Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 16
Driving Pressure-Guided Ventilation in Obese Patients Undergoing Laparoscopic Sleeve Gastrectomy: A Randomized Controlled Trial
Authors Yang G, Zhang P, Li L, Wang J, Jiao P, Wang J, Chu Q
Received 24 January 2023
Accepted for publication 19 May 2023
Published 24 May 2023 Volume 2023:16 Pages 1515—1523
DOI https://doi.org/10.2147/DMSO.S405804
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Gian Paolo Fadini
Guanyu Yang,* Pin Zhang,* Liumei Li, Jingjing Wang, Pengfei Jiao, Jie Wang, Qinjun Chu
Department of Anesthesiology and Perioperative Medicine, Zhengzhou Central Hospital, Zhengzhou, Henan, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Qinjun Chu, Tongbai North Road 16, Zhongyuan District, Zhengzhou, Henan, People’s Republic of China, Tel/Fax +86 13643711142, Email [email protected]
Purpose: This study aims to compare the conventional lung protective ventilation strategy (LPVS) with driving pressure-guided ventilation in obese patients undergoing laparoscopic sleeve gastrectomy (LSG).
Methods: Forty-five patients undergoing elective LSG under general anesthesia were randomly assigned to the conventional LPVS group (group L) or the driving pressure-guided ventilation group (group D) using random numbers generated by Excel. The primary outcome was the driving pressure of both groups 90 min after pneumoperitoneum.
Results: After 30 min of pneumoperitoneum, 90 min of pneumoperitoneum, 10 min of closing the pneumoperitoneum, and restoring the supine position, the driving pressure of group L and group D were 20.0 ± 2.9 cm H2O vs 16.6 ± 3.0 cm H2O (P < 0.001), 20.7 ± 3.2 cm H2O vs 17.3 ± 2.8 cm H2O (P < 0.001), and 16.3 ± 3.1 cm H2O vs 13.3 ± 2.5 cm H2O (P = 0.001), respectively; the respiratory compliance of groups L and D were 23.4 ± 3.7 mL/cm H2O vs 27.6 ± 5.1 mL/cm H2O (P = 0.003), 22.7 ± 3.8 mL/cm H2O vs 26.4 ± 3.5 mL/cm H2O (P = 0.005), and 29.6 ± 6.8 mL/cm H2O vs 34.7 ± 5.3 mL/cm H2O (P = 0.007), respectively. The intraoperative PEEP in groups L and group D was 5 (5– 5) cm H2O vs 10 (9– 11) cm H2O (P < 0.001).
Conclusion: An individualized peep-based driving pressure-guided ventilation strategy can reduce intraoperative driving pressure and increase respiratory compliance in obese patients undergoing LSG.
Keywords: lung protective ventilation strategy, driving pressure-guided ventilation, respiratory compliance, driving pressure, obese
Corrigendum for this paper has been published.
Introduction
As living standards improve, so does the proportion of obese people,1 and because the complications associated with obesity significantly impact their lives, making weight loss a popular topic among obese individuals. Laparoscopic sleeve gastrectomy (LSG) is a new option for obese patients due to its significant weight loss and improved comorbidity,2 however, the incidence of postoperative pulmonary complications (PPCs) is as high as 18% among obese patients.3 The occurrence of PPCs prolongs hospital stays and increases mortality,4,5 and without effective measures, this healthcare burden will increase as the number of obese patients undergoing surgery increases.
Lung protective ventilation strategy (LPVS) includes low tidal volumes (VT), positive end-expiratory pressure (PEEP), recruitment maneuver (RM), and like.6 Numerous studies7–9 have demonstrated that LPVS can reduce the incidence of PPCs. The driving pressure is the pressure responsible for opening the alveoli.10 In recent years, the driving pressure-guided ventilation strategy has emerged as a new LPVS that reduces the driving pressure during mechanical ventilation by adjusting the parameters of mechanical ventilation to minimize PPCs.11
Little research has been conducted on applying driving pressure-guided ventilation strategy in obese patients. This research aimed to compare the effects of a driving pressure-guided ventilation strategy and a conventional LPVS in obese patients undergoing LSG.
Materials and Methods
Study Population
This randomized, controlled study was conducted at Zhengzhou Central Hospital and approved by the hospital’s medical ethics committee (Zhengzhou Central Hospital Affiliated with Zhengzhou University, Zhengzhou, China; approval number: 202205; date: 10/01/2022). The study protocol was registered in the Chinese Clinical Trial Registry with the registration number ChiCTR2200057246 (date: 04/03/2022). The methodology was executed according to international guidelines for randomized clinical studies, CONSORT Guidelines (Table S1 CONSORT checklist), and the Declaration of Helsinki.
Patients undergoing LSG between March 5, 2022, and April 5, 2022, were recruited for this study. Before the commencement of the clinical trial, all patients signed an informed consent form. The following were the criteria for inclusion: 1) Patients aged 18–60 years; 2) body mass index (BMI) > 30 kg/m2; 3) American Society of Anesthesiologists (ASA) physical status II or III. Exclusion criteria included: 1) preoperative hemoglobin concentration below 110 g/L; 2) preoperative coagulation abnormalities; 3) history of upper respiratory tract infection within two weeks before surgery; 4) preoperative computed tomography demonstrating pulmonary atelectasis or severe respiratory disease; 5) concomitant neurological disease or previous history of cerebrovascular disease; 6) serious heart, liver, kidney, and other systemic diseases; and 7) refusal to sign the informed consent form.
Blinding Methods
The patients were randomly assigned to the conventional LPVS group (group L) or the driving pressure-guided ventilation group (group D) using random numbers generated by Excel. Based on the list of random numbers, opaque, sealed, and sequentially numbered envelopes containing cards with the allocation information were prepared. Upon the arrival of an eligible patient in the operating room, the investigator nurse designated participants by opening envelopes containing pre-written assignments. During the entire observation period, including all postoperative follow-up periods, patients, surgeons, and post-anesthesia care unit (PACU) personnel were blinded to the grouping.
Study Protocol of Each Ventilator Strategy
After endotracheal intubation, patients in both groups received RM (sustained inflation, 30–40 cm H2O, 20–30 s), followed by volume control mode mechanical ventilation. The parameters for breathing are as follows: VT of 6–8 mL/kg of lean body weight (LBW), the respiration rate of 12–20 breaths/min, inspired oxygen fraction of 0.61 (1:1 oxygen/air mixture), the inspiratory interval of 30%, inspiration-to-expiration ratio of 1:2, and flow rate of 2 L/min.
Group L: PEEP of 5 cm H2O was set 20 min after establishing pneumoperitoneum and completing surgical position adjustment and maintained throughout the procedure.
Group D: A driving pressure-guided ventilation strategy was initiated 10 min after the pneumoperitoneum was established and the surgical position was adjusted. The PEEP was sequentially increased from 4 cm H2O to 12 cm H2O, and each PEEP level was maintained for thirteen respiratory cycles. The plateau pressure (Pplat) corresponding to the fourth respiratory cycle through the thirteenth respiratory cycle was recorded, and the driving pressure corresponding to each of these ten respiratory cycles was calculated using a simplified algorithm of driving pressure (driving pressure = Pplat - PEEP). The driving pressure that occurs most frequently in ten respiratory cycles is the driving pressure corresponding to this PEEP level. Then, the driving pressures corresponding to the nine PEEP levels are compared, and the PEEP level corresponding to the lowest driving pressure is selected and maintained throughout the entire procedure.
Anesthesia and Surgery
When the patients were admitted to the operating room, an intravenous line was determined with an 18-gauge IV cannula on the forearm. Ringer’s lactate solution at a rate of 2–4 mL/kg/h was used to maintain the basal fluid. Rescue vasoactive agents such as atropine, ephedrine, or epinephrine were available. All patients started induction of general anesthesia after receiving bilateral paravertebral nerve blocks at T9. Later, all patients had a pneumoperitoneum established by the surgeon and adjusted to the surgical positional (reverse Trendelenburg and right-lateral tilt positions).
Based on the patient’s LBW, propofol 2 mg/kg, sufentanil 0.5 μg/kg, and rocuronium 1 mg/kg were administered intravenously as anesthetic induction. Mechanical ventilation was initiated after endotracheal intubation. Anesthesia was maintained with propofol (2–6 mg.kg−1.h−1), sevoflurane (1–2%), remifentanil (0.05–0.15 μg.kg−1.min−1) and intermittent bolus injection of rocuronium. The bispectral index (BIS) value was maintained within 40–60. Intraoperative blood pressure fluctuation was maintained at ± 20% of the baseline.
Flurbiprofen (100 mg) was administered intravenously half an hour before the completion of surgery. For postoperative analgesia, each patient received a patient-controlled intravenous analgesia (PCIA) pump (REHN11, Renxian Medical Corporation, Jiangsu, China) according to the same protocol. After surgery, all patients were extubated after receiving an intravenous sugammadex injection (Bridion, Merck Sharp Dohme, New Jersey, USA) at 2 mg/kg of LBW and then transported to the PACU. All patients were evaluated by the anesthesiologist in PACU and returned to the ward by the nurse anesthetist after meeting the PACU discharge criteria.
Measurements
Mean artery pressure (MAP), heart rate (HR), end-tidal carbon dioxide (ETCO2), VT, Pplat, and peak airway pressure (Ppeak) were recorded at five-time points: 10 min after induction of anesthesia (T0), 10 min after pneumoperitoneum (T1), 30 min after pneumoperitoneum (T2), 90 min after pneumoperitoneum (T3), and 10 min after pneumoperitoneum was closed and supine position was restored (T4), respectively. The driving pressure and respiratory compliance were calculated using the formula (respiratory compliance = VT/driving pressure). The incidence of PPCs within three days after surgery was recorded (three or more new findings: cough, increased sputum, dyspnea, chest pain, temperature > 38 °C, and heart rate > 100 beats/min).12
The primary outcome was the driving pressure of the two groups at T3. Secondary outcomes included PEEP, respiratory compliance, and the incidence of PPCs.
Statistical Analysis
The sample size was determined using data from the preliminary study. Patients in group L had a T3 time point driving pressure of 20.7 ± 3.4 cm H2O, while patients in group D was 16.8 ± 3.8 cm H2O. Assuming alpha = 0.05, beta = 0.1, a two tails test is performed, and the calculated sample size for each group was 19. To allow for a possible 20% dropout rate, we enrolled 46 participants in this study.
R software was applied to analyze all statistical data. The measurement data of normal distribution were expressed as mean ± standard deviation, and the comparison of mean between the two groups was performed by two independent samples t-test. The median (interquartile range) was used for measurement data of non-normal distribution, and the Mann–Whitney U-test was used for comparison between groups. Categorical variables were compared using the chi-square test or Fisher’s exact test. P < 0.05 was considered statistically significant.
Results
In the present study, 56 patients undergoing LSG were evaluated; however, six patients were excluded because they did not satisfy the inclusion criteria, and four refused to participate. 46 patients were randomly allocated into two groups. In group L, one patient was excluded due to anaphylactic shock. Finally, 45 patients completed the study: group L (n = 22) and group D (n = 23). The flowchart is shown in Figure 1. The two groups had no statistically significant differences in patient demographics and operative characteristics (Table 1).
 |
Table 1 Patient Demographics and Operation Characteristics |
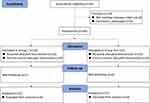 |
Figure 1 The CONSORT study diagram. Notes: CONSORT figure adapted from Schulz KF, Altman DG, Moher D, CONSORT 2010 Statement: Updated Guidelines for Reporting Parallel Group Randomised Trials. PLoS Med. 2010;7(3): e1000251. Copyright: © 2010 Schulz et al. Creative Commons Attribution License.13 |
Figures 2A and B show that both groups’ driving pressure and respiratory compliance differences at T0-T1 were insignificant (P > 0.05). However, at T2-T4, group D had significantly lower driving pressure and significantly higher respiratory compliance (P < 0.01). As shown in Figures 2C and D, there was no statistically significant difference in Pplat and Ppeak between the two groups from T0-T4 (P > 0.05).
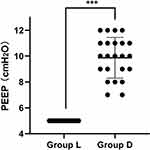
Figure 3 illustrates the PEEP distribution during mechanical ventilation. In group D, the median PEEP was 10 (9–11) cm H2O compared with 5 (5–5) cm H2O in group L (P < 0.001).
Figure 4 shows no statistically significant difference in MAP, HR, ETCO2, and VT between the two groups from T0-T4 (P > 0.05).
None of the patients was observed to have PPCs within three days postoperatively.
Discussion
In this randomized controlled trial, we found that a driving pressure-guided ventilation strategy achieved by individualized PEEP settings can reduce driving pressure and improve respiratory compliance in obese patients undergoing LSG compared to a conventional LPVS.
In obese patients, the accumulation and abnormal distribution of large amounts of adipose tissue in the chest stiffens the chest wall, resulting in decreased respiratory compliance;14 on the other hand, the accumulation of fat in the abdomen and viscera increase intra-abdominal pressure and diaphragm, thereby limiting the movement of the thorax, also resulting in a decreased respiratory compliance.15 Driving pressure is defined as the VT divided by the respiratory compliance, so when the VT is fixed, the lower the driving pressure, the greater the respiratory compliance. A driving pressure-guided ventilation strategy allows the patient to sustain a relatively low level of driving pressure intraoperatively to achieve a high level of respiratory compliance. “Functional lung size” proposed by Park et al9 and referred to the total volume of alveoli during normal ventilation, above which ventilation can lead to barotrauma and below which ventilation can lead to atelectasis,16 while ventilation according to the functional lung size achieves the highest respiratory compliance and prevents alveolar overinflation or hypoventilation, which is the aim of the driving pressure-guided ventilation strategy. Although improving respiratory compliance is not the same as reducing the incidence of PPCs, it is a crucial component of this process.
Atelectasis is one of the important factors causing PPCs. The incidence of atelectasis is typically reduced by PEEP, lowering the concentration of inhaled oxygen and RM, and like.17–19 Obese patients are a high-risk group for atelectasis, and studies15,20 have shown that obese patients, especially morbidly obese patients, already have a certain area of atelectasis before surgery, mainly due to the decreased compliance of the patient’s respiratory system. Larger intraoperative atelectasis may result in complications after surgery, including difficult extubation, hypoxemia, hypercapnia, and others,21 resulting in a greatly increased incidence of PPCs. In the present study, the individualized PEEP setting avoided the over-expansion of alveoli caused by excessive PEEP and the ineffective opening of alveoli caused by inadequate PEEP. The driving pressure-guided ventilation strategy was used to maintain the uniform alveoli opening and reduce the incidence of atelectasis by individualizing the PEEP setting. In this study, an RM was performed after the patient’s anaesthesia induction and intraoperative ventilation with an air-oxygen mixture to further reduce the incidence of atelectasis.
Driving pressure is an indicator that has received much attention in recent years in the context of lung protection. Studies5,10 have shown that higher driving pressure is firmly associated with the development of PPCs during mechanical ventilation. In contrast, VT and PEEP are only associated with the development of PPCs if they result in a change in driving pressure. The results of several randomized controlled studies12,22,23 have demonstrated that a driving pressure-guided ventilation strategy is superior to a conventional LPVS in reducing the incidence of PPCs. One study24 showed a 3.4% increase in the incidence of major morbidity in patients for every 1 cm H2O increase in driving pressure. In this study, after completing the intervention, the mean reduction in driving pressure in group D compared to group L was approximately 3.2 cm H2O.
The current guideline recommendation for PEEP during mechanical ventilation is 5 cm H2O.25 However, numerous studies26–28 have demonstrated that obese patients require a higher level of PEEP. This study investigated the appropriate PEEP during mechanical ventilation in obese patients, using driving pressure as a guide. The results of the study revealed a median PEEP of 10 (9–11) cm H2O, which, on the one hand, supports the need for a higher level of PEEP in obese patients in terms of driving pressure and, on the other hand, offers some guidance for the choice of PEEP during mechanical ventilation in obese patients.
The lung-protective effect of the driving pressure-guided ventilation strategy as a new LPVS has been demonstrated in high-risk surgeries (abdominal, thoracic) in PPCs,;12,22,23,29 however, it has been investigated less in obese patients, who are a high-risk group for PPCs. The driving pressure-guided ventilation strategy can be achieved through PEEP, VT, and other variables.11 In this study, we chose PEEP, which is presently the most popular method. This trial was designed with a PEEP range of 4–12 cm H2O because this is the most common range in clinical practice, and higher PEEP is associated with more complications. The results indicated a minimum PEEP of 7 cm H2O, a median of 10 cm H2O, and a maximum of 12 cm H2O (probably because of the pre-determined upper PEEP limit of 12 cm H2O) when the driving pressure was lowest. Therefore, this study provides some reference to investigate further the application of driving pressure-guided ventilation strategy in obese patients. Based on this study, the pre-set PEEP could be increased by 7–15 cm H2O to determine whether a medium to a high level of PEEP is a more optimal choice for obese patients.
This study has the following three limitations: First, the driving pressure rather than the incidence of PPCs is the primary outcome of this study, mainly because the incidence of PPCs in obese patients undergoing LSG in our center is very low, which may be due to the young age of the patients and the short operation time. Additionally, all patients underwent nerve blocks before surgery. Muscle relaxation antagonism was performed after surgery, and the patients could drink water and do activities 2 h after surgery, greatly reducing the incidence of PPCs. Second, in this study, although we were tempted to perform blood gas analysis to monitor the arterial partial pressure of oxygen to compare oxygenation, we did not do so mainly because an arterial line is not routinely placed for LSG, performing an invasive arterial puncture is both damaging and expensive for the patient; Third, in this study, the patient’s atelectasis was not evaluated, primarily because accurate images could not be obtained by ultrasound scanning of the lungs of obese patients. Equipment such as electrical impedance tomography was unavailable at our institution.
Conclusion
In conclusion, an individualized peep-based driving pressure-guided ventilation strategy can reduce intraoperative driving pressure and improve respiratory compliance in obese patients undergoing LSG.
Data Sharing Statement
The original data is available upon request by email from (Qinjun Chu: [email protected]).
Acknowledgments
We thank bariatric surgery for its assistance in our patient recruitment process. We also thank Home for Researchers editorial team (www.home-for-researchers.com) for language editing service.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
There is no funding to report.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Kim KM, Choi JJ, Lee D, et al. Effects of ventilatory strategy on arterial oxygenation and respiratory mechanics in overweight and obese patients undergoing posterior spine surgery. Sci Rep. 2019;9(1):16638.
2. Baheeg M, Elgohary SA, Tag-Eldin M, et al. Effect of laparoscopic sleeve gastrectomy vs laparoscopic Roux-en-Y gastric bypass on weight loss in Egyptian patients with morbid obesity. Ann Med Surg. 2022;73:103235. doi:10.1016/j.amsu.2021.103235
3. Ball L, Hemmes SNT, Serpa Neto A, et al. Intraoperative ventilation settings and their associations with postoperative pulmonary complications in obese patients. Br J Anaesth. 2018;121(4):899–908. doi:10.1016/j.bja.2018.04.021
4. LAS VEGAS investigators. Epidemiology, practice of ventilation and outcome for patients at increased risk of postoperative pulmonary complications: las Vegas - an observational study in 29 countries. Eur J Anaesthesiol. 2017;34(8):492–507. doi:10.1097/EJA.0000000000000646
5. Fernandez-Bustamante A, Frendl G, Sprung J, et al. Postoperative Pulmonary Complications, Early Mortality, and Hospital Stay Following Noncardiothoracic Surgery: a Multicenter Study by the Perioperative Research Network Investigators. JAMA Surg. 2017;152(2):157–166. doi:10.1001/jamasurg.2016.4065
6. Sato H, Nakamura K, Baba Y, et al. Low tidal volume ventilation with low PEEP during surgery may induce lung inflammation. BMC Anesthesiol. 2016;16:47. doi:10.1186/s12871-016-0209-y
7. Goldenberg NM, Steinberg BE, Lee WL, et al. Lung-protective ventilation in the operating room: time to implement? Anesthesiology. 2014;121(1):184–188. doi:10.1097/ALN.0000000000000274
8. Futier E, Constantin JM, Paugam-Burtz C, et al. A trial of intraoperative low-tidal-volume ventilation in abdominal surgery. N Engl J Med. 2013;369:428–437. doi:10.1056/NEJMoa1301082
9. Severgnini P, Selmo G, Lanza C, et al. Protective mechanical ventilation during general anesthesia for open abdominal surgery improves postoperative pulmonary function. Anesthesiology. 2013;118:1307–1321. doi:10.1097/ALN.0b013e31829102de
10. Amato MB, Meade MO, Slutsky AS, et al. Driving pressure and survival in the acute respiratory distress syndrome. N Engl J Med. 2015;372:747–755. doi:10.1056/NEJMsa1410639
11. Ahn HJ, Park M, Kim JA, et al. Driving pressure guided ventilation. Korean J Anesthesiol. 2020;73(3):194–204. doi:10.4097/kja.20041
12. Xu Q, Guo X, Liu J, et al. Effects of dynamic individualized PEEP guided by driving pressure in laparoscopic surgery on postoperative atelectasis in elderly patients: a prospective randomized controlled trial. BMC Anesthesiol. 2022;22:72. doi:10.1186/s12871-022-01613-9
13. Schulz KF, Altman DG, Moher D. CONSORT 2010 Statement: Updated Guidelines for Reporting Parallel Group Randomised Trials. PLoS Med. 2010;7(3):e1000251.
14. Zerah F, Harf A, Perlemuter L, et al. Effects of obesity on respiratory resistance. Chest. 1993;103:1470–1476. doi:10.1378/chest.103.5.1470
15. Pelosi P, Croci M, Ravagnan I, et al. Total respiratory system, lung, and chest wall mechanics in sedated-paralyzed postoperative morbidly obese patients. Chest. 1996;109(1):144–151. doi:10.1378/chest.109.1.144
16. Gattinoni L, Pesenti A. The concept of “baby lung”. Intensive Care Med. 2005;31:776–784. doi:10.1007/s00134-005-2627-z
17. Tusman G, Böhm SH. Alveolar recruitment strategy improves arterial oxygenation during general anaesthesia. Br J Anaesth. 1999;82:8–13. doi:10.1093/bja/82.1.8
18. Huhle R, Serpa Neto A, Schultz MJ, et al. Is mechanical power the final word on ventilator-induced lung injury?-no. Ann Transl Med. 2018;6:394. doi:10.21037/atm.2018.09.65
19. Koo CH, Park EY, Lee SY, et al. The Effects of Intraoperative Inspired Oxygen Fraction on Postoperative Pulmonary Parameters in Patients with General Anesthesia: a Systemic Review and Meta-Analysis. J Clin Med. 2019;8:583. doi:10.3390/jcm8050583
20. Guivarch E, Voiriot G, Rouzé A, et al. Pulmonary Effects of Adjusting Tidal Volume to Actual or Ideal Body Weight in Ventilated Obese Mice. Sci Rep. 2018;8:6439. doi:10.1038/s41598-018-24615-5
21. Ferrando C, Romero C, Tusman G, et al. The accuracy of postoperative, non-invasive Air-Test to diagnose atelectasis in healthy patients after surgery: a prospective, diagnostic pilot study. BMJ Open. 2017;7:e015560. doi:10.1136/bmjopen-2016-015560
22. Park M, Ahn HJ, Kim JA, et al. Driving Pressure during Thoracic Surgery: a Randomized Clinical Trial. Anesthesiology. 2019;130:385–393. doi:10.1097/ALN.0000000000002600
23. Zhang C, Xu F, Li W, et al. Driving Pressure-Guided Individualized Positive End-Expiratory Pressure in Abdominal Surgery: a Randomized Controlled Trial. Anesth Analg. 2021;133:1197–1205. doi:10.1213/ANE.0000000000005575
24. Blank RS, Colquhoun DA, Durieux ME, et al. Management of One-lung Ventilation: impact of Tidal Volume on Complications after Thoracic Surgery. Anesthesiology. 2016;124:1286–1295. doi:10.1097/ALN.0000000000001100
25. Young CC, Harris EM, Vacchiano C, et al. Lung-protective ventilation for the surgical patient: international expert panel-based consensus recommendations. Br J Anaesth. 2019;123(6):898–913. doi:10.1016/j.bja.2019.08.017
26. Elshazly M, Khair T, Bassem M, Mansour M. The use of intraoperative bedside lung ultrasound in optimizing positive end expiratory pressure in obese patients undergoing laparoscopic bariatric surgeries. Surg Obes Relat Dis. 2021;17(2):372–378. doi:10.1016/j.soard.2020.09.023
27. Elokda SA, Farag HM. Preemptive alveolar recruitment maneuver followed by PEEP in obese patients undergoing laparoscopic gastric banding. Does it make a difference? A randomized controlled clinical study. Open Anesthesia J. 2019;13:31–39. doi:10.2174/2589645801913010031
28. Nestler C, Simon P, Petroff D, et al. Individualized positive end-expiratory pressure in obese patients during general anaesthesia: a randomized controlled clinical trial using electrical impedance tomography. Br J Anaesth. 2017;119(6):1194–1205. doi:10.1093/bja/aex192
29. Douville NJ, McMurry TL, Ma JZ, et al. Airway driving pressure is associated with postoperative pulmonary complications after major abdominal surgery: a multicentre retrospective observational cohort study. BJA Open. 2022;4:100099. doi:10.1016/j.bjao.2022.100099
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.