Back to Journals » Journal of Pain Research » Volume 7
Dosing considerations with transdermal formulations of fentanyl and buprenorphine for the treatment of cancer pain
Authors Skaer T
Received 3 January 2014
Accepted for publication 23 May 2014
Published 19 August 2014 Volume 2014:7 Pages 495—503
DOI https://doi.org/10.2147/JPR.S36446
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Video abstract presented by Tracy L Skaer
Views: 3764
Tracy L Skaer
College of Pharmacy, Washington State University, Spokane, WA, USA
Abstract: Opioids continue to be first-line pharmacotherapy for patients suffering from cancer pain. Unfortunately, subtherapeutic dosage prescribing of pain medications remains common, and many cancer patients continue to suffer and experience diminished quality of life. A large variety of therapeutic options are available for cancer pain patients. Analgesic pharmacotherapy is based on the patient's self-report of pain intensity and should be tailored to meet the requirements of each individual. Most, if not all, cancer pain patients will ultimately require modifications in their opioid pharmacotherapy. When changes in a patient's medication regimen are needed, adequate pain control is best maintained through appropriate dosage conversion, scheduling immediate release medication for withdrawal prevention, and providing as needed dosing for breakthrough pain. Transdermal opioids are noninvasive, cause less constipation and sedation when compared to oral opioids, and may improve patient compliance. A relative potency of 100:1 is recommended when converting the patient from oral morphine to transdermal fentanyl. Based on the limited data available, there is significant interpatient variability with transdermal buprenorphine and equipotency recommendations from oral morphine of 75:1–110:1 have been suggested. Cancer patients may require larger transdermal buprenorphine doses to control their pain and may respond better to a more aggressive 75–100:1 potency ratio. This review outlines the prescribing of transdermal fentanyl and transdermal buprenorphine including how to safely and effectively convert to and use them for those with cancer pain.
Keywords: opioids, analgesic pharmacotherapy, immediate release medication, pain treatment modification, breakthrough pain, opioid withdrawal, equipotency ratio
Introduction
Opioids continue to be mainstay pharmacotherapy for moderate-to-severe cancer pain. A substantial amount of uncontrolled pain continues to be reported in at least 33% of newly diagnosed cancer patients, and in 65%–85% of those with metastatic disease.1,2 A wide variety of pharmacotherapy options are currently available to manage cancer pain.3 Unfortunately, many experience subtherapeutic levels and continue to suffer from inadequate pain control.2–4 Patients who worry about exacerbations of their pain with ambulation often hesitate in participating in daily activities. As their averting behavior increases, family and social relationships are impacted and quality of life is reduced.4,5
Oral (PO) sustained-release (SR) preparations of morphine are considered to be the practitioner’s first choice for substantial cancer pain and, although transdermal opioids (TD-Os) (primarily transdermal fentanyl [TD-Fe]) are primarily recommended for use in those unable to take PO medications, the use of transdermal preparations have substantially increased in recent years.4–14 Most, if not all, cancer pain patients will ultimately require changes to their opioid therapy throughout the course of their illness.4,13,14
The term “opioid switching” is used to describe the practice of substituting one World Health Organization (WHO) Pain Ladder step III choice opioid (ie, PO and TD-Os for moderate to severe pain) with another, and when patients are unable to achieve significant pain relief without experiencing substantial adverse effects (AE).3,4,9,13 This is not to be mistaken with the patient who is stabilized on PO morphine with limited to no AE and chooses to receive their medication via a transdermal route of administration. Opioid substitution, also called incomplete cross-tolerance, has been shown to improve opioid response.14–17
TD-Os’ place in therapy
TD-Os are considered a WHO Pain Ladder Step III choice (opioids for moderate to severe pain).3,18 TD-O administration provides a slow and steady increase in opiate plasma levels, extended half-lives of several days, and a long latent period before full pharmacologic effects are achieved. Based on the current literature available on the use of TD-Fe and transdermal buprenorphine (TD-Bu) for cancer pain, no significant differences in efficacy were discerned between the transdermal preparations in comparison to other opioids.11,12,18–20
TD-Os have lower rates of constipation and sedation when compared to PO opioids, are noninvasive, and offer a convenient dosing schedule to assist with patient adherence.7,10–14 There is limited data available demonstrating that TD-Bu may have fewer reports of nausea and treatment discontinuance due to AE when compared to TD-Fe, but more research is needed to validate these results.18,19
Converting to TD-Os
When substituting one opioid for another, it is important to utilize safe and effective conversion ratios.21–24 Dosage calculation errors can result in under- or over-dosing, undue distress in the patient, therapy failure, non-adherence, and/or discontinuance. Unfortunately, intrapatient variability and incomplete cross-tolerance have contributed to the lack of a consensus guideline on opioid equianalgesic dosing.24 Historically, a conversion ratio of 6:1 has been used for pain to substitute between PO and intravenous (IV) morphine.25
The 6:1 ratio was derived from acute repeated crossover administration (also called “relative potency assays”) and has since been found to be inadequate for cancer pain.26,27 A ratio of 3:1 (PO to IV morphine) has been shown to be more effective and the most often utilized to relieve cancer pain.28–30 Practical equianalgesic dosing ratios were derived from randomized controlled trials (RCTs) that compared the efficacy of two opioid medications or from observational case series describing opioid substitution during chronic administration.23 A systematic review by Mercadante and Caraceni provides important insight from six RCTs with crossover designs and from 26 case series examining opioid switching.23 The strongest evidence was found from patients who were stabilized at equianalgesic doses of oxycodone and morphine (four RCTs), oxycodone and hydromorphone (one RCT), and hydromorphone and morphine (one RCT) before opioid substitution occurred.23
The first step in conversion is to calculate the patient’s 24-hour PO morphine usage (mg/day) and then apply the appropriate conversion ratio to determine the TD-O dose. When converting to TD-O pharmacotherapy, the patient is at increased risk of suffering significant breakthrough pain and opioid withdrawal.22 In order to reduce the chances of opioid withdrawal, patients should be prescribed an instant release (IR) opioid given on a regular schedule every 3 or 4 hours and not as needed (PRN) until the TD-O reaches therapeutic levels. Additionally, supplemental doses of IR opioid PRN for breakthrough pain relief should be given during initiation of TD-O therapy (first 18 hours for TD-Fe and first 48 hours for TD-Bu) followed by every 2–3 hours PRN therafter.22 During dosage titration of TD-Os, the practitioner should note the daily amount of the IR breakthrough pain medication utilized during the first 48–72 hours after beginning TD-O pharmacotherapy, and consider increasing the TD-O dose if clinically necessary. Moreover, TD-Os are long acting and can take 5–6 days to reach steady state serum concentrations. Steady state concentrations occur during the second patch application of TD-Fe when dosed every 3 days and during the first application of TD-Bu when given every 7 days.22,30 TD-Bu for cancer pain is dosed on two fixed days of the week (ie, Tuesday and Saturday).10
TD-Fe conversion ratios
Donner et al examined 98 cancer pain patients who were switched from SR PO morphine to TD-Fe.31 They found that a 2:1 dosage conversion ratio (2 mg/day of PO morphine equals 1 μg/hr of TD-Fe), representing a relative potency of 100:1, provided an appropriate starting dose for cancer pain.31 The starting TD-Fe dose was determined based on pre-enrollment dose of SR PO morphine and then converted to TD-Fe using the 2:1 PO morphine (mg/day) to TD-Fe (μg/hr) dosage conversion rate (100:1 relative potency). Patients were provided a liquid formulation of IR PO morphine PRN to address any breakthrough pain. TD-Fe was found to be comparable to SR morphine for treatment of cancer pain with pain levels and the number of reported pain attacks unchanged.31 However, TD-Fe patients had significantly higher (P<0.05) usage of IR PO morphine PRN for breakthrough pain as compared to SR morphine. The researchers were unable to determine a reason for this finding but hypothesized that either patients were accustomed to using an PO IR opioid for breakthrough pain, or that some patients did not achieve effective pain relief over the 72-hour dosing period and may have benefited from 48-hour dosing of their TD-Fe.31
Constipation and the use of laxatives was significantly reduced (P≤0.05) in those receiving TD-Fe.31 No clinical differences were discovered in regards to vital signs (respiratory rate, blood pressure, and heart rate), other adverse events (vomiting, diarrhea, dizziness, dyspnea, sweating, pruritus, dry mouth, fatigue), and reports of respiratory depression. There were three patients (3% of participants) who reported experiencing morphine withdrawal symptoms within the first day after switching to TD-Fe.31
Clinical experience with TD-Fe for cancer pain in Germany also concluded that the 2:1 dosage conversion ratio was safe and effective.32 An observational study was conducted on 1,828 cancer patients who were switched from opioid naïve (WHO Step I), codeine (WHO Step II), or PO morphine (WHO Step III) to TD-Fe for their pain.33 The authors reported that a 3:1 exchange ratio utilized to begin TD-Fe pharmacotherapy created subtherapeutic serum levels.33 All patients in the study required dosage increases equal to the 2:1 ratio during the first 2 days of TD-Fe pharmacotherapy.33 Overall, there were no significant differences in the side effect profiles between the opioid naïve and PO morphine group (32% versus 30.1%, respectively). Patients switched from PO codeine to TD-Fe had a higher incidence of side effects (45%) although this finding was not reported as statistically significant. Constipation was the most common adverse effect reported with an average incidence of 16.6% across all groups.33
Given the information reported, Figure 1 outlines a possible dosing algorithm for TD-Fe. Table 1 provides the relative analgesic potencies compared to PO morphine of commonly prescribed opioids in the treatment of cancer pain.22,23,32–38 Patients should first be stabilized on their current opioid therapy prior to switching.24 When the initial dose of TD-Fe is determined, the practitioner should evaluate the patient’s clinical status and adjust the dosage to the nearest dosage using available strengths (12.5, 25, 50, 75, and 100 μg/hr). Opioid naïve patients should be started on no more than 25 μg/hr of TD-Fe. It may be necessary to combine patch strengths to reach the nearest dose. Figure 2 provides some examples of TD-Fe dosage conversion.
  | Figure 1 Dosing algorithm for TD-Fe in the cancer patient. |
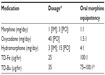  | Table 1 Recommended dosage conversion rates from oral morphine to other selected opioids for the treatment of cancer-related pain |
  | Figure 2 Examples of determining the appropriate initial fentanyl patch size. |
TD-Bu rotation
Buprenorphine, a partial mu-receptor agonist, does not appear to produce clinically negative effects on pain control during opioid switching.34,35 TD-Bu is available in several dosing strengths (5, 10, 15, 20, 35, 52.5, and 70 μg/hr). Published data for conversion to TD-Bu is less robust and a variety of conversion ratios have been utilized.23 There have been two N of 1 studies performed in cancer patients with stable pain control that support the use of a 75:1 relative analgesic potency for switching from PO morphine to TD-Bu.34,35 Thus, a dose of 60 mg/day of PO morphine would equate to 0.8 mg/day of TD-Bu or 35 μg/hr.34,35 The first study involved converting 10 cancer patients who were receiving stable doses of PO Morphine or TD-Fe for more than 6 days with no more than 2 daily IR PO morphine breakthrough doses to TD-Bu.34 No statistically significant changes in pain, symptom intensity, and global satisfaction with analgesic pharmacotherapy were observed.34 Constipation was significantly (P=0.014) improved with the conversion to TD-Bu.34 In the other N of 1 study, a within-patient two-way crossover design comparing TD-Bu and TD-Fe in six cancer patients was utilized.35 These patients had been stabilized on either TD-Bu or TD-Fe for at least 6 days with acceptable analgesia and without relevant AE, and were using two or fewer breakthrough doses of IR PO morphine each day.35 There were no statistically significant changes in pain and symptom intensity reported during switching. The authors concluded that cancer patients receiving stable doses of TD-Fe or TD-Bu can be safely switched to an alternate TD-O at using the 100:1 relative potency for TD-Fe and a 75:1 relative potency for TD-Bu.35
A study by Aurilio et al examined 16 patients switched from TD-Fe to TD-Bu and 16 others from TD-Bu to TD-Fe; all as a result of ineffective analgesia due to AE.36 Unfortunately, the final conversion ratios varied based on the direction of the switch and patient selection. Therefore, the dosing conversion-related conclusions could not be made in this study.36
A retrospective study of a German patient database involving 2,198 non-cancer and 2,544 cancer patient records suggested an equipotency ratio of PO morphine to TD-Bu of 110:1 to 115:1.37 A study limitation was the inability to identify patients concurrently using more than one patch of the same strength. Therefore, the “calculated” equipotent doses between PO morphine and TD-Bu were conservative and most likely higher than reported.37
Freye et al evaluated TD-Bu in 42 patients suffering from severe musculoskeletal (N=24), cancer (N=10), or neuropathic (N=8) pain.38 No conversion recommendations were provided and opioid transfers were made solely on practitioner’s clinical judgment.38 The researchers found that based on the dosages of TD-Bu prescribed, lower TD-Bu doses were needed. The majority (71%) of patients in this study obtained sufficient relief with 52.5 μg/hr of TD-Bu (25% reduction in dose or 100:1 ratio); however, it should be noted that this dose was increased to a 75:1 ratio or 70 μg/hr for the ten cancer patients enrolled in the study, suggesting that this population may require more aggressive dosing.38
Four patient case evaluations reported a less aggressive morphine to TD-Bu relative potency ratio.39 It was difficult to determine which ratio the researchers were supporting (110–115:1 in the abstract and a 100:1 final ratio reported in the conclusions). It is important to note that all of the patients studied had chronic non-cancer pain and research has shown that cancer patients require higher doses to achieve adequate pain relief.40–43 Given that there is limited evidence available on the conversion ratios to TD-Bu, these data are of some importance here. In the first case, the prescribed TD-Bu dose of 17.5 μg/hr was equal a 76:1 equipotency ratio (PO morphine 32 mg/day to TD-Bu 0.42 mg/day).22,23,39 In the fourth reported case a dose of 50 μg/hr of TD-Fe was switched to 52.5 μg/hr of TD-Bu using a 100:1 relative potency. The 100:1 potency resulted in 70% relief from pain with no adverse consequences in this particular patient. For over a year, this patient reported reasonable pain control and only used breakthrough medication approximately once weekly.39 Significant interpatient variability in therapeutic response to TD-Bu was reported between each of the cases and patients were also taking several adjuvant pain medications or treatments (ie, amitriptyline, gabapentin, non-steroidal anti-inflammatory drugs, carbamazepine, acupuncture) to assist with pain management. Thus, the data reported from these four case studies are quite mixed and an accurate equipotency ratio for TD-Bu cannot be pinpointed by these findings.
Given the limited data available, a precise recommendation for TD-Bu conversions cannot be made at this time. Cancer pain patients may require more aggressive conversion ratios but more research is needed in this patient population.
Breakthrough pain and withdrawal
Breakthrough or transient pain is described as brief exacerbations of pain that occur in the background of stabilized pain management adequately controlled by around-the-clock (ATC) SR opioid therapy.40,41 Transient pain is treated with adjunctive PRN doses of PO IR opioids (morphine, oxycodone, or hydrocodone), as well as buccal or intra-nasal fentanyl in concert with an appropriate ATC SR opioid therapy.4 IR transmucosal fentanyl doses should be titrated according to patient response.4 Adequate pain relief from first dose of TD-O should be evaluated at initiation of pharmacotherapy and continued over the first 3–5 days of administration.42 The goal is to limit the number of breakthrough pain doses such that the patient’s life is disrupted as little as possible during their day and at rest.
If more than two doses are required for breakthrough pain over a 24-hour period in order to achieve adequate pain control once steady state has been reached, then the clinician may consider increasing the TD-O dose.22 TD-Fe dosage increases are usually 25%–33% of the patch strength and based on the patient’s response to therapy.22 The ideal dose of TD-O is individualized and determined by continued evaluation of pain control and use of breakthrough pain pharmacotherapy.
For minimal withdrawal symptoms, patients should be counseled to utilize their scheduled IR opioid every 3–4 hours and not PRN during the transition period.43 The withdrawal prevention or bridge dose of IR PO opioid should be equal to 10%–15% of the previous 24 hour opioid dose taken prior to beginning TD-Fe pharmacotherapy. Patients can also self-medicate with additional doses or IR opioids about every 2 hours, PRN, if they experience breakthrough pain during this initial period and thereafter.22
TD-O patch considerations
Proper skin adhesion is essential to ensure TD-O patch efficacy.44 First, the hair on the skin at the application site should be carefully clipped; shaving of the skin is not recommended as abrasions may occur.44,45 TD-Os should be applied to clean, dry, unbroken, and undamaged skin.45,46 The plastic backing should be removed and the patch secured firmly with hand pressure over 30 seconds. Per the transdermal therapeutic system Multicentre Study Group, there were no issues associated with patch adherence in the majority (82%) of TD-Fe patients.46 In warm weather climates or for those experiencing significant sweating, the patch may also be held in place with a small amount of adhesive tape. Rotation of the application site is recommended with each TD-O patch change in order to minimize subcutaneous deposits of opioid medication.44
TD-O patches can be worn during bathing, showering, or swimming but patients should avoid hot water and external heat sources (eg, electric blankets, heating pads, saunas, hot water spas, hot springs, sunbathing).44,45 Several transdermal medication formulations have been found to contain metals (eg, aluminum, titanium dioxide).46,47 These metals have the potential to conduct a current during magnetic resonance imaging (MRI) procedures or when external defibrillation is used in cardiac resuscitation.46 There have been several cases of skin burns in patients wearing TD-O patches during MRI scans prompting the Institute for Safe Medication Practices and Food and Drug Administration (FDA) to issue alerts to remove the patches prior to MRI procedures.47,48
TD-O patches should never be cut for dosage adjustments or applied when altered or damaged.47,49 When a patch is removed for disposal, it should be folded in half such that the adhesive side sticks to itself and then flushed down the toilet immediately.46 Leftover patches that are no longer needed can be removed from their packaging and disposed of in the same manner.47
TD-Fe’s analgesic effects usually last for 3 days for the majority of cancer pain patients and is therefore dosed every 72 hours.10 For those with poor pain control, it is recommended that the dosage of TD-Fe be increased rather than reducing the dosing interval.50,51 However, a small number of patients may experience a reduction in pain control between 48 hours and 60 hours post-application.49,50 Therefore, the practitioner may contemplate a 48-hour dosing interval if the patient is consistently requiring more than four daily doses of breakthrough pain medication taken between 48–72 hours following patch application even with several dosing modifications.46,50 TD-Bu patches are usually changed twice weekly on fixed days of the week to assist with patient adherence.10
Adverse effects and interactions
As mentioned earlier, TD-Os are well tolerated and are associated with reduced AE, especially constipation (16%–22%), nausea (2%–9%) and sedation (2%–11%) when compared to PO opioid analgesics.7,10–14,30 Minor application site-related dermatologic reactions such as rash (5.6%), itching (13.6%), erythema (8.2%), irritation (2.7%), and dermatitis (0.8%) have been noted with TD-Os.11,22,45
An RCT by Tassinari et al demonstrated that patients receiving morphine or methadone developed clinically significant central nervous system (CNS) AE, whereas no CNS side effects were reported in the TD-Fe cohort.11 A meta-analysis conducted by the same group of researchers found that cancer and non-cancer patients experienced significantly less constipation and urinary retention, as well as preferring the use of TD-Fe over PO SR morphine.12 No significant differences were discerned between PO SR opiates and TD-Os (TD-Fe and TD-Bu) in overall AE, overall gastrointestinal AE, overall neurologic AE, nausea, somnolence, hypoventilation, withdrawal from trial, and changes in opioid therapy.12
All opioids can cause physical dependence and patients should be monitored for symptoms such as agitation, anxiety, insomnia, hyperkinesia, tremors, and gastrointestinal problems at 2 days to 2 weeks following discontinuance of TD-O pharmacotherapy.44 In healthy volunteers, buprenorphine does exhibit a “ceiling effect” for respiratory depression but not for analgesia.51,52 Clinically significant respiratory depression can be seen with TD-Bu in patients prescribed concomitant CNS depressants.53 Removal of the final TD-Bu patch produces a gradual reduction in serum levels, as well as a very slow dissociation from the opioid receptor, making the risk of withdrawal symptoms upon patch discontinuation low.52
TD-Os are processed by the liver’s CYP450 enzyme system and any medication known to affect the CYP3A enzyme (Table 2) will interact with TD-Os.53 An FDA black box warning exists prohibiting the concomitant use of CYP34A inhibitors with TD-Fe.54,55 There is also concern in patients with CYP3A5 polymorphism as the capability of CYP3A5 to metabolize opioids has not been fully evaluated.53 Any substances or medications known to cause CNS depression (eg, alcohol, benzodiazepines, skeletal muscle relaxants) can cause additive effects with TD-Os.54 Deaths have occurred with co-administration of benzodiazepines and TD-Os.54
  | Table 2 Significant CYP3A interactions with transdermal opioids |
As with all opioid medication, TD-Os carry a risk of overdose and the potential for death. The use of TD-Os is increasing and so are the number of TD-O related deaths.55 In order to reduce the risk of fatality, the FDA has issued several safety warnings over the past 9 years.56–58 In September 2013, the FDA sent out a safety communication indicating that all fentanyl patches where to undergo a color change in an effort to prevent accidental exposure which can cause serious harm and death in children, pets, and others.59 From 1997 to 2012, 26 young children have had accidental overdoses of TD-Fe; ten of these children died as a result. The FDA continues to raise public awareness via their TD-Fe Safe Use Initiative along with the TD-Fe Medication Guide and Instructions for Use (provided with each prescription).58,59 However, it is also very important that health care professionals provide information to patients and their caregivers on the appropriate storage and disposal each time they write a prescription for TD-Os.
Special populations
TD-Os should be used with caution in cachectic and/or liver dysfunction patients. Limited research has demonstrated that TD-Fe is not as well absorbed through the skin in cachectic patients which may be a result of their reduced adipose tissue stores.60 Cachectic patients should be carefully monitored as they may be at risk for inadequate analgesia even with seemingly large doses of TD-O. Frail elderly are often on multiple medications and have several comorbidities increasing their risk of medication- and disease-related interactions.61 The elderly commonly experience increased medication sensitivity, reduced medication response, and harmful adverse reactions. PO SR opioids and TD-Os are the preferred medications for this population and must be carefully monitored and titrated. Dosage adjustments of TD-Os are not required in those with renal impairment; however, opioids are metabolized by the liver and should be carefully monitored in those with hepatic impairment.62 Limited data exist for the use of TD-Os in the pediatric population. While these medications may be a convenient choice for the treatment of cancer pain in children, research is currently not available to support their widespread use.63,64
Conclusion and recommendations
Opioid medications are first-line pharmacotherapy for cancer pain sufferers. Most if not all cancer pain patients will ultimately transition between opioids, including transdermal formulations. It is important to appropriately convert and monitor them during these transitional periods. Every cancer patient requires individualized dosing of their TD-O in order to reduce the risk of subtherapeutic dosing and withdrawal. TD-Fe and TD-Bu formulations offer safe and effective therapeutic options in the treatment of cancer pain. More aggressive dosing of TD-Fe is required in this patient population using a 100:1 equianalgesic ratio. There is significant interpatient variability with TD-Bu and equipotency ratio recommendations range from 75:1–110:1. Cancer patients may require larger TD-Bu doses to control their pain and may respond better to a 75–100:1 dosing ratio. Patients prefer the favorable side-effect profile and convenience of TD-Os making these formulations a beneficial WHO step III choice for moderate to severe cancer pain.
Disclosure
The author reports there was no funding received to write this manuscript or conflict of interest of any kind.
References
Cleeland CS, Gonin R, Hatfield AK, et al. Pain and its treatment in outpatients with metastatic cancer. N Engl J Med. 1994;330(9):592–596. | |
Van den Beuken-van Everdingen MH, de Rijke JM, Kessels AG, Schouten HC, van Kleef M, Patijn J. Prevalence of pain in patients with cancer: a systematic review of the past 40 years. Ann Oncol. 2007;18(9):1437–1449. | |
Mercadante S, Fultaro F. World Health Organization guideline: a reappraisal. Ann Oncol. 2005;16(Suppl 4):iv132–iv135. | |
Caraceni A, Hanks G, Kaasa S, et al. Use of opioid analgesics in the treatment of cancer pain: evidence-based recommendations from the EAPC. Lancet Oncol. 2012;13(2):e58–e68. | |
Quigley C. The role of opioids in cancer pain. BMJ. 2005;331(7520):825–829. | |
Hui D, Bruera E. Transdermal fentanyl: not ready for front line. J Palliat Care. 2009;25(4):300. | |
Tassinari D, Drudi F, Rosati M, Maltoni M. Transdermal opioids as front line treatment of moderate to severe cancer pain: a systemic review. Palliat Med. 2011;25(5):478–487. | |
Wiffen PJ, McQuay HJ. Oral morphine for cancer pain. Cochrane Database Syst Rev. 2007;(4):CD003868. | |
Jadad AR, Browman GP. The WHO analgesic ladder for cancer pain management: stepping up the quality of its evaluation. JAMA. 1995;274(23):1870–1873. | |
Cachia E, Ahmedzai SH. Transdermal opioids for cancer pain. Curr Opin Support Palliat Care. 2011;5(1):15–19. | |
Tassinari D, Sartori S, Tamburini E, et al. Adverse effects of transdermal opiates treating moderate-severe cancer pain in comparison to long-acting morphine: a meta-analysis and systematic review of the literature. J Palliat Med. 2008;11(3):492–501. | |
Tassinari D, Sartori S, Tamburini E, et al. Transdermal fentanyl as a front-line approach to moderate-severe pain: a meta-analysis of randomized clinical trials. J Pallliat Care. 2009;25(3):172–180. | |
Mercadante S, Bruera E. Opioid switching: a systematic and critical review. Cancer Treat Rev. 2006;32(4):304–315. | |
Dale O, Moksnes K, Kaasa S. European Palliative Care Research Collaborative pain guidelines: opioid switching to improve analgesia or reduce side-effects. A systematic review. Palliat Med. 2011;25(5):494–503. | |
de Stoutz N, Bruera E, Suarez-Almazor M. Opioid rotation for toxicity reduction in terminal cancer patients. J Pain Symptom Manage. 1995;10(5):378–384. | |
Bruera E, Pereira J, Watanabe S, Belzile M, et al. Opioid rotation in patients with cancer pain. A retrospective comparison of dose ratios between methadone, hydromorphone, and morphine. Cancer. 1996;78(4):852–857. | |
Mercadante S. Opioid rotation in cancer pain: rationale and clinical aspects. Cancer. 1999;86(9):1856–1866. | |
Mercadente S, Porzio G, Ferrera P, et al. Sustained release oral morphine versus transdermal fentanyl and oral methadone in cancer pain management. Eur J Pain. 2008;12(8):1040–1046. | |
Wolff RF, Aune D, Truyers C, et al. Systematic review of efficacy and safety of buprenorphine versus fentanyl or morphine in patients with chronic moderate to severe pain. Curr Med Res Opin. 2012;28(5):833–845. | |
Koyyalagunta D, Bruera E, Solanki DR, et al. A systematic review of randomized trials on the effectiveness of opioids in cancer pain. Pain Physician. 2012;15(Suppl 3):ES39–ES58. | |
Slatkin NE. Opioid switching and rotation in primary care: implementation and clinical utility. Curr Med Res Opin. 2009;25(9):2133–2150. | |
Skaer TL. Transdermal opioids for cancer pain. Health Qual Life Outcomes. 2006;4:24. | |
Mercadante S, Caraceni A. Conversion ratios for opioid switching in the treatment of cancer pain: a systematic review. Palliat Med. 2011;25(5):504–515. | |
Bradley AM, Valgus JM, Bernard S. Converting transdermal fentanyl: avoidance of underdosing. J Palliat Med. 2013;16(4):409–411. | |
Ashburn M, Lipman A. Management of pain in the cancer patient. Anesth Analg. 1993;76(2):402–416. | |
Fine PG, Portenoy RK. Ad Hoc Expert Panel on Evidence Review and Guidelines for Opioid Rotation. Establishing best practices for opioid rotation: conclusions of an expert panel. J Pain Symptom Manage. 2009;38(3):418–425. | |
Knotkova H, Fine PF, Portenoy RK. Opioid rotation: the science and the limitations of the equianalgesic dose table. J Pain Symptom Manage. 2009;38(3):426–439. | |
Hanks GW, Fallon MT. Transdermal fentanyl in cancer pain. Conversion from oral morphine. J Pain Symptom Manage. 1995;10(2):87. | |
Webster LR, Fine PG. Overdose deaths demand a new paradigm for opioid rotation. Pain Med. 2012;13(4):571–574. | |
Grond S, Radbruch L, Lehmann KA. Clinical pharmacokinetics of transdermal opioids: focus on transdermal fentanyl. Clin Pharmacokinet. 2000;38(1):59–89. | |
Donner B, Zenz M, Tryba M, Strumpf M. Direct conversion from oral morphine to transdermal fentanyl: A multicenter study in patients with cancer pain. Pain. 1996;64(3):527–534. | |
Radbruch L, Elsner F. Clinical experience with transdermal fentanyl for the treatment of cancer pain in Germany. Keio J Med. 2004;53(1):23–29. | |
Mystakidou K, Parpa E, Tsilika E, et al. Pain management of cancer patients with transdermal fentanyl: a study of 1828 step I, II, and III transfers. J Pain. 2004;5(2):119–132. | |
Mercadante S, Casuccio A, Tirelli W, Giarrantano A. Equipotent doses to switch from high doses of opioids to transdermal buprenorphine. Support Care Cancer. 2009;17(6):715–718. | |
Mercadante S, Porzio G, Fulfano F, et al. Switching from transdermal drugs: an observational ‘N of 1’ study of fentanyl and buprenorphine. J Pain Symptom Manage. 2007;34(5):532–538. | |
Aurilio C, Pace MC, Pota V, et al. Opioids switching with transdermal systems in chronic cancer pain. J Ex Clini Cancer Res. 2009;28:61. | |
Sitti R, Likar R, Nautrup BP. Equipotent dose of transdermal fentanyl and transdermal buprenorphine in patients with cancer and noncancer pain: results of a retrospective cohort study. Clin Ther. 2005;27(2):225–237. | |
Freye E, Anderson-Hillemacher A, Ritzdorf I, Levy JV. Opioid rotation from high-dose morphine to transdermal buprenorphine (Transtec) in chronic pain patients. Pain Pract. 2007;7(2):123–129. | |
Likar R, Krainer B, Sitti R. Challenging the equipotency calculation for transdermal buprenorphine: four case studies. Int J Clin Pract. 2008;62(1):152–156. | |
Davies AN, Dickman A, Reid C, et al. The management of cancer-related breakthrough pain: recommendations of a task group of the Science Committee of the Association for Palliative Medicine of Great Britain and Ireland. Eur J Pain. 2009;13(4):331–338. | |
Haugen DF, Hjermstad MJ, Hagen N, et al. Assessment and classification of cancer breakthrough pain: a systematic literature review. Pain. 2010;149(3):476–482. | |
Kornick CA, Santiago-Palma J, Moryl N. et al. Benefit-risk assessment of transdermal fentanyl for the treatment of chronic pain. Drug Saf. 2003;26(13):951–973. | |
Breitbart W, Chandler S, Eagel B, et al. An alternative algorithm for dosing of transdermal fentanyl for cancer-related pain. Oncology (Williston Park). 2000;14(5):695–705. | |
Payne R, Chandler S, Einhaus M. Guidelines for the clinical use of transdermal fentanyl. Anticancer Drugs. 1995;6(3):50–53. | |
Wen W, Lynch SY, Munera C, et al. Application site adverse events associated with the buprenorphine transdermal system: a pooled analysis. Expert Opin Drug Saf. 2013;12(3):309–319. | |
Durand C, Alhanmmad A, Willett K. Practical considerations for optimal transdermal drug delivery. Am J Health Syst Pharm. 2012;69(2):116–124. | |
FDA [homepage on the Internet]. Public Health Advisory: risk of burns during MRI scans from Transdermal drug patches with metallic backings. Available from: http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/DrugSafetyInformationforHeathcareProfessionals/PublicHealthAdvisories/ucm111313.htm. Accessed May 19, 2014. | |
Hong I, Gabay M, Ladolce A. Safety concerns involving transdermal patches and magnetic resonance imaging (MRI). Hospital Pharmacy. 2010;45(10):771–778. | |
Southam MA. Transdermal fentanyl therapy: System design, pharmacokinetics and efficacy. Anticancer Drugs. 1995;6(Suppl 3):29–34. | |
Portenoy RK, Southam M, Gupta SK, et al. Transdermal fentanyl for cancer pain. Repeated dose pharmacokinetics and efficacy. Anesthesiology. 1993;78(1):36–43. | |
Dahan A, Yassen A, Romberg R, et al. Buprenorphine induces ceiling in respiratory depression but not in analgesia. Br J Anaesth. 2006;96(5):627–632. | |
Dahan A. Opioid-induced respiratory effects: new data on buprenorphine. Palliat Med. 2006;20(Suppl 1):S3–S8. | |
Overholser BR, Foster DR. Opioid pharmacokinetic drug-drug interactions. Am J Manage Care. 2011;17(Suppl 11):S276–S287. | |
McCance-Katz EF, Sullivan L, Nallani S. Drug interaction of clinical importance among the opioids, methadone, and buprenorphine, and other frequently prescribed medications: a review. Am J Addict. 2010;19(1):4–16. | |
Jumbelic MI. Deaths with transdermal fentanyl patches. Am J Forensic Med Pathol. 2010;31(1):18–21. | |
FDA [homepage on the Internet]. Safety warnings regarding use of fentanyl transdermal (skin) patches. Available from: http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/DrugSa fetyInformationforHeathcareProfessionals/PublicHealthAdvisories/ucm051739.htm. Accessed May 19, 2014. | |
FDA [homepage on the Internet]. FDA issues second safety warning on fentanyl skin patch: deaths and serious injuries from improper use. Available from: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2007/ucm109046.htm. Accessed May 19, 2014. | |
FDA [homepage on the Internet]. FDA reminds the public about the potential for life-threatening harm from accidental exposure to fentanyl transdermal systems (“patches”). Available from: http://www.fda.gov/drugs/drugsafety/ucm300747.htm. Accessed May 19, 2014. | |
FDA [homepage on the Internet]. FDA Drug Safety Communication: FDA requiring color changes to Duragesic (fentanyl) pain patches to aid safety-emphasizing that accidental exposure to used patches can cause death. Available from: http://www.fda.gov/drugs/drugsafety/ucm368902.htm. Accessed May 19, 2014. | |
Heiskanen T, Matzke S, Haakana S, et al. Transdermal fentanyl in cachectic cancer patients. Pain. 2009;144(1–2):218–222. | |
van Ojik AL, Jansen PAF, Bruwers JR, van Roon EN. Treatment of chronic pain in older people evidence-based choice of strong-acting opioids. Drugs Aging. 2012;29(8):615–625. | |
Plosker Gl. Buprenorphine 5, 10, and 20 μg/h transdermal patch: a review of its use in the management of chronic non-malignant pain. Drugs. 2011;71(18):2491–2509. | |
Michel E, Anderson B, Zernikow B. Buprenorphine TTS for children – a review of the drug’s clinical pharmacology. Paediatr Anaesth. 2011;21(3):280–290. | |
Zernikow B, Michel E, Craig F, Anderson BJ. Pediatric palliative care: use of opioids for the management of pain. Paediatr Drugs. 2009;11(2):129–151. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
