Back to Journals » HIV/AIDS - Research and Palliative Care » Volume 12
Drug-Resistant Tuberculosis and HIV Infection: Current Perspectives
Authors Singh A, Prasad R , Balasubramanian V, Gupta N
Received 30 July 2019
Accepted for publication 9 December 2019
Published 13 January 2020 Volume 2020:12 Pages 9—31
DOI https://doi.org/10.2147/HIV.S193059
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Bassel Sawaya
Abhijeet Singh, 1 Rajendra Prasad, 1, 2 Viswesvaran Balasubramanian, 1 Nikhil Gupta 3
1Department of Pulmonary Medicine, Vallabhbhai Patel Chest Institute, University of Delhi, New Delhi, Delhi 110007, India; 2Department of Pulmonary Medicine, King George Medical University, Lucknow, Uttar Pradesh 226003, India; 3Department of Internal Medicine, Ram Manohar Lohia Institute of Medical Sciences, Lucknow, Uttar Pradesh 226010, India
Correspondence: Rajendra Prasad
Department of Pulmonary Medicine, Vallabhbhai Patel Chest Institute, University of Delhi, New Delhi, Delhi 110007, India
Tel +91 9415 021 590
Email [email protected]
Abstract: Drug-resistant tuberculosis (DR-TB), including multidrug-resistant TB (MDR-TB) and extensively drug-resistant TB (XDR-TB), is considered a potential obstacle for elimination of TB globally. HIV coinfection with M/XDR-TB further complicates the scenario, and is a potential threat with challenging management. Reports have shown poor outcomes and alarmingly high mortality rates among people living with HIV (PLHIV) coinfected with M/XDR-TB. This coinfection is also responsible for all forms of M/XDR-TB epidemics or outbreaks. Better outcomes with reductions in mortality have been reported with concomitant treatment containing antiretroviral drugs for the HIV component and antitubercular drugs for the DR-TB component. Early and rapid diagnosis with genotypic tests, prompt treatment with appropriate regimens based on drug-susceptibility testing, preference for shorter regimens fortified with newer drugs, a patient-centric approach, and strong infection-control measures are all essential components in the management of M/XDR-TB in people living with HIV.
Keywords: drug-resistant, HIV, multidrug-resistant, extensively drug-resistant, tuberculosis
Introduction
Drug-resistant tuberculosis (DR-TB) is defined as a case of TB excreting bacilli resistant to one or more anti-TB drugs. DR-TB exists in various forms: monodrug-resistant TB (mono-DR-TB), polydrug-resistant TB (poly-DR-TB), rifampicin-resistant TB (RR-TB), multidrug-resistant TB (MDR-TB), and extensively drug-resistant TB (XDR-TB).1 Mono-DR-TB is a case of TB showing resistance to single first-line anti-TB drugs only. Isoniazid (H) resistance is a type of mono-DR-TB and has recently become an important concern, as there is a risk of amplification of resistance involving additional drugs if left untreated. Poly-DR-TB is a case of TB resistant to more than one first-line anti-TB drug other than both rifampicin (R) and H. RR-TB is defined as a case of TB showing either resistance to R only or accompanied by resistance to other anti-TB drugs in various forms such as R mono-DR-TB, R poly-DR-TB, MDR-TB, and XDR-TB. The last two are important forms of DR-TB. MDR-TB is defined as a case of TB showing either exclusive resistance to both H and R or accompanied by resistance to other first-line anti-TB drugs. XDR-TB refers to resistance to at least one of three fluoroquinolones (FQs) and at least one of three second-line injectable drugs (SLIDs), in addition to multidrug resistance. A subset of MDR-TB patients may have additional resistance to second-line anti-TB drugs — at least one of three FQs (ofloxacin [Ofx], levofloxacin [Lfx], moxifloxacin [Mfx]) or one of three SLIDs (capreomycin [Cm], kanamycin [Km], amikacin (Am)] but not to both — also known as pre-XDR-TB. MDR-TB and XDR-TB are considered a global health problem with notoriously difficult and challenging treatment.2 A dynamic interaction exists between TB and HIV infection. TB accelerates the progression of disease in people living with HIV (PLHIV), and PLHIV have increased susceptibility to TB infection. TB is a major cause of mortality among PLHIV, while HIV is responsible for failure of TB-control programs in achieving targets, particularly in high-burden countries. TB and HIV coinfection enhances the risk of harboring and acquiring M/XDR-TB strains.3,4 HIV coinfection with M/XDR-TB further complicates the situation, and is considered a significant threat with challenging management. Proper adherence to treatment remains an issue, due to higher pill burden, overlapping or additive adverse drug reactions (ADRs) and drug–drug interactions. The current review addresses the relationship between HIV and M/XDR-TB, collaborative TB–HIV activities, diagnostic and clinical guidelines for management of M/XDR-TB in HIV-infected patients, potential drug interactions, ADRs and monitoring requirements in the concomitant treatment of M/XDR-TB and HIV, and infection-control implications in the context of HIV and M/XDR-TB.
Search Strategy
A search strategy was adopted involving principal electronic databases (PUBMED, EMBASE, Web of Science, ISI Web of Knowledge, Google and Google Scholar) and World Health Organization (WHO) documents (Drug resistance surveillance reports, Guidelines on management of PLHIV, Global TB reports and Programmatic management of DR-TB). Articles in English language from 1980 till now, were identified using the terms in combination: “Tuberculosis”, “Drug-sensitive”, “Drug-resistant”, “Multi-drug resistant” or “MDR-TB”, “Extensively-drug resistant” or “XDR-TB”, “HIV” or “PLHIV” or “AIDS”, “Coinfection”, “Epidemiology”, “Prevalence”, “Global burden”, “Epidemic”, “Outbreak”, “Clinical features”, “Diagnosis”, “Laboratory diagnostics”, “Genotypic tests”, “Management”, “First line”, “Second line”, “Anti-tubercular therapy” or “Anti-tuberculous therapy”, “Anti-retroviral therapy” or “ART”, “Adverse drug reactions” or “Adverse events”, “Treatment outcome”, “Chemoprophylaxis” and “Infection control”. Manual search was also performed in addition. 165 articles including 5 systematic reviews have been identified. All articles with resulting titles, abstracts and full texts, when available were read and kept for references.
Global Burden of HIV and TB Coinfection
The incidence of drug-susceptible TB (DS-TB) and HIV coinfection has increased over the past two decades, as both are strongly linked. It is estimated that PLHIV, especially with fewer than 200/cm3 CD4 count, show a 19 (15–22)-fold increased risk of developing active TB compared with those who are HIV-negative.5 According to the WHO, 8.6% (7.4%–10%) of 10 million (range, 9–11.1 million) incident cases with active TB were alsocoinfected with HIV in 2018. A third of 37 million PLHIV are infected with TB bacillus. Sub-Saharan Africa is the region with the highest burden of coinfection, comprising 71% of global coinfected cases. Among 30 high-burden countries with TB and HIV coinfection, HIV infection has been detected in 70% of patients with TB. South Africa (177,000) has reported the highest number of incident TB cases among PLHIV, followed by India (92,000) and Mozambique (58,000). Also, in 2018 0.25 million (16.8%) of a total 1.5 million deaths from TB showed HIV coinfection.5 Of 862,000 new TB cases among PLHIV, 477,461 (56%) were notified, of which 86% were placed on antiretroviral therapy (ART). The global cure rate for TB in PLHIV has been reported to be 75%. Although the mortality rate has reduced by 60% worldwide since 2000, there is marked regional variation. This reduction is most evident in Europe and lowest in sub-Saharan Africa.
Association Between HIV and DR-TB: Epidemiology and Risk Factors
PLHIV are also vulnerable to DR-TB infection. There are several epidemiological reasons that M/XDR-TB may be associated with HIV.6 The reasons suggested are rapid progression of disease due to harboring of DR strains, particularly in the immunocompromised compared to immunocompetent state; drug malabsorption of anti-TB drugs, such as R and ethambutol (E), leading to drug resistance and treatment failure; early reactivation of an infection due to increased vulnerability in an immunocompromised state acquired from community or institutional transmission; direct contact with DR-TB cases, suggesting primary or transmitted resistance; confounding by common risk factors, such as intravenous drug abuse, imprisonment, low socioeconomic status, alcoholism, and frequent hospitalization; repeated exposure to DR isolates; and poor adherence to treatment. The magnitude of global burden of DR-TB and HIV coinfection has not been exactly defined. The primary reason for the lack of data is that HIV infection and anti-TB drug-susceptibility testing (DST) are not adequately accessible for joint surveillance under routine conditions. Epidemiological studies from different countries have shown discordant associations. There has been heterogeneity in setting, demographic profile, methodology, and analysis of data. In the fourth WHO–International Union Against Tuberculosis and Lung Disease global drug-surveillance report, 24 countries reported data on MDR-TB stratified by HIV status.7 Only eleven countries, the majority from Eastern European and Central Asian regions, reported strong associations between HIV and drug resistance. There was a lower association for DR-TB infection in PLHIV from the US. Additional information on overlapping risk factors for coinfection, including history of hospitalization or imprisonment, was not available initially for this analysis, so specific reasons for the association were not known. There was heterogeneous geographic distribution, with the majority confined to high-risk groups, even in countries showing a high prevalence of MDR-TB along with an emerging HIV epidemic. Detailed analysis of surveillance data from Kazakhstan showed no change in the proportion of TB patients with drug resistance from 2007 to 2011 in PLHIV, with average prevalence of 36.1% for both new and retreatment cases, although there was a progressive increase in the burden of TB and HIV coinfection.8 The prevalence of MDR-TB was 20% higher in HIV-positive patients than HIV-negative ones. HIV was not an independent risk factor for MDR-TB, although such risk factors as male sex, young adult, urban residence, homelessness, and history of incarceration and drug abuse were largely overlapping. The last two subgroups were at particular risk of HIV and MDR-TB coinfection. Enhanced efforts are necessary to provide care to these high-risk groups. Further, a systematic review and meta-analysis has recently reported that HIV-positive cases have a 24% higher risk of MDR-TB compared to HIV-negative ones.9 Another study observed no significant association between MDR-TB and HIV coinfection.6 Institution-based studies have shown a stronger association than community- or population-based studies. With high prevalence of HIV seropositivity and M/XDR-TB, countries from sub-Saharan African and Asia-Pacific regions have reported very low prevalence of coinfection.9 Factors responsible for underestimation of prevalence were delay in diagnosis, increased susceptibility to infection or progression of disease, and lack of infrastructure. The WHO has projected 14 countries for high burden for MDR-TB and HIV coinfection. These are Angola, China, the Democratic Republic of the Congo, Ethiopia, India, Indonesia, Kenya, Mozambique, Myanmar, Nigeria, Papua New Guinea, South Africa, Thailand, and Zimbabwe. A few studies have estimated the burden of drug resistance among PLHIV ranging from 5.6% to 24% with genotypic tests, such as the cartridge-based nucleic acid–amplification test (CBNAAT), including automated GeneXpert MTB/RIF assays and polymerase chain reaction–based line-probe assays (LiPAs).10–15 However, existing data remain sparse, as implementation of these tests remains suboptimal, particularly in resource-limited settings assumed to have a high burden of disease. Community-based surveillance at national level should be conducted for all regions worldwide to estimate the burden of coinfection in the near future.
Epidemiology of DR-TB and HIV Coinfection Outbreaks
Reports have shown poor outcomes and alarmingly high mortality rates among PLHIV coinfected with M/XDR-TB.16–23 HIV is also responsible for all forms of M/XDR-TB epidemics or outbreaks.7,24–28 TB in PLHIV is mostly smear-negative, and delayed diagnosis due to lack of rapid diagnostic tests and appropriate treatment has led to high mortality in PLHIV. Epidemics of DR-TB and HIV have not only affected different regions of the world but also converged in a deadly syndemic, particularly in sub-Saharan African and Eastern European regions, over the last decade, leading to poor treatment outcomes and high mortality.29–31 The epidemic of HIV infection in the European region is concentrated within high-risk groups, whereas that in the sub-Saharan African region has affected the general population.32 Outbreaks of MDR-TB among HIV-infected patients between 1985 and 1993 have been observed, even in developed countries, such as the US.33–36 A Bayesian modeling study reported that HIV epidemics facilitate TB outbreaks only by enhancing increased susceptibility to infections, but that HIV coinfection was not a direct driver for evolution and transmission of DR strains.37 Development of acquired drug resistance is of concern, due to poor compliance with treatment, deficiencies or gaps in existing knowledge regarding management of diseases, and lack of uniform standards of care or guidelines, causing faulty practices. Other factors responsible for acquired drug resistance are suboptimal anti-TB treatment due to poor maintenance of quality of drugs; prescription of fixed-dose combinations unadjusted for appropriate body weight; intermittent or standardized TB regimens; undetected resistance to H and second-line anti-TB drugs, particularly FQs and SLIDs, at baseline in retreatment cases and high-risk groups; pharmacokinetic and pharmacodynamic variability; and poor drug penetration to target lesions, rendering drugs ineffective.38–40 All these factors remain common in people living with or without HIV infection. Primary or transmitted resistance is also responsible for epidemics or outbreaks, as detected by molecular fingerprinting.41,42 HIV-infected cases progress rapidly to disease, and in settings where M/XDR-TB is prevalent, this may lead to increased numbers of DR-TB patients, either in the general population or local populations, such as hospitals, prisons, and intravenous drug abusers, or an outbreak. PLHIV may also be more likely to be exposed to M/XDR-TB patients, due to either increased hospitalization in settings with poor infection control or association with colleagues with M/XDR-TB, particularly in prison settings. Temporal trends and transmission patterns were analyzed in a study from the US with restriction fragment–length polymorphism among TB patients and clustering of MDR-TB cases observed among HIV patients.33 Spread of drug resistance was caused by direct transmission from HIV-seronegative patients. The largest global outbreak of M/XDR-TB occurred in Tugela Ferry of the KwaZulu-Natal region in South Africa in an HIV-positive population, characterized by a high mortality rate of 99%. The main driver responsible for this deadly convergence was not only the evolution of drug-resistance mutations but also transmitted resistance among the surrounding population, unveiled by genotypic tests, including next-generation sequencing.40–42 Studies from other cities, such as Tomsk, Russia and Shanghai, China, also reported similar findings.43,44 A study from Mumbai, India reported an alarmingly high burden of MDR-TB, MDR-TB with additional resistance to FQs or SLIDs, and XDR-TB, with prevalence rates of 38%, 21%, and 6%, respectively, among HIV-infected patients attending public ART centers, likely representing ongoing transmission in the community and health facilities.39 Prevalence rates of mono-DR-TB and poly-DR-TB were 21% and 12%, respectively. Further, the prevalence rate of MDR-TB was 11.4% among new TB cases and 36.4% among retreatment TB cases. Despite existing weakness in the collection of data, the association between HIV and M/XDR-TB is a matter of concern, particularly given the implications for the clinical management of these patients.
Clinical Features and Diagnosis of M/XDR-TB in PLHIV
An accurate diagnosis of DS-TB as well as DR-TB in HIV-infected people is more difficult and may be confused with other pulmonary or systemic infections. The clinical features, such as cough, fever, night sweats, and weight loss, can be observed in both forms of TB, even in HIV patients. These features can be used for active screening, with reported sensitivity of 85%–93% and specificity of 30%–40%.45 Radiological presentations, such as upper-lobe infiltrates or cavitation in either form of TB, remain similar in HIV-infected patients with CD4 count >350/cm3 and uninfected patients. The presentation is more likely to be extrapulmonary or atypical, such as middle- or lower-lobe infiltrates, pleural effusion, mediastinal lymphadenopathy, or interstitial nodular opacities, especially as immunosuppression advances (CD4 <200/cm3).46 However, cavitation can be observed in advanced disease. Sputum-smear examination is usually positive in early disease and negative in advanced disease, but contrary results can be obtained. Phenotypic tests, such as conventional solid Lowenstein–Jensen medium culture and DST remain the gold standard for diagnosis, but usually take 2–6 weeks. This can result in misdiagnosis or delays in diagnosis, and in turn higher morbidity and mortality. Other phenotypic tests, such as the Mycobacterium growth-indicator tube, Bactec Radiometric 960, and microscopic observation broth drug-susceptibility assay are more sensitive, have faster turnover, and show rapid results, but are expensive and more prone to contamination.47 Algorithms have been devised by the WHO with the aim of enhancing diagnostic yield in PLHIV with smear-negative pulmonary and extrapulmonary TB.48 Clinical criteria should be initially used for establishing diagnosis, followed by additional laboratory data, such as culture and radiography. The positive predictive value of clinical criteria is 89%–96% in these cases when compared with culture as the gold standard.49 For patients with advanced HIV disease with extrapulmonary involvement, mycobacterial culture of other fluids (eg, blood, pleural fluid, ascitic fluid, cerebrospinal fluid, and bone-marrow aspirates) and histopathology (eg, lymph-node biopsies) may be helpful in diagnosis. It is recommended that all PLHIV with TB be screened for drug resistance with culture and DST. Programs without facilities or resources to screen all PLHIV for M/XDR-TB should put significant efforts into obtaining them, especially if M/XDR-TB rates are moderate or high. Some programs may adopt a strategy of targeted DST for patients at increased risk of M/XDR-TB, such as those in whom treatment has failed or who are contacts of M/XDR-TB cases. Programs may also choose to use targeted DST for those with lower CD4 counts (<200 cells/mm3), as these patients are at very high risk of death due to unrecognized M/XDR-TB. Genotypic tests have been endorsed by the WHO, facilitating prompt diagnosis of M/XDR-TB.47 The CBNAAT can establish diagnoses in smear-positive and even smear-negative pulmonary TB and extrapulmonary TB, decreasing the time a patient may be on an inadequate regimen and the period during which the patient may be spreading M/XDR-TB.14 Therefore, the CBNAAT, especially GeneXpert MTB/RIF, should be used as an initial diagnostic test in PLHIV and M/XDR-TB coinfection.50 First-line LiPAs can detect additional H monoresistance and second-line LiPAs resistance to FQs and SLIDs in pre-XDR-TB and XDR-TB cases.51 LiPAs have a better diagnostic yield in smear-positive cases and on culture isolates. It requires higher technical expertise and cautious interpretation of results in PLHIV compared to the CBNAAT. A diagnostic approach should initially include the CBNAAT followed by LiPAs to first- and second-line anti-TB drugs to facilitate early diagnosis with universal DST and initiation of appropriate treatment. Newer tests, such as GeneXpert Ultra and urine TB lipoarabinomannan, have shown promising results in HIV patients with profound mmunosuppression (CD4 count <100 cells/mm3), with better diagnostic yield.52,53 Targeted next-generation sequencing and whole-genome sequencing (WGS) have emerged as potential diagnostic modalities and can detect organisms responsible, strain relatedness, and number of mutations conferring resistance to major anti-TB drugs, such as R, H, Z, FQs, and SLIDs, with rapid turnover of 1–2 days. Resistance can be detected by WGS for even bedaquiline (Bdq) and delamanid (Dlm) lacking validated DST. However, there are various concerns causing hindrance, such as expenditure, availability at few centers, integration into the existing diagnostic algorithm, technical or operational skills, and requirement of expert guidance in clinical interpretation of sequencing data. A study from China reported that the Beijing strain was the most frequently isolated lineage for drug resistance among patients with TB with or without HIV coinfection.54 Strains from coinfected patients were scattered from those of the general community without any clusters, suggesting an inability to detect transmission among PLHIV, despite a high burden of disease.55 This could be attributed to the limited genomic database of ongoing transmission of TB among PLHIV. Next-generation sequencing or WGS can be used as an initial diagnostic test in PLHIV with DR-TB coinfection, and may be preferred to the CBNAAT after overcoming existing limitations. Extensive effort is required to generate genomic databases worldwide. The utility of genotypic tests, including WGS, in programmatic conditions in resource-limited settings needs to be determined in the near future, with the major concerns of cost, requirement of operational skills, and quality control in mind.
Concomitant Treatment of M/XDR-TB and HIV
Treatment of M/XDR-TB in PLHIV is similar to those without HIV. Treatment should be offered immediately with anti-TB and ART whenever this coinfection is diagnosed. The classification of anti-TB drugs recommended by WHO for designing regimens is given in Table 1. Classification of ART drugs is given in Table 2. The M/XDR-TB component should be treated with conventional regimens, with treatment duration of at least 18–20 months containing a combination of second-line anti-TB drugs that may also include newer drugs, such as Bdq and Dlm.1,51,56 Duration of treatment has to be decided on the basis of treatment response, and should be continued for 15–17 months after culture conversion. Various anti-TB regimens are described in Table 3. The regimen can be individualized or standardized, depending on drug-sensitivity patterns and availability of resources at a particular center. At least five to six drugs should be used, as each active drug in a regimen boosts the chance of cure by 65%.57 A conventional regimen containing at least five effective anti-TB drugs, including Z, and four core second-line anti-TB drugs — one chosen from group A, one from group B, and at least two from group C — is recommended during the intensive phase. If the regimen remains ineffective, drugs from groups D2 and D3 may be used.51 The regimen may be further strengthened with high-dose H and/or E. Individualized regimens should be preferred, although their implementation remains challenging in resource-limited settings requiring support of quality-assured laboratory facilities and expertise in interpretation of results. Standardized regimens can be prescribed empirically in cases of strong suspicion of drug resistance based on the most prevalent local drug-resistance pattern. Once DST results are available, the regimen can be subsequently modified. Proper care should be taken not to compromise the regimen. Drugs should be administered daily under supervision. The conventional regimens are lengthy, expensive, and more toxic, leading to poor adherence and making treatment challenging for M/XDR-TB and HIV coinfection. The WHO has recommended a standardized shorter and economical 9–12 months treatment regimen for MDR‑TB cases containing a combination of second-line anti-TB drugs to improve treatment outcomes of MDR‑TB.1,51,58 The currently recommended shorter MDR-TB regimen consists of an intensive phase of 4 months (extended up to 6 months in cases of nonconversion of sputum smear) with Km, gatifloxacin (Gfx) or Mfx, prothionamide (Pto)/ethionamide (Eto), clofazimine (Cfz), high-dose H, Z, and E followed by a continuation phase of 5 months with Gfx or Mfx, Cfz, E, and Z. These are indicated only if the treatment history does not contain exposure to second-line anti-TB drugs for >1 month and also no documented resistance to SLIDs and FQs is confirmed by second-line LiPA complemented by second-line phenotypic liquid-culture DST. Shorter regimens are not recommended for extrapulmonary M/XDR-TB (except lymph nodes and pleura) in PLHIV, as evidence regarding efficacy is still lacking. Other situations where shorter regimens are avoided include preference by the clinician and patient for longer regimens, confirmed resistance or suspected ineffectiveness or intolerance to drug(s), unavailability at the center, and such conditions as pregnancy, disseminated, meningeal, or central nervous system TB. Anti-TB drugs have been reclassified by the WHO based on emerging evidence regarding treatment outcome and profile of ADRs, especially with linezolid (Lzd), Cfz, Bdq, and Dlm.1 The conventional regimen contains all three drugs from group A and one from group B. If one of the drugs cannot be used from group A, then both drugs from group B should be considered. The number is reduced to four if these drugs can be used to design an effective regimen. Reduction in the number of drugs can improve adherence by reducing pill burden, particularly in M/XDR-TB and HIV coinfection. Exclusion of SLIDs, such as Km or Cm, and preference for complete oral drugs is recommended for designing a regimen to achieve better outcomes. High-dose H, Gfx, and clarithromycin (Clr) have been omitted from a recent classification, because of high frequency of H monoresistance, lack of evidence, poor efficacy, and high incidence of ADRs. Treatment outcome and monitoring of ADRs also have to be followed as per the recommendations by the WHO.1 Overall, it is recommended that shorter regimens be used as the first preference in eligible MDR-TB cases with or without HIV coinfection, otherwise all oral conventional regimens containing Bdq and Dlm should be offered. If second-line LiPA shows resistance to FQs including Mfx, then high-dose Mfx can still be used before switching to other drugs. Various trials on newer shorter regimens are ongoing in order to improve treatment outcomes. These trials are conducted by Research Excellence to Stop Tuberculosis Resistance, which is an initiative adopted under the End TB strategy by the WHO to promote and conduct research on therapy for rapid control of DR-TB.59 Few of upcoming shorter regimens can treat DS-TB as well in addition to DR-TB, facilitating approach of universal treatment in subsequent years. These regimens are currently in phase 2A/2B trials and require to undergo phase 3 trials for further validation. The approach of universal treatment might have potential advantages in PLHIV such as reduction in drug burden as well as incidence of ADRs and shorter culture conversion time leading to decreased risk of transmission of infection.
 |
Table 1 Grouping of antitubercular drugs for design of regimens for treatment of drug-resistant tuberculosis |
 |
Table 2 Grouping of antiretroviral drugs for design of regimens for treatment of HIV |
 |  |  |
Table 3 Regimens for treating DR-TB and HIV components in patients with established coinfection |
Life-long uninterrupted ART remains the cornerstone treatment for the HIV component. It contains at least three ART drugs from different classes.60 Various ART regimens are described in Table 3. The WHO has recommended first-line regimens comprising two nucleoside reverse-transcriptase inhibitors (NRTIs)/one NRTI and a nucleotide RTI (NtRTI), and a nonnucleoside RTI or integrase inhibitors. The currently approved regimen includes tenofovir (TDF), lamivudine (3TC), and efavirenz (Efv)/nevirapine (Nvp) or dolutegravir (Dtg), if available. Second-line regimens are indicated when there is resistance or intolerance to NRTIs. Failure of first-line ART followed by switching to second- or third-line ART is assessed by viral load testing, documentation of exposure history to drugs, enhanced adherence counseling, and also genotyping if available.60 Genotyping needs to be implemented at a programmatic level in view of early detection of probable resistance to ART drugs, including integrase inhibitors, even without previous exposure. Unnecessary switching to different regimens must be avoided. Currently recommended second-line regimens for adults include two NRTIs, such as zidovudine (Azt) with 3TC, and a boosted protease inhibitor (PI), with lopinavir/ritonavir (Lpv/r) or atazanavir/ritonavir (Atv/r) preferred. Boosted PI-based regimens are preferred in children. However, certain considerations should be kept in mind while managing these cases: ART plays a pivotal role, as mortality in M/XDR-TB in PLHIV without the use of ART is extremely high; ADRs are reported frequently in PLHIV, as multiple medications (nine or ten) are involved in M/XDR-TB with recognized high-toxicity risks and interactions, often combined with ART; some toxicities are common to both anti-TB treatment and ART, which may result in additive rates of ADRs; monitoring needs to be more intense for both response to therapy and ADRs; use of thiacetazone (T) is not routinely recommended for HIV-infected patients, as it has a propensity to cause skin rash and immunoreconstitution inflammatory syndrome (IRIS), which might complicate the therapy.
Initiating ART Treatment in Patients with M/XDR-TB
Concomitant use of ART with an appropriate anti-TB treatment improves survival for both DS-TB and DR-TB. However, the probability of overlapping or additive ADRs could compromise the treatment of HIV or M/XDR-TB if both are started simultaneously. However, undue delay in initiation of ART can result in increased risk of HIV-associated mortality, especially among patients with advanced disease. The exact timing for the introduction of ART in newly diagnosed HIV patients receiving DR-TB treatment remains undefined. ART should be initiated in all TB patients, including DR-TB, irrespective of CD4 count.61 Anti-TB therapy should be started first, followed by ART as soon as possible within the first 8 weeks of treatment. ART should be immediately introduced within the first 2 weeks of initiating TB treatment when there is advanced disease with CD4 count <50/cm3. This is also applicable for the pediatric population with HIV and DR-TB coinfection. Efv should be used as the preferred NRTI in patients starting ART while on anti-TB therapy, whereas Nvp should be avoided. Shorter regimens containing newer drugs should be preferred if there is no contraindication and an adequate supply of drugs.
M/XDR-TB in Patients Already Receiving ART
There are two issues to consider in patients who are diagnosed with M/XDR-TB while on ART. The first is whether modification of ART is needed due to drug–drug interactions or to decrease the potential of overlapping toxicities. The second issue is whether the presentation of active M/XDR-TB in a patient on ART constitutes ART failure and resistance.
Drug–Drug Interactions in the Treatment of HIV and M/XDR-TB
Drug–drug interactions between second-line anti-TB drugs and ART are not well known, as information is limited.62 R derivatives are the potent cytochrome (CYP450) inducers that lead to subtherapeutic concentrations of ART drugs. NRTIs, PIs, integrase strand–transferase inhibitors, and the CCR5 antagonist maraviroc are more prone to interactions with R than NRTIs, NtRTI, and fusion inhibitors.63 The last three groups are not metabolized by CYP450 enzymes. Rhas been reported to reduce levels of both Nvp and Efv on coadministration. The effect is stronger for Nvp than Efv. Nvp-based regimens are associated with virological failure and death more frequently than Efv-based regimens. R has significant pharmacokinetic interactions with PIs, which are all metabolized by CYP3A4 and have weak inhibitory activity. Of all PIs, ritonavir has a potent inhibitory effect on CYP3A4 and Pgp. It is frequently used with other PIs to pharmacologically boost their concentrations. Rifabutin, another rifamycin derivative, is a less potent inducer of the CYP450 system and can be preferred to R in tubercular patients treated with PIs containing ART regimens. Rifapentine is metabolized by CYP3A4 enzymes, and reduction is required when coadministered with PIs. Use of triple or quadruple NRTI/NtRTI regimens with R or non-rifamycin–based anti-TB regimens could be other options, but efficacy of these regimens remains questionable. R is not routinely used in M/XDR-TB treatment. Therefore, the probability of clinically significant drug interactions becomes minimal. They are prescribed only in the treatment of R-sensitive poly-DR-TB and mono-DR-TB. Relevant drug–drug interactions are described in Table 4. Buffered didanosine (ddl), which contains an aluminum magnesium–based antacid, may result in decreased FQ absorption when administered concomitantly. It should be given 6 h before or 2 h after FQ administration. Enteric-coated formulations can be preferred to buffer one, as they can be used concomitantly without this precaution. Eto/Pto is thought to be metabolized by the CYP450 system, although data are lacking regarding responsible CYP enzymes. The rationale to modify doses of Eto/Pto and certain ART drugs during concomitant treatment has not been clearly specified. Clr acts as a substrate and inhibitor of CYP3A, and has multiple drug interactions with PIs and NRTIs. Therefore, Clr should be avoided, because of both its poor efficacy against M/XDR-TB and multiple drug interactions. The newer drug Bdq is metabolized by CYP3A4, which is also inhibited or induced by various ART drugs, leading to significant drug–drug interactions.64 Efv induces CYP3A4, leading to decreased levels of Bdq, whereas Nvp and Lpv/r have modest effects. Therefore, it has been recommended that ART can be switched from generic fixed-dose combination Efv-containing regimens to twice-daily Nvp with separate companion pills. Dlm has not shown any clinically relevant interactions when coadministered with other anti-TB drugs or ART.65 Dtg-based ART regimens are expected to be safe when coadministered with those containing Bdq and Dlm.
 |
Table 4 Potential drug–drug interactions between antiretroviral and antitubercular drugs |
Potential Overlying and Additive Toxicity of ART and Anti-TB Therapy
HIV patients experience more frequent ADRs to both anti-TB and other non-TB medications for other opportunistic infections, and the risk of ADRs increases with the degree of immunosuppression.66–68 A recent systematic review and meta-analysis reported at least one ADR in 83% of patients, which is significantly higher than a previous one that reported at least one ADR in 57% of included patients.69,70 The higher incidence was because the former study focused on high-burden HIV settings. The risk factors responsible for higher incidence of ADRs were overlapping or additive toxicity from treatment, poor general condition, low CD4 count, and body-mass index. No statistically significant association has been reported between the occurrence of ADRs and recognized risk factors. Identifying one or more offending drugs responsible for ADRs in patients receiving concomitant therapy for DR-TB and HIV is very challenging. Many of the medications used to treat coinfection have overlapping or additive ADRs, as mentioned in Table 5. The typical strategy of stopping all medications and rechallenging them one by one is not possible in these patients, as the risk of emergence of resistance, especially for ART, is very high.71 It should be noted that information regarding the frequency of ADRs is relatively scarce. Most drugs have to be included in the regimens, outweighing the benefit over risk, despite awareness regarding the high probability of overlapping ADRs. If two drugs with overlapping toxicity are considered essential for therapy, intense monitoring of ADRs is to be considered, rather than disallowing a certain combination. Lzd, when used for M/XDR-TB with HIV-coinfected patients, requires intense monitoring, as there is potential for significant worsening of peripheral neuropathy associated primarily with either HIV infection or combination with stavudine (d4T) and also bone-marrow depression when coadministered with Azt. Active TB drug-safety monitoring and management should be an essential component of management of DR-TB and HIV-coinfected patients treated with anti-TB regimens containing newer or repurposed drugs.1
 |  |  |
Table 5 Common adverse reactions to antiretroviral and antitubercular drugs |
Monitoring of M/XDR-TB and HIV Therapy in Coinfected Patients
ART and anti-TB therapy must be taken daily without exception to prevent the evolution of drug resistance. The directly observed–treatment strategy is an important component of the therapy. National programs should adopt this strategy or alternative methods of adherence support to provide anti-TB medications and ART. This is particularly essential for patients with DR-TB being treated with second-line anti-TB drugs resulting in larger pill burden and increased incidence of ADRs when given concomitantly with ART. The efficacy of therapy should ideally be determined by estimation of levels of serum-drug concentrations for both ART and anti-TB therapy. Extensive clinical supervision and decentralized or patient-centric care with an aim to ensure high levels of adherence, consistent virological suppression, and immunological recovery is required.60 Integrated home-based treatment can also be an effective alternative strategy to improve adherence. Issues responsible for unfavorable outcomes include poor tolerance of regimen, larger pill burden, and social stigma related to both the diseases, leading to serious discrimination and high risk of mortality. Therefore, these patients may require additional socioeconomic, nutritional, and psychosocial support to improve treatment outcomes.
Collaborative TB/HIV Activities
The WHO has endorsed certain collaborative activities to reduce the joint burden of TB and HIV coinfection.56,72 These activities constitute the backbone of the TB/HIV collaborative adopted under the Stop TB strategy, which along with the implementation of effective directly observed treatment–strategy programs, will strengthen and increase the success of M/XDR-TB and HIV control and treatment activities. The management of TB in PLHIV relies on three components known as the “three Is”: intensified case-finding, isoniazid preventive therapy, and infection control. The Joint United Nations Programme on HIV/AIDS (UNAIDS) has also collaborated with the WHO to reduce the burden of TB and HIV coinfection.73 Three components have been added to the three Is, currently designated the six Is: initiating ART, integration of TB/HIV, and involvement of the community. The End TB strategy has been further introduced by the WHO with the objective of eliminating TB as a public health problem.74 UNAIDS has set a goal to end HIV as a public health threat by 2030.73 The principle of “hit hard, hit early” has been implemented to reduce the burden of HIV infection. The United Nations aims to end AIDS epidemics by 2020 with the adoption of the 90-90-90 strategy (90% of estimated PLHIV should know their status, 90% of which should be initiated on ART, 90% of which should demonstrate viral suppression).75 The following activities are based on TB/HIV activities and are adapted to be specifically applicable to M/XDR-TB.56,72
Voluntary HIV Testing and Counseling in All TB Suspects
Voluntary HIV testing and counseling is recommended for all TB suspects with clinical symptoms, especially in high-burden countries for TB and HIV coinfection. Diagnosed TB patients showing relapse or failure to treatment should also be screened for HIV coinfection. Testing for HIV should be performed simultaneously and preferably at the same center, along with sputa for smear microscopy or culture and also CBNAAT, if available. This will avoid referring patients elsewhere for HIV testing and counseling to prevent attrition or loss to follow-up.
Standard Protocols to Diagnose Pulmonary and Extrapulmonary TB
New protocols need to be devised, especially for enhancing the diagnosis and treatment of PLHIV with smear-negative pulmonary and extrapulmonary TB. Mycobacterial cultures (conventional or liquid) of sputum or other fluids and tissue apart from smear microscopy are recommended to help in the diagnosis of sputum smear–negative and extrapulmonary TB. Smear microscopy carries inadequate diagnostic accuracy in the majority of HIV-coinfected patients, particularly those with advanced immunosuppression. The CBNAAT should be considered up front in the protocol for establishing rapid diagnosis by detecting R resistance.56 Only Ethiopia and South Africa have been able to scale up GeneXpert MTB/RIF in primary health care.76 Nearly 500,200 PLHIV have been offered the CBNAAT for diagnosis in India.5 A study reported that GeneXpert MTB/RIF utilization was low due to lack of technical expertise, even though the majority of sites had access to the test.77 A concerning issue is that there is a high probability of amplification of drug resistance leading to suboptimal treatment, as current diagnostic policies rely primarily on the CBNAAT for rapid diagnosis, which detects only R resistance, so the possibility of the more frequent H monoresistance at baseline cannot be ruled out.39 Samples should also be analysed with first-line LiPAs if possible. Second-line LiPA are also advised, in order to rule out additional resistance at baseline if there is documented multidrug resistance on the CBNAAT and firstline LiPA. Findings of genotypic tests should be cautiously interpreted and complemented with gold-standard phenotypic tests. WGS can be used as an initial diagnostic test, especially in high burden countries with epidemics or outbreaks of DR-TB and HIV coinfection. Utilization of WGS with preference over GeneXpert in programmatic conditions needs to be determined in the near future, with major concerns of expenditure and requirement of operational skills, as well as quality control. A consolidated algorithm to diagnose DR-TB in PLHIV is given in Figure 1. Neither phenotypic nor genotypic tests are standardized in many laboratory settings for Z, S, Mfx, Cfz, Lzd, Bdq, and Dlm. National TB-control programs must scale up laboratory resources, training of health-care workers, quality-assured laboratory DST reporting for both first- and second-line anti-TB drugs, and DST-tailored individualized treatment. Prompt initiation of appropriate anti-TB regimens tailored to DST results followed by initiation of ART can reduce mortality and improve treatment outcomes. Although performing DST for all DS-TB and DR-TB remains the standard of care, it is infeasible in many resource-limited settings. Programs ideally should estimate the burden of M/XDR-TB and HIV epidemics.
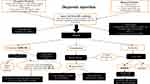 |
Figure 1 Algorithm for diagnosis of drug-resistant tuberculosis in people living with HIV. |
Prompt Introduction of ART in M/XDR-TB/HIV Patients as Soon as Anti-TB Drugs Are Tolerated
Guidelines recommend the prompt initiation of ART in HIV-infected patients with M/XDR-TB. Other opportunistic infections apart from TB, if present, should also be aggressively managed.
Empirical Therapy with Second-Line Anti-TB Drugs
Patients with a very high risk of M/XDR-TB or residing in high-burden settings can be empirically started on second-line regimens based on locally prevalent drug-resistance patterns. This strategy can be applied to all patients with or without HIV. Empirical therapy should be avoided in scenarios with documented resistance to SLIDs and FQs, and individualized regimens tailored to DST patterns should be preferred.
Cotrimoxazole Prevention Therapy
Cotrimoxazole prevention therapy should be provided to all patients with HIV infection. No significant interaction has been observed with any second-line anti-TB agents. Strict monitoring of ADRs is indispensable, as there are overlapping toxicities among ART, anti-TB, therapy, and cotrimoxazole prevention therapy.
Treatment Supervision by a Specialized Team
Team members should be well trained in providing treatment to patients with M/XDR-TB and HIV coinfection. Strict monitoring of treatment response, occurrence of potential additive ADRs, and nutritional status should be assessed periodically.
Implementation of Nutritional and Socioeconomic Support
This component deserves special attention. M/XDR-TB and HIV–coinfected patients encounter multiple issues. They usually have low socioeconomic status and associated wasting, diarrheal diseases, and malabsorption syndromes, leading to decreased efficacy of anti-TB drugs. Additive ADRs affect treatment adherence, leading to more frequent visits. Therefore, patients should be offered additional socioeconomic and nutritional support.
Effective Infection Control
Infection-control measures should be implemented to reduce the risk of Mycobacterium tuberculosis transmission in HIV-endemic settings. Infection-control measures recommended by the WHO should be ensured.
Promotion of Managerial Activities
National, state, or local coordinating bodies, community groups, and key stakeholders should have an integrated approach in the planning and monitoring of M/XDR-TB and HIV activities and programs. Managerial activities should be promoted on a larger scale. The burden of TB coinfection with HIV poses a great challenge in the elimination of both components by 2035 and 2030, respectively. DR-TB patients should be targeted separately, due to emergence of primary resistance, apart from management of DS-TB to break the chain of transmission. Various measures, such as rapid detection with genotypic tests, universal DST to prescribe appropriate individualized regimens, innovation of shorter or completely oral conventional regimens fortified with newer drugs to improve adherence, decentralized or patient-centric approaches, infection control, and preventive treatment for DR-TB contacts, have been emphasized to drastically reduce the burden of DR-TB.78 Engagement of the private sector for notification and management of DR-TB cases is also essential to reduce the burden of coinfection. Ideally, routine diagnosis should be scaled up at the programmatic level for the patient’s benefit. These measures are difficult to implement in resource-limited settings, where allocating sufficient resources for funding remains a challenge. Better community or local surveillance data and promotion of genotypic analysis in the near future may help in developing an understanding of the relationship between HIV and M/XDR-TB.
Treatment Outcomes for M/XDR-TB and HIV Coinfection
ART for HIV appears to benefit coinfected M/XDR-TB patients, and various studies have shown favorable outcomes.32,79–90 Treatment outcomes have been reported from different regions worldwide, with marked interregional variation. A systematic review and meta-analysis revealed a pooled treatment-success rate of 56.9% (95% CI 46.2%–67.6%): 49.9% (95% CI 38.5%–61.2%) among adults and 83.4% (95% CI 74.7%–92%) among children.89 That rate is almost similar to that reported among MDR-TB patients in general, regardless of HIV status. Mortality was 38% in adults (95% CI 28%–48.1%), and 11.4% (95% CI 5.8%–17.1%) in children.89 Mortality was higher for XDR-TB (82%) than MDR-TB (67%) with HIV coinfection. Another study from sub-Saharan African countries reported pooled cure and mortality rates of 34.9% and 18.1%, respectively.32 MDR-TB and HIV–coinfected patients had more unfavorable outcomes than HIV-negative MDR-TB patients (RR 0.87, 95% CI 0.97–0.96). However, the pooled cure rate from the European region is even lower (16%).90 Factors responsible for the variable results are difference in populations affected, sample size, extent of disease burden, and quality of treatment services offered. Shorter regimens might show promising results, particularly in MDR-TB patients with HIV coinfection, but current evidence is sparse in programmatic settings.58,91 A study from Africa reported a high level of success among HIV-positive patients with shorter regimens.91 Resistance to FQs, whether at baseline or acquired, was responsible for unfavorable outcomes. An important concern remains: whether an MDR-TB regimen will work in all settings, particularly outside trial conditions. Further research is required to assess its efficacy. The factors responsible for unfavorable outcomes were atypical clinical manifestations of TB and lack of rapid and sensitive genotypic tests, leading to delayed management and poor adherence to treatment. Adequate adherence to treatment remains challenging, because of the long and difficult treatment associated with M/XDR-TB and HIV coinfection; additional comorbid illnesses, such as diabetes mellitus, anemia, renal or hepatic failure, and other opportunistic infections; a higher pill burden resulting from the coadministration of anti-TB and ART drugs; and the potential for overlapping adverse events and drug–drug interactions. Other factors showing poor outcomes were advanced age, profound immunosuppression (CD4 count <100 cells/cm3), unemployment, male sex, smoking, pregnancy, drug abuse, coinfection with hepatitis B or C, and a higher degree of drug resistance, including to second-line drugs.92,93 High-burden countries do not have adequate infrastructure to detect or manage an outbreak. Outbreaks should be prevented to reduce the impact on mortality.
Tuberculosis Immunoreconstitution Inflammatory Syndrome
TB-IRIS is an unusually exaggerated immunoresponse against viable or dead M. tuberculosis antigens, and occurs frequently in HIV-infected patients compared with uninfected ones. It has emerged as an important complication of ART. It is relatively common, reported in up to a third of patients, in mild–moderate forms in patients with TB started on ART.94–96 However, it is relatively rare in its severe forms, and observed particularly when the site of involvement is the central nervous system. IRIS has been observed for other opportunistic infections or HIV-associated illnesses apart from TB. A retrospective meta-analysis of 54 cohorts showed that the incidence of TB-IRIS in PLHIV on ART treatment was 15.7% (95% CI 9.7%–24.5%), with mortality of 3.2% (95% CI 0.7%–9.2%).97 Two forms of IRIS exist: paradoxical and unmasking. The paradoxical form is either appearance of new lesions or deterioration/recurrence of preexisting pulmonary or extrapulmonary tubercular lesions in patients undergoing anti-TB treatment. It may occur during or even after completion of anti-TB therapy. It generally presents within 3 months of the initiation of ART, and is more common with CD4 count <50 cells/cm3.94,95 This syndrome can present as a paradoxical worsening of the patient’s clinical status, often due to a previously subclinical and unrecognized opportunistic infection. Clinical features include hastening of symptoms, deteriorating pulmonary lesions, or exacerbation of inflammation, such as enlarging lymphadenopathy or pleural effusion, at the sites of involvement. The unmasking form is an inflammatory manifestation of an apparently asymptomatic subclinical infection occurring after initiation of ART, resulting in clinical worsening. Patients with advanced disease may show clinical deterioration caused by other reasons. It is important to note that IRIS is a diagnosis of exclusion. Clinical suspicion of IRIS is high, provided there should be initial improvement of symptoms after adequate therapy, followed by paradoxical worsening and other possible factors responsible for reducing efficacy, such as poor compliance and drug malabsorption or drug toxicity, are ruled out. Other causes mimicking TB-IRIS include superimposed infections, treatment failure, and relapse and progression of disease due to drug resistance. Strict criteria should be defined to diagnose this entity. Management of IRIS is complicated. Prognosis depends on the existing clinical profile, extent of disease, and site of involvement. Various treatment modalities have been used depending on the severity. These include observation with continuation of ART and anti-TB therapy. Nonsteroidal anti-inflammatory drugs can be used in mild disease and corticosteroids in moderate–severe disease. Interruption of ART is usually required in a few cases.
Chemoprophylaxis of Drug-Resistant Tuberculosis
PLHIV are vulnerable to infection with TB bacillus, including DR ones. The annual risk of developing TB infection in the HIV-infected population is ten times that of the uninfected.98,99 Screening is required for ruling out not only active infection but also latent infection. Chemoprophylaxis is indicated if the latent infection is detected by immunologic testing, such as tuberculin skin test or IFNγ-release assay. Appropriate chemoprophylaxis has been reported to reduce the risk of TB infection by 35%, with larger benefit in the skin test–positive population (risk reduction of 52%), with the risk of mortality reduced by 35%.100–102 It has also been observed to reduce the risk of HIV disease progression by 31%. Therefore, chemoprophylaxis is an important preventive therapy in DS-TB and HIV coinfection. Limited evidence is available regarding the role of chemoprophylaxis in preventing active disease among people with or without HIV infection havin contacts with M/XDR-TB patients.103,104 People in whom latent infection is diagnosed are often infected with the same strain as the index case. Prompt treatment of the index case with DR-TB should be the priority. If contact is asymptomatic, no prophylactic treatment is recommended, and the patient should be placed under clinical follow-up. The follow-up duration for close contacts of M/XDR-TB patients is not defined, but should be at least 2 years.105 Children <3 years of age suspected of contact with patients with M/XDR-TB can be given chemoprophylaxis to prevent progression of disease. If the contact is symptomatic, presumptive TB infection should be suspected and investigated further with the CBNAAT or appropriate investigation. Prompt initiation of treatment with a regimen designed to treat the index case is recommended in cases of development of active disease till DST results are collected. There is a lack of consensus on designing standardized chemoprophylaxis regimens related to the selection of second-line anti-TB drugs, dosages, and duration of therapy. The proposed recommendation is to use two to three oral drugs (FQs, E, Z, high-dose H, Eto, and Cs) for 6–12 months, based on the susceptibility pattern of the index case of DR-TB. The reduction of risk of developing infection has been reported to be 37%–90%.100,101,104 These regimens are also associated with ADRs. FQs are included in all regimens with fewest ADRs. However, preventive therapy containing FQs can be ineffective in particular settings where probability of baseline resistance to FQs remains high.39 Various clinical trials are ongoing for effective preventive therapy for DR-TB with FQs' (TB CHAMP and VQUIN trials) and even with newer drugs like Dlm (PHOENIXMDR-TB trial).78 National programs should emphasize chemoprophylaxis in preventing latent infection among HIV-endemic areas where the probability of DR-TB coinfection remains high. Further research is required to prove the efficacy of chemoprophylaxis in the prevention of infection in PLHIV with contacts with M/XDR-TB.
Infection Control
Infection control is one of the essential components of management of patients with TB with or without HIV coinfection. It restricts the transmission of infection from patients to high-risk groups, especially health-care workers. There is an increased risk of transmission of infection, even for PLHIV harboring DR strains, although evidence is sparse. Such factors as delayed diagnosis of M/XDR-TB causing prolonged duration of infectiousness leading to increased exposure, overcrowding of wards, and merging of patients with TB and HIV are responsible for nosocomial transmission. These practices enhance infection epidemics or outbreaks, particularly in HIV-endemic settings. Implementation of adequate infection-control measures at health centers or hospitals significantly reduces nosocomial transmission.106 Interventions for infection control should be adopted at three levels: administrative, environmental/engineering, and personal respiratory protection. Administrative controls include patient screening, cough hygiene, segregation and fast-tracking of symptomatics, and education of patients and their relatives. Environmental controls include indoor patient segregation, adequate bed spacing, and ensuring effective ventilation, either natural or mechanical, including negative-pressure rooms, with a focus on high-risk areas. Ultraviolet germicidal irradiation devices could be an alternative option for ventilation, which are effective but complex and costly. Personal respiratory protection including N95 particulate respirators, is indicated when the risk to health-care workers is especially high. The feasibility of these interventions is yet to be determined by robust randomized controlled trials, so the existing recommendations are based on consensus. Community-based treatment programs should implement these measures on a larger scale. Home-based measures, such as separate living quarters, personal respiratory protection for visitors, and adequate ventilation, have also been devised.107
Conclusion
DR-TB in HIV-infected patients is highly lethal and a growing problem in many parts of the world. Rapid diagnosis with genotypic tests, prompt treatment with appropriate regimens, including shorter regimens fortified with newer drugs, intense monitoring, management of ADRs, sound patient support, and strong infection-control measures are all essential components in the management of DR-TB in PLHIV. TB- and HIV-control programs should curb the epidemic of HIV-associated M/XDR-TB in the near future.
Disclosure
The authors report no conflicts of interest in this work.
References
1. World Health Organization. WHO consolidated guidelines on drug-resistant tuberculosis treatment. Geneva: WHO; 2019 (WHO/CDS/TB/2019.7); Available from: https://apps.who.int>iris>bitstream>handle>9789241550529-eng.
2. Dye C, Bassili A, Bierrenbach AL, et al. Measuring tuberculosis burden, trends and the impact of control programmes. Lancet Infect Dis. 2008;8(4):233–243. doi:10.1016/S1473-3099(07)70291-8
3. Wells CD, Cegielski JP, Nelson LJ, et al. HIV infection and multi-drug resistant tuberculosis: the perfect storm. J Infect Dis. 2007;196(Suppl 1):S86–S107. doi:10.1086/518665
4. Dubrovina I, Miskinis K, Gilks C, et al. Drug-resistant tuberculosis and HIV in Ukraine: a threatening convergence of two epidemics. Int J Tuberc Lung Dis. 2008;12(7):756–762.
5. World Health Organization. Global tuberculosis report 2019. Geneva: WHO; 2019 (WHO/CDS/TB/2019.15). Available from: https://www.who.int/tb/publications/global_report/en/.
6. Suchindran S, Brouwer ES, Van Rie A. Is HIV infection a risk factor for multi-drug-resistant tuberculosis? A systematic review. PLoS One. 2009;4(5):e5561. doi:10.1371/journal.pone.0005561
7. World Health Organization. Anti-tuberculosis drug resistance in the world. Fourth global report. WHO/IUATLD global project on anti-tuberculosis drug resistance surveillance, 2002–2007. Geneva: WHO; 2008 (WHO/HTM/TB/2008.394). Available from: https://www.who.int/tb/publications/tb-drugresistance-fourthreport/en/.
8. Dean AS, Zignol M, Falzon D, Getahun H, Floyd K. HIV and multidrug-resistant tuberculosis: overlapping epidemics. ERJ. 2014;44(1):251–254. doi:10.1183/09031936.00205413
9. Mesfin YM, Hailemariam D, Biadglign S, Kibret KT. Association between HIV/AIDS and multi-drug resistance tuberculosis: a systematic review and meta-analysis. PLoS One. 2014;9(1):e82235. doi:10.1371/journal.pone.0082235
10. Akanbi MO, Achenbach C, Taiwo B, et al. Evaluation of Gene Xpert for routine diagnosis of HIV-associated tuberculosis in Nigeria: a prospective cohort study. BMC Pulm Med. 2017;17:87. doi:10.1186/s12890-017-0430-6
11. Magee MJ, Blumberg HM, Broz D, et al. Prevalence of drug resistant tuberculosis among patients at high-risk for HIV attending outpatient clinics in Delhi India. Southeast Asian J Trop Med Public Health. 2012;43(2):354–363.
12. Dagnra AY, Mlaga KD, Adjoh K, Kadanga E, Disse K, Adekambi T. Prevalence of multidrug-resistant tuberculosis cases among HIV-positive and HIV-negative patients eligible for retreatment regimen in Togo using GeneXpert MTB/RIF. New Microbe New Infect. 2015;8:24–27. doi:10.1016/j.nmni.2015.09.001
13. Raizada N, Sachdeva KS, Sreenivas A, et al. Catching the missing million: experiences in enhancing TB & DR-TB detection by providing upfront Xpert MTB/RIF testing for people living with HIV in India. PLoS One. 2015;10(2):e0116721. doi:10.1371/journal.pone.0116721
14. Lawn SD, Brooks SV, Kranzer K, et al. Screening for HIV-associated tuberculosis and rifampicin resistance before antiretroviral therapy using the xpert MTB/RIF assay: a prospective study. PLoS Med. 2011;8(7):e1001067. doi:10.1371/journal.pmed.1001067
15. Saldanha N, Runwal K, Ghanekar C, Gaikwad S, Sane S, Pujari S. High prevalence of multi drug resistant tuberculosis in people living with HIV in Western Indias. BMC Infect Dis. 2019;19:391.
16. Chum HJ, O’Brien RJ, Chonde TM, Graf P, Rieder HL. An epidemiological study of tuberculosis and HIV infection in Tanzania, 1991–1993. AIDS. 1996;10(3):299–309. doi:10.1097/00002030-199603000-00009
17. Kenyon TA, Mwasekaga MJ, Huebner R, Rumisha D, Binkin N. Low levels of drug resistance amidst rapidly increasing tuberculosis and human immunodeficiency virus co-epidemics in Botswana. Int J Tuberc Lung Dis. 1999;3(1):4–11.
18. Churchyard GJ, Corbett EL, Klinschmidt I, Mulder D, De Cock KM. Drug-resistant tuberculosis in South African gold miners: incidence and associated factors. Int J Tuberc Lung Dis. 2000;4(5):433–440.
19. Mac-Arthur A, Gloyd S, Perdiagao P, et al. Characteristics of drug resistance and HIV among tuberculosis patients in Mozambique. Int J Tuberc Lung Dis. 2001;5(10):894–902.
20. Campos PE, Suarez PG, Sanchez J, et al. Multi-drug resistant Mycobacterium tuberculosis in HIV-infected persons, Peru. Emerg Infect Dis. 2003;9(12):1571–1578. doi:10.3201/eid0912.020731
21. Espinal MA, Laserson K, Camacho M, et al. Determinant of drug-resistant tuberculosis: analysis of 11 countries. Int J Tuberc Lung Dis. 2001;5(10):887–893.
22. Quy HT, Buu TN, Cobelens FGJ, et al. Drug resistance among smear-positive tuberculosis patients in Ho Chi Minh City, Vietnam. Int J Tuberc Lung Dis. 2006;10(2):160–166.
23. Masjedi MR, Farnia P, Sorooch S, et al. Extensively drug-resistant tuberculosis: 2 years of surveillance in Iran. Clin Infect Dis. 2006;43(7):841–847. doi:10.1086/cid.2006.43.issue-7
24. Edlin BR, Tokars JI, Grieco MH, et al. An outbreak of multidrug-resistant tuberculosis among hospitalized patients with the acquired immunodeficiency syndrome. N Engl J Med. 1992;326(23):1514–1521. doi:10.1056/NEJM199206043262302
25. Moro ML, Gori A, Errante I, et al. An outbreak of multidrug resistant tuberculosis involving HIV infected patients of two hospitals in Milan, Italy. AIDS. 1998;12(9):1095–1102. doi:10.1097/00002030-199809000-00018
26. Gandhi NR, Moll A, Sturm AW, et al. Extensively drug-resistant tuberculosis as a cause of death in patients co-infected with tuberculosis and HIV in a rural area of South Africa. Lancet. 2006;368(9547):1575–1580. doi:10.1016/S0140-6736(06)69573-1
27. Shah NS, Wright A, Bai GH, et al. Worldwide emergence of extensively drug-resistant tuberculosis. Emerg Infect Dis. 2007;13(3):380–387. doi:10.3201/eid1303.061400
28. Gandhi NR, Shah NS, Andrews JR, et al. HIV co-infection in multidrug-and extensively drug-resistant tuberculosis results in high early mortality. Am J Respir Crit Care Med. 2010;181(1):80–86. doi:10.1164/rccm.200907-0989OC
29. Horsburgh CR
30. Nunes EA, De Capitani EM, Coelho E, et al. Patterns of anti-tuberculosis drug resistance among HIV-infected patients in Maputo, Mozambique, 2002–2003. Int J Tuberc Lung Dis. 2005;9(5):494–500.
31. Nelson LJ, Talbot EA, Mwasekaga MJ, et al. Anti-tuberculosis drug resistance and anonymous HIV surveillance in tuberculosis patients in Botswana, 2002. Lancet. 2005;366(9484):488–490. doi:10.1016/S0140-6736(05)67062-6
32. Chem ED, Van Hout MC, Hope V. Treatment outcomes and anti-retroviral uptake in multidrug-resistant tuberculosis and HIV co-infected patients in Sub-Saharan Africa: a systematic review and meta-analysis. BMC Infect Dis. 2019;19(723):1–8. doi:10.1186/s12879-019-4317-4
33. Frieden TR, Sterling T, Pablos-Mendez A, Kilburn JO, Cauthen GM, Dooley SW. The emergence of drug-resistant tuberculosis in New York City. N Engl J Med. 1993;328(8):521–526. doi:10.1056/NEJM199302253280801
34. Coronado VG, Beck-Sague CM, Hutton MD, et al. Transmission of multidrug-resistant Mycobacterium tuberculosis among persons with human immunodeficiency virus infection in an urban hospital: epidemiologic and restriction fragment length polymorphism analysis. J Infect Dis. 1993;168(4):1052–1055. doi:10.1093/infdis/168.4.1052
35. Busillo C, Lessnau K-D, Sanjana V. Multidrug-resistant Mycobacterium tuberculosis in patients with human immunodeficiency virus infection. Chest. 1992;102(3):797–801. doi:10.1378/chest.102.3.797
36. Shafer RW, Small PM, Larkin C, et al. Temporal trends and transmission patterns during the emergence of multidrug-resistant tuberculosis in New York City: a molecular epidemiologic assessment. J Infect Dis. 1995;177(1):171–176.
37. Eldholm V, Rieux A, Monteserin J, et al. Impact of HIV co-infection on the evolution and transmission of multidrug-resistant tuberculosis. Elife. 2016;5:e16644. doi:10.7554/eLife.16644
38. Rakesh PS, Balakrishnan S, Jayasankar S, Asokan RV. TB management by private practitioners - Is it bad everywhere? Indian J Tuberc. 2016;63(4):251–254. doi:10.1016/j.ijtb.2016.09.009
39. Isaakidis P, Das M, Kumar AMV, et al. Alarming levels of drug-resistant tuberculosis in HIV-infected patients in Metropolitan Mumbai, India. PLoS One. 2014;9(10):e110461. doi:10.1371/journal.pone.0110461
40. Dheda K, Gumbo T, Maartens G, et al. The epidemiology, pathogenesis, transmission, diagnosis, and management of multidrug-resistant, extensively drug-resistant, and incurable tuberculosis. Lancet Respir Med. 2017;5(4):291–360. doi:10.1016/S2213-2600(17)30079-6
41. Shah NS, Auld SC, Brust JC, et al. Transmission of extensively drug resistant tuberculosis in South Africa. N Engl J Med. 2017;376(3):243–253. doi:10.1056/NEJMoa1604544
42. Cohen KA, Abeel T, Manson McGuire A, et al. Evolution of extensively drug-resistant tuberculosis over four decades: whole genome sequencing and dating analysis of mycobacterium tuberculosis isolates from KwaZulu-Natal. PLoS Med. 2015;12(9):e1001880. doi:10.1371/journal.pmed.1001880
43. Kimerling ME, Slavuckij A, Chavers S, et al. The risk of MDR-TB and poly-resistant tuberculosis among the civilian population of Tomsk city, Siberia, 1999. Int J Tuberc Lung Dis. 2003;7(9):866–872.
44. Zhao M, Li X, Xu P, et al. Transmission of MDR and XDR tuberculosis in Shanghai, China. PLoS One. 2009;4(2):e4370. doi:10.1371/journal.pone.0004370
45. Cain KP, McCarthy KD, Heilig CM, et al. An algorithm for tuberculosis screening and diagnosis in people with HIV. N Engl J Med. 2010;362:707–716. doi:10.1056/NEJMoa0907488
46. Kwan CK, Ernst JD. HIV and tuberculosis: a deadly human syndemic. Clin Microbiol Rev. 2011;24(2):351–376. doi:10.1128/CMR.00042-10
47. Parsons LM, Somoskövi A, Gutierrez C, et al. Laboratory diagnosis of tuberculosis in resource-poor countries: challenges and opportunities. Clin Microbiol Rev. 2011;24(2):314–350. doi:10.1128/CMR.00059-10
48. WHO. Improving the diagnosis and treatment of smear-negative pulmonary and extra-pulmonary tuberculosis among adults and adolescents: recommendations for HIV-prevalent and resource-constrained settings, (WHO/HTM/TB/2007.379; WHO/HIV/2007.01). Geneva: World Health Organization; 2007. Available from: https://www.who.int/hiv/pub/tb/pulmonary/en/.
49. WHO. Guidance on provider-initiated HIV testing and counselling in health facilities. Geneva: World Health Organization, 2007. Available from: https://apps.who.int/iris/handle/10665/43688.
50. WHO. Automated real-time nucleic acid amplification technology for rapid and simultaneous detection of tuberculosis and rifampicin resistance: xpert MTB/RIF assay for the diagnosis of pulmonary and extra-pulmonary TB in adults and children. Geneva: WHO; 2013. Available from: https://apps.who.int/iris/handle/10665/112472.
51. World Health Organization. WHO treatment guidelines for drug-resistant tuberculosis;
52. Dorman SE, Schumacher SG, Alland D, et al. Xpert MTB/RIF Ultra for detection of Mycobacterium tuberculosis and rifampicin resistance: a prospective multi-centre diagnostic accuracy study. Lancet Infect Dis. 2018;18(1):76–84. doi:10.1016/S1473-3099(17)30691-6
53. Lawn SD, Kerkhoff AD, Burton R, et al. Diagnostic accuracy, incremental yield and prognostic value of determine TB-LAM for routine diagnostic testing for tuberculosis in HIV-infected patients requiring acute hospital admission in South Africa: a prospective cohort. BMC Med. 2017;15(1):67. doi:10.1186/s12916-017-0822-8
54. Mai TQ, Van Anh NT, Hien NT, et al. Drug resistance and Mycobacterium tuberculosis strain diversity in TB/HIV co-infected patients in Ho Chi Minh city, vietnam. J Glob Antimicrob Resist. 2017;10:154–160. doi:10.1016/j.jgar.2017.07.003
55. Mai TQ, Martinez E, Menon R, et al. Mycobacterium tuberculosis drug resistance and transmission among human immunodeficiency virus-infected patients in Ho Chi Minh City, vietnam. Am J Trop Med Hyg. 2018;99(6):1397–1406. doi:10.4269/ajtmh.18-0185
56. World Health Organization. Companion handbook to the WHO guidelines for the programmatic management of drug-resistant tuberculosis. Geneva: WHO, 2014. (WHO/HTM/TB/2014.11). Available from: https://www.who.int/tb/publications/pmdt_companionhandbook/en/.
57. Yuen CM, Kurbatova E, Tupasi T, et al. Association between regimen composition and treatment response in patients with multidrug-resistant tuberculosis: a prospective cohort study. PLoS Med. 2015;12(12):e1001932. doi:10.1371/journal.pmed.1001932
58. Khan FA, Hamid Salim MA, Du Cros P, et al. Effectiveness and safety of standardised shorter regimens for multidrug-resistant tuberculosis: individual patient data and aggregate data meta-analyses. Eur Respir J. 2017;50:1700061. doi:10.1183/13993003.00061-2017
59. Research Excellence to Stop TB Resistance. Drug-resistant tuberculosis: clinical trials progress report. RESIST TB; 2018. Available from: http://resisttb.org/docs/RESIST-TB-CTPR_6_6_2018.pdf.
60. WHO. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a Public Health approach—second edition. Geneva: World Health Organization, June, 2016. Available from: https://apps.who.int/iris/handle/10665/208825.
61. Blanc FX, Sok T, Laureillard D, et al. CAMELIA (ANRS 1295–CIPRA KH001) Study Team. Earlier versus later start of antiretroviral therapy in HIV-infected adults with tuberculosis. N Engl J Med. 2011;365(16):1471–1481. doi:10.1056/NEJMoa1013911
62. Dooley KE, Flexner C, Andrade AS. Drug interactions involving combination antiretroviral therapy and other anti-infective agents: repercussions for resource-limited countries. JID. 2008;198(7):948–961. doi:10.1086/591459
63. Semvua HH, Kibiki GS, Kisanga ER, et al. Pharmacological interactions between rifampicin and antiretroviral drugs: challenges and research priorities for resource-limited settings. Ther Drug Monit. 2015;37:22–32. doi:10.1097/FTD.0000000000000108
64. O’Donnell MR, Padayatchi N, Daftary A, et al. Antiretroviral switching and bedaquiline treatment of drug-resistant tuberculosis HIV co-infection. Lancet HIV. 2019;6(3):e201–e204. doi:10.1016/S2352-3018(19)30035-9
65. Mallikaarjun S, Wells C, Petersen C, et al. Delamanid co-administered with antiretroviral drugs or anti-tuberculosis drugs shows no clinically relevant drug-drug interactions in healthy subjects. Antimicrob Agents Chemother. 2016;60(10):5976–5985. doi:10.1128/AAC.00509-16
66. Hoffmann CJ, Christopher J, Charalambous S, et al. Hepatotoxicity in an African antiretroviral therapy cohort: the effect of tuberculosis and hepatitis B. AIDS. 2007;21(10):1301–1308. doi:10.1097/QAD.0b013e32814e6b08
67. Dean GL, Edwards SG, Ives NJ, et al. Treatment of tuberculosis in HIV-infected persons in the era of highly active antiretroviral therapy. AIDS. 2002;16(1):75–83. doi:10.1097/00002030-200201040-00010
68. McIlleron H, Meintjes G, Burman WJ, Maartens G. Complications of antiretroviral therapy in patients with tuberculosis: drug interactions, toxicity and immune reconstitution inflammatory syndrome. J Infec Dis. 2007;196(Suppl 1):S63–S75. doi:10.1086/518655
69. Schnippel K, Firnhaber C, Berhanu R, et al. Adverse drug reactions during drug-resistant TB treatment in high HIV prevalence settings: a systematic review and meta-analysis. J Antimicrob Chemother. 2017;72:1871–1879. doi:10.1093/jac/dkx107
70. Wu S, Zhang Y, Sun F, et al. Adverse events associated with the treatment of multidrug-resistant tuberculosis: a systematic review and meta-analysis. Am J Ther. 2016;23:e521–30. doi:10.1097/01.mjt.0000433951.09030.5a
71. Dia-Jeanette T. Mycobacterial Disease in HIV positive patients. J Pharm Practice. 2006;19(1):10–16. doi:10.1177/0897190005284100
72. WHO/PEPFAR/UNAIDS/The Global Fund. A guide to monitoring and evaluation for collaborative TB/HIV activities (2015 Revision). Geneva: WHO; 2015. (WHO/HTM/TB/2015.02). https://www.who.int>hiv>pub>tb>hiv_tb_monitoring_guide.
73. UNAIDS. UNAIDS warns that countries will miss the 2020 target of reducing HIV-associated TB deaths by 75% unless urgent action is taken. March 24, 2017.
74. Uplekar M, Weil D, Lonnroth K, et al. WHO’s new end TB strategy. Lancet. 2015;385(9979):1799–1801. doi:10.1016/S0140-6736(15)60570-0
75. UNAIDS. 90–90–90: an ambitious treatment target to help end the AIDS epidemic. JC; 2014: 2684. https://www.unaids.org/en/resources/documents/2017/90-90-90.
76. Cox H, Dickson-Hall L, Ndjeka N, et al. Delays and loss to follow-up before treatment of drug-resistant tuberculosis following implementation of Xpert MTB/RIF in South Africa: a retrospective cohort study. PLoS Med. 2017;14(2):e1002238. doi:10.1371/journal.pmed.1002238
77. Clouse K, Blevins M, Lindegren ML, et al. Low implementation of Xpert MTB/RIF among HIV/TB co-infected adults in the International epidemiologic Databases to Evaluate AIDS (IeDEA) program. PLoS One. 2017;12(2):e0171384. doi:10.1371/journal.pone.0171384
78. Kendall EA, Sahu S, Pai M, et al. What will it take to eliminate drug-resistant tuberculosis? Int J Tuberc Lung Dis. 2019;23(5):535–546. doi:10.5588/ijtld.18.0217
79. Jones JL, Hanson DL, Dworkin MS, DeCock KM. HIV-associated tuberculosis in the era of highly active antiretroviral therapy. The Adult/Adolescent spectrum of HIV disease group. Int J Tuberc Lung Dis. 2000;4(11):1026–1031.
80. Waisman JL, Palmero DJ, Albert FA, Güemes JLG, Francos JL, Negroni R. [Mejor pronóstico en pacientes con tuberculosis multirresistente relacionados con el VIH/SIDA tratados con terapia antirretroviral altamente activa]. Medicina (B Aires). 2001;61(6):810–814.
81. Trickey A, May MT, Vehreschild J, et al. Antiretroviral Therapy Cohort Collaboration (ART-CC). Cause-specific mortality in HIV-positive patients who survived ten years after starting antiretroviral therapy. PLoS One. 2016;11(8):e0160460. doi:10.1371/journal.pone.0160460
82. Brust JCM, Shah NS, Mlisana K, et al. Improved survival and cure rates with concurrent treatment for MDR-TB/HIV co-infection in South Africa. Clin Infect Dis. 2018;66(8):1246–1253. doi:10.1093/cid/cix1125
83. Shin SS, Modongo C, Boyd R, et al. High treatment success rates among HIV infected multidrug-resistant tuberculosis patients after expansion of antiretroviral therapy in Botswana, 2006–2013. J Acquir Immune Defic Syndr. 2017;74(1):65–71. doi:10.1097/QAI.0000000000001169
84. Ahuja SD, Ashkin D, Avendano M, et al. Multidrug resistant pulmonary tuberculosis treatment regimens and patient outcomes: an individual patient data meta-analysis of 9,153 patients. PLoS Med. 2012;9(8):e1001300. doi:10.1371/journal.pmed.1001300
85. Brust JC, Gandhi NR, Carrara H, Osburn G, Padayatchi N. High treatment failure and default rates for patients with multidrug-resistant tuberculosis in KwaZulu-Natal, South Africa, 2000–2003. Int J Tuberc Lung Dis. 2010;14(4):413–419.
86. Daniels JF, Khogali M, Mohr E, et al. Time to ART initiation among patients treated for rifampicin-resistant tuberculosis in Khayelitsha, South Africa: impact on mortality and treatment success. PLoS One. 2015;10(11):e0142873. doi:10.1371/journal.pone.0142873
87. O’Donnell MR, Padayatchi N, Kvasnovsky C, Werner L, Master I, Horsburgh CR
88. Carlucci JG, Blevins PM, Kipp AM, et al. Tuberculosis treatment outcomes among HIV/TB Co-infected Children in the International Epidemiology Databases to Evaluate AIDS (IeDEA) network. J Acquir Immune Defic Syndr. 2017;75(2):156–163. doi:10.1097/QAI.0000000000001335
89. Isaakidis P, Casas EC, Das M, Tseretopoulou X, Ntzani EE, Ford N. Treatment outcomes for HIV and MDR-TB co-infected adults and children: systematic review and meta-analysis. Int J Tuberc Lung Dis. 2015;19(8):969–978. doi:10.5588/ijtld.15.0123
90. Magis-Escurra C, Günther G, Lange C, et al. Treatment outcomes of MDR-TB and HIV co-infection in Europe. Eur Respir J. 2017;49:1602363. doi:10.1183/13993003.02363-2016
91. Trebucq A, Schwoebel V, Kashongwe Z, et al. Treatment outcome with a short multidrug-resistant tuberculosis regimen in nine African countries. Int J Tuberc Lung Dis. 2018;22(1):17–25. doi:10.5588/ijtld.17.0498
92. Gandhi NR, Andrews JR, Brust JCM, et al. Risk factors for mortality among MDR- and XDR-TB patients in a high HIV-prevalence setting. Int J Tuberc Lung Dis. 2012;16(1):90–97. doi:10.5588/ijtld.11.0153
93. Azeez A, Ndege J, Mutambayi R. Associated factors with unsuccessful tuberculosis treatment outcomes among tuberculosis/HIV co-infected patients with drug-resistant tuberculosis. Int J Mycobacteriol. 2018;7(4):347–354. doi:10.4103/ijmy.ijmy_140_18
94. Narita M, Ashkin D, Hollender ES, Pitchenik AE. Paradoxical worsening of tuberculosis following antiretroviral therapy in patients with AIDS. Am J Resp Crit Care Med. 1998;158(1):157–161. doi:10.1164/ajrccm.158.1.9712001
95. Lawn SD, Myer L, Bekker LG, Wood R. Tuberculosis-associated immune reconstitution disease: incidence, risk factors and impact in an antiretroviral treatment service in South Africa. AIDS. 2007;21(3):335–341. doi:10.1097/QAD.0b013e328011efac
96. Navas E, Martín-Dávila P, Moreno L, et al. Paradoxical reactions of tuberculosis in patients with the acquired immunodeficiency syndrome who are treated with highly active antiretroviral therapy. Arch Intern Med. 2002;162(1):97–99. doi:10.1001/archinte.162.1.97
97. Muller M, Wandel S, Colebunders R, et al. Immune re-constitution inflammatory syndrome in patients starting antiretroviral therapy for HIV infection: a systematic review and meta-analysis. Lancet Infect Dis. 2010;10(4):251–261. doi:10.1016/S1473-3099(10)70026-8
98. Horsburgh CR
99. Durovni B, Saraceni V, Moulton LH, et al. Effect of improved tuberculosis screening and isoniazid preventive therapy on incidence of tuberculosis and death in patients with HIV in clinics in Rio de Janeiro, Brazil: a stepped wedge, cluster-randomised trial. Lancet Infect Dis. 2013;13(10):852–858. doi:10.1016/S1473-3099(13)70187-7
100. Ayele HT, Mourik MS, Debray TP, Bonten MJ. Isoniazid prophylactic therapy for the prevention of tuberculosis in HIV infected adults: a systematic review and meta-analysis of randomized trials. PLoS One. 2015;10(11):e0142290. doi:10.1371/journal.pone.0142290
101. Fraser A, Paul M, Attamna A, Leibovici L. Drugs for preventing tuberculosis in people at risk of multiple drug- resistant pulmonary tuberculosis. Cochrane Database Syst Rev. 2006;19(2):CD005435.
102. Marks SM, Mase SR, Morris SB. Systematic review, meta-analysis, and cost-effectiveness of treatment of latent tuberculosis to reduce progression to multidrug-resistant tuberculosis. Clin Infect Dis. 2017;64(12):1670–1677. doi:10.1093/cid/cix208
103. Fox G, Dobler C, Marais B, Denholm J. Preventive therapy for latent tuberculosis infection—the promise and the challenges. Int J Infec Dis. 2017;56:68–76. doi:10.1016/j.ijid.2016.11.006
104. Padmapriyadarsini C, Das M, Nagaraja SB, et al. Is chemoprophylaxis for child contacts of drug-resistant TB patients beneficial? A systematic review. Tuberc Res Treat. 2018;3905890:1–8. doi:10.1155/2018/3905890
105. World Health Organization. Latent tuberculosis infection: updated and consolidated guidelines for programmatic management. Geneva: WHO; 2018 (WHO/CDS/TB/2018.4). Available from: https://apps.who.int›iris›bitstream›9789241550239-eng.
106. Basu S, Andrews JR, Poolman EM, et al. Prevention of nosocomial transmission of extensively drug-resistant tuberculosis in rural South African district hospitals: an epidemiological modelling study. Lancet. 2007;370(9597):1500–1507. doi:10.1016/S0140-6736(07)61636-5
107. Shin S, Furin J, Bayona J, Mate K, Kim JY, Farmer P. Community-based treatment of multidrug-resistant tuberculosis in Lima, Peru: 7 years of experience. Soc Sci Med. 2004;59(7):1529–1539. doi:10.1016/j.socscimed.2004.01.027
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
