread of electric current from the unused electrode to an adjacent metallic surgical instrument either directly or indirectly through insulators), dielectric burns (unintended injury to the surgical team through breakdown of nonconductive material like rubber gloves), operating theater fires, or animal/surgical team burns.
Sets 2 and 3: Results were similar to set 1, but we did, however, find out that we had to use extreme caution when using these extraordinary long shafts. Personal injury or injury to the nursing staff is possible with the unused end of an unusually long shaft.
Histopathological findings
Sections examined from the skin of the rats in all three sets revealed an intact covering of stratified squamous epithelium, with no ulceration or erosions. The skin adnexa, including hair follicles and sebaceous glands, were also intact. There was no evidence of excessive fibrosis or inflammation in the dermis (Figure 4).
Discussion
Conventional electrosurgery has been available in operating theaters for almost a century and although the shape and design of ESUs have changed dramatically, the fundamental principles of electrosurgery have not changed much, and with the exception of the introduction of adaptive generators, innovations have been limited.1 Basically, electrosurgery is the process where a high-frequency electrical current is applied to biologic tissues to achieve specific thermal effects like cutting or coagulation. One electrode delivers the current to the tissues while the other electrode returns the current to the machine. The human body acts as the source of electrical resistance (impedance) and completes the circuit.6
Biophysical basis
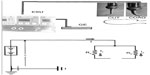
To help explain our design in precise electrical terms, we have to understand that it is analogous to connecting an electrical circuit in parallel. In parallel circuits voltage is equal across all branches of the circuit. The total current is the sum of currents flowing though all branches (Figure 4), and the current in each branch in a parallel circuit is totally independent of the other branch and is calculated by Ohm’s law as follows:
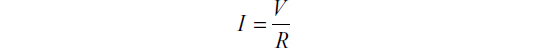
In layman’s terms, in parallel circuits each component behaves as if it is connected alone to the electric source and current flow is dependent mainly on the resistor in individual components so the total current could simply be calculated as follows:
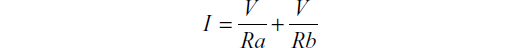
where I is the total current across the circuit, V is the voltage which in parallel circuits is the same in all branches, Ra is the impedance of the active electrode, and Rb is the impedance of the inactive electrode (Figure 5). There will be little resistance in the “inactive” branch which is only due to the resistance of the electrode material itself which is very low. Current will flow in both branches, with considerably more flowing in the inactive electrode because according to Ohm’s law, current flows in the path of least resistance. Although we could infer from the previously mentioned formula that for a given energy output on the ESU, the final effect on tissues would be less than when using a traditional electrosurgical pencil; in fact, that this is of little clinical relevance because the final effect of electrosurgery on tissues depends on several variables, which include, in addition to the current, the tissue resistance, the speed of motion through tissues, the pressure applied by the surgeon’s electrosurgical instrument on tissues, the type of waveform (ie, continuous or intermittent), and finally electrode design and geometry.1,7–9 The effect of all these factors could be summarized by Joule’s first law:
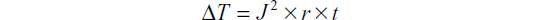
where ΔT denotes the local rise in temperature, J is the current density (current/cross-sectional area), t is the time or duration of application of the current, and r is tissue resistance.1,4,10,11 It is clear from the equation mentioned earlier that the square of current density is positively correlated with the final rise in temperature; therefore, electrode size or shape has a direct bearing on the final tissue effect, as the heat produced is inversely proportional to the surface area of the electrode. The smaller the electrode, the more localized is the current concentration, which ultimately creates more intense heating at tissue level.1,12
Electrodes could be broadly classified into two categories: spherical or ball electrodes for coagulation and cylindrical electrodes for cutting.4 The cutting mode works by sending electric sparks that produce intense localized heat at the surgical site high enough to vaporize tissues ahead of the active electrode, thus behaving like a traditional scalpel blade; however, it is inconceivable that blunt, usually rounded ball, electrodes can cut through any tissue at all even if the ESU is in the cutting mode. Conversely, ultrathin cutting electrodes with their markedly localized tissue heating effect would not provide the ideal desiccation tool.12
Theoretical bases aside, both electrode categories claim special usage in everyday oculoplastic practice. The merits of using ultrathin microdissection needles for precise soft tissue dissections, and the safety of using ball electrodes while applying desiccation in the vicinity of the globe has led some authors to claim that electrode geometry is the most important factor controlling the quality of hemostasis or the finesse of a cut.13
Surgeons handle hemostasis differently. Certainly, a case could be made for the simultaneous use of a bipolar and a monopolar handpiece; the monopolar handpiece for cutting and the bipolar forceps for coagulation, but both having cords in juxtaposition in the surgical field could induce capacitative or direct leakage of electrical current from the monopolar to the bipolar cord, or inadvertent activation of the either tool, and subsequent burns.1,14 Besides, a single handpiece that does both jobs simplifies matters and relieves the operating room staff from the complexity of having too many tools in the operative field.
Alternatively, other surgeons use a monopolar handpiece alone. A meticulous surgeon would regularly change electrodes, and alter the mode dial on the ESU or pencil every time the situation demands it, which may be quite cumbersome. Time-conscious surgeons usually compromise with several maneuvers. Some simply change the mode dial on the machine, or on the pencil while using a single universal needle or blade electrode as a trade-off, while others use the electrode tip for cutting, and then maneuver the handpiece and use the side of the same electrode for coagulation. Manipulating the handpiece may not always be a viable option in every surgical situation. Again our design enables simultaneous cutting or coagulation with the proper electrodes at the discretion of the surgeon without wasting time locating, attaching, or removing several electrodes, but it is not without drawbacks.
Potential risks
With the exception of handpiece “insulation failure” which still lingers as one of the few remaining dangers of modern electrosurgery,7 electrosurgical injuries in the operating theater have been practically eliminated;15 therefore, it may be counterintuitive to create an artificial “insulation failure” environment by having two electrodes that are simultaneously active at the same time, with one of them hanging uninsulated in the air. Given our understanding that electricity always flows along the path of least resistance,16 we would expect current flow through the unused electrode to be extremely high and therefore any accidental contact with this low impedance electrode could in theory subject the recipient to stray radiofrequency current and extremely high temperatures.2,5 However, as we mentioned earlier, we did not encounter a single case of capacitative failure or dielectric breakdown or any accidental burn to the surgical team or our animals. Technically, none of these idiosyncrasies traditionally linked to the use of ESUs could be encountered because the unused electrode is always well away from the surgical field or any metal instrument, so capacitative or direct coupling or unintended burns are not physically possible (Figure 3). More importantly, we have found that reducing the length of the handpiece down to 12 cm (set 1) would allow it to rest comfortably in the operator’s hand without being too long or too short to further minimize the risk of any potential burn to the scrubbed nurse or the surgeon, respectively (Figure 3). Alternatively, an autoclavable plastic cap could be used as a temporary cover for the unused electrode. In fact, we could counter argue that by creating a controlled defect in the insulation, our design merely exploits in a precise and exact manner the insulation failure issue which still haunts modern electrosurgery.14
A further improvement that would possibly safeguard against any potential hazard could be the addition of a single-pole double-throw switch to act as a safety valve against accidental injury by the unused electrode; however, our limited technical staff and our low resources prevented us from developing such a switch. Besides, we believe that the addition of a switch defies our notion of design simplicity.
Conclusion
In conclusion, although our design offers the clear advantage of using two different electrodes simultaneously, this comes at the price of a theoretical burn risk which we acknowledge was never encountered so far. It would be rather rash to assume that the design in question is superior to the traditional bipolar/monopolar combination frequently used by many surgeons around the world, or the gold standard electrosurgery pencil with the typical cutting/coagulation buttons near the tip.17 There may be advantages and disadvantages inherent in each design. As with all surgical disciplines, different surgeons may employ or favor a slightly dissimilar surgical approach for the management of the same disease, and while we may proceed at length to offer spirited debates about the advantages of our design, we acknowledge that all prior well-established designs yield an equally decent job, and they are all a testament to the success of electrosurgery and a tribute to all those innovators at the turn of the previous century who made it possible.
Acknowledgment
Our special thanks to Mr Tom Henderson from the physics classroom project (http://www.physicsclassroom.com/) for his guidance and valuable assistance in understanding parallel circuits.
Disclosure
The authors report no conflicts of interest in this work.
References
Massarweh NN, Cosgriff N, Slakey DP. Electrosurgery: history, principles, and current and future uses. J Am Coll Surg. 2006;202:520–530. | |
Smith TL, Smith JM. Electrosurgery in otolaryngology – head and neck surgery: principles, advances, and complications. Laryngoscope. 2001;111:769–780. | |
Jones CM, Pierre KB, Nicoud IB, et al. Electrosurgery. Curr Surg. 2006;63:458–463. | |
Palanker DV, Vankov A, Huie P. Electrosurgery with cellular precision. IEEE Trans Biomed Eng. 2008;55:838–841. | |
Association of Surgical Technologists. AST Recommended Standards of Practice for Use of Electrosurgery. Available from: http://www.ast.org/pdf/Standards_of_Practice/RSOP_Electrosurgery.pdf. Accessed September 14, 2013. | |
Usatine RB, Hainer BL. Electrosurgery of the skin. Am Fam Physician. 2002;66:1259–1267. | |
Wu MP, Ou CS, Chen SL, et al. Complications and recommended practices for electrosurgery in laparoscopy. Am J Surg. 2000;179:67–73. | |
Munro MG. Energy sources for operative laparoscopy. In: Gomel V, Taylor PJ, editors. Diagnostic and Operative Gynecologic Laparoscopy. St Louis, MO: Mosby-Year Book;1995:26–56. | |
Soderstrom R. Principles of electrosurgery as applied to gynecology. In: Rock JA, Thompson JD, editors. Te Linde’s Operative Gynecology. 8th ed. Philadelphia: Lippincott-Raven; 1997:321–326. | |
Smith TL, Smith JM. Radiofrequency electrosurgery. Oper Tech Otolaryngol Head Neck Surg. 2000;11:66–70. | |
Eggleston JL, Von Maltzahn WW. “Electrosurgical Devices.” In: Bronzino JD, editor. The Biomedical Engineering Handbook. 2nd ed. Boca Raton, FL: CRC Press LLC; 2000;81:1–7. | |
Wang K, Advincula AP. “Current thoughts” in electrosurgery. Int J Gynaecol Obstet. 2007;97:245–250. | |
Professional Medical Education Association. Practical Electrosurgery Manual – Laser Training. Available from: http://www.lasertraining.org/Administrative/Library/Practical%20Electrosurgery%20Manual.pdf. Accessed January 7, 2013. | |
Vilos G, Latendresse K, Gan BS. Electrophysical properties of electrosurgery and capacitative induced current. Am J Surg. 2001;182:222–225. | |
Nduka CC, Super PA, Monson JR, Darzi AW. Cause and prevention of electrosurgical injuries in laparoscopy. J Am Coll Surg. 1994;179:161–170. | |
Ansell Healthcare Europe. Electrosurgery and Latex Gloves. Available from: http://www.anselleurope.com/medical/downloads/Electrosurgery_EN.pdf. Accessed January 18, 2013. | |
Niamtu J 3rd. Radiowave surgery versus CO laser for upper blepharoplasty incision: which modality produces the most aesthetic incision? Dermatol Surg. 2008;34:912921. | |
Davison J, Zamah N. Electrosurgery: Principles, Biologic Effects and Results in Female Reproductive Surgery. Available from: http://www.glowm.com/index.html?p=glowm.cml/section_view&articleid=21. Accessed November 10, 2013. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.