Back to Journals » Journal of Pain Research » Volume 13
Does Vitamin D Affect Diabetic Neuropathic Pain and Balance?
Authors Sari A , Akdoğan Altun Z, Arifoglu Karaman C , Bilir Kaya B , Durmus B
Received 28 January 2019
Accepted for publication 29 November 2019
Published 16 January 2020 Volume 2020:13 Pages 171—179
DOI https://doi.org/10.2147/JPR.S203176
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Michael Schatman
Aylin Sari, 1 Zeynep Akdoğan Altun, 1 Cigdem Arifoglu Karaman, 1 Basak Bilir Kaya, 1 Bekir Durmus 2
1Department of Physical Medicine and Rehabilitation, Erenkoy Physical Medicine and Rehabilitation Hospital, Istanbul, Turkey; 2Department of Physical Medicine and Rehabilitation, Istinye University Medical Faculty, Istanbul, Turkey
Correspondence: Aylin Sari
Department of Physical Medicine and Rehabilitation, Erenkoy Physical Medicine and Rehabilitation Hospital, Semsettin Gunaltay Avenue Sultan Street No. 14, Kadikoy, Istanbul, Turkey
Tel +905058396368
Fax +902164783123
Email [email protected]
Purpose: This randomized, placebo-controlled study examined the effect of vitamin D replacement therapy on neuropathic symptoms and balance in patients with diabetic neuropathic pain and low vitamin D levels.
Patients and Methods: Among the 258 patients, the results in a total of 57 volunteers (32 in the treatment and 25 in the control arm) meeting the inclusion criteria are reported. Symptoms of neuropathic pain were assessed using Douleur Neuropathique 4 (DN4) questionnaire, and presence of polyneuropathy (PNP) was determined by performing electromyography (EMG). Balance was assessed using Berg balance test (BBT). After undergoing these examinations, the patients in the treatment group were intramuscularly (IM) injected with 300,000 IU vitamin D in a liquid formulation and those in the placebo group were IM injected with physiological saline. The DN4 and BBT were repeated after 12 weeks, and the results were compared.
Results: The patients in the treatment group showed a significant decrease in total DN4 scores from baseline to the study endpoint compared with the patients in the placebo group (p=0.008). The patients in the treatment group also showed a significant increase in BBT scores from baseline to the study endpoint compared with the patients in the placebo group (p=0.001). Furthermore, in subgroup analysis, these patients showed a significant decrease in electric shock and burning sensation scores from baseline to the study endpoint compared with the patients in the placebo group (p=0.006, p=0.001, respectively).
Conclusion: In patients with diabetic neuropathic pain, vitamin D levels should be measured and vitamin D replacement therapy should be administered as required to resolve neuropathic symptoms and to improve balance.
Keywords: diabetic neuropathic pain, vitamin D, balance, neuropathic pain symptoms
A Letter to the Editor has been published for this article.
Introduction
Diabetic neuropathy (DN) is a very common long-term complication of diabetes mellitus (DM) that affects approximately 50% patients with DM and is associated with a significant reduction in the quality of life of patients.1–3
DN may induce several symptoms, such as foot and hand muscle weakness, balance disturbance, and neuropathic pain including alterations in touch, pain, or heat sensations; burning; pins and needles; tingling; or numbness.4,5
DN involves both small and larger nerve fibers. The small nerve fibers include C-fibers associated with electric shock or burning symptoms. Pathological changes in these fibers cannot be detected by performing EMG. Involvement of the large nerve fibers may impair balance because of their effect on deep senses and can be determined by performing EMG.1–3
Although the pathophysiology of diabetic neuropathy is quite complicated, new studies have shown that vitamin D deficiency is an independent predictor of DN development.4,5
In recent years, vitamin D has been described as a neurotrophic hormone. It has a neuroprotective effect through upregulation of vitamin D receptor (VDR) expression and downregulation of L-type calcium channel expression.6 It has been shown in vivo studies that vitamin D improves axonogenesis and sensory neural response in peripheral nerve and improves electrophysiological recovery.7,8 The study investigating the relationship between DN and vitamin D has found lower serum vitamin D in patients with DN than those without DN.9 DN is associated with decreased Nerve Growth Factor (NGF) expression in human nerve cells10 and vitamin D increases NGF synthesis in human cells.11,12 Vitamin D deficiency promotes DN development by triggering hyperglycemia and inflammation.13 It has also been reported that vitamin D deficiency may be associated with increased pain sensitization.14 In another study, vitamin D supplementation has been reported to have beneficial effects on neuropathic pain and prevent neuronal degeneration.15 Patients with DN have more balance disturbance than normal healthy subjects and diabetic individuals without neuropathy.16,17 Recent studies have shown a relationship between vitamin D and balance. A possible mechanism was considered as the association of VDR receptor in muscle tissue and central nervous system.18,19 Based on all these findings, vitamin D replacement therapy in patients with DN lack of this vitamin may resolve neuropathic pain to some extent and may improve balance. The present study examined the effect of vitamin D replacement therapy on neuropathic pain and balance in patients with DN who presented to our outpatient unit with neuropathic pain symptoms and low vitamin D levels.
Materials and Methods
This study was conducted at the Physical Therapy and Rehabilitation Outpatient Unit of Erenkoy Physical Therapy and Rehabilitation Hospital between October 2013 and June 2018. Power analysis was performed to determine the sample size of the study. Results of the power analysis indicated that the study had to include a total of 40 cases, with 20 cases in each study group, at α = 0.05 to achieve a power of 80%. We screened 258 patients with type 2 DM and neuropathic symptoms who visited the outpatient unit. DN4 was administered to the patients and of these102 patients had neuropathic pain with DN4. Therefore, their 25(OH)D levels were measured. In this study 25(OH)D levels were measured twice; first, before the treatment to determine low 25(OH)D levels (<30 ng/mL) and second, 12 weeks after treatment. Of the 102 patients, 83 patients had low 25(OH)D levels (<30 ng/mL) for the season. In the present study, 25(OH)D clinical cutoff levels were defined using Endocrine Society guidelines (optimal level, ≥30 ng/mL), which was similar to that in several previous studies.20 Eighty-three patients were referred to the EMG center of the outpatient unit. In all, 65 patients were confirmed as having PNP by performing EMG and were included in the study. These patients were randomized to treatment (n = 33) and placebo (n = 32) groups. The patients in the treatment group were intramuscularly (IM) injected with 300,000 IU vitamin D in a 2 mL liquid formulation and those in the placebo group were IM injected with 2 mL physiological saline. Intramuscularly (IM) injections were administered at the ventrogluteal site. All the patients were invited for a follow-up visit after 12 weeks. One patient in the placebo group withdrew consent, and six patients in the placebo group were lost to follow-up. Moreover, one patient in the treatment group had 25(OH)D level below 30 ng/mL after treatment and therefore was removed from the study. All the patients were evaluated for adverse reactions and no significant adverse reaction was observed. Thus, follow-up assessments were performed in 32 and 25 patients in the treatment and placebo groups, respectively (Figure 1).
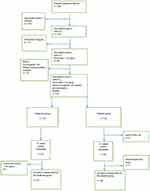 |
Figure 1 Flowchart of the study. |
The study was conducted according to the criteria set by the declaration of Helsinki and each subject signed a written informed consent before participating in the study. The study design was approved by the ethics committee of Yeditepe University. Inclusion criteria were as follows: confirmed diagnosis of type 2 DM, age between 18 and 80 years, presence of neuropathic pain symptoms for >3 months, low levels of 25(OH)D (below <30 ng/mL for the season before the study), and confirmed diagnosis of PNP by performing EMG. Pregnant or lactating women; patients with renal failure, hyper- or hypoparathyroidism, hyper- or hypothyroidism, and polyneuropathy due to conditions other than diabetes; patients receiving vitamin D supplementation; and patients with 25(OH)D levels below 30 ng/mL after vitamin D replacement therapy were excluded from the study. The following parameters were recorded for all the study patients: age; sex; disease duration; occupation; education level; income; marital status; concomitant conditions; ongoing treatments; body mass index (BMI); HbA1c levels; fasting blood glucose levels (FBG); 25(OH)D levels; alkaline phosphatase levels (ALP); parathormone (PTH) levels; calcium (Ca) and phosphorus (P) levels; cholesterol panel; vitamin B12 levels, Berg balance test scores, DN4 questionnaire scores; tea, coffee, and alcohol intake; and cigarette smoking. After 12 weeks, 25(OH)D levels and Berg balance test and DN4 questionnaire scores were re-assessed for all the patients.
Serum 25(OH)D levels were measured by performing radioimmunoassay (RIA) with RIA CT kits (Biosource Europe SA, Nivelles, Belgium). A 25(OH)D level of >30 ng/mL was considered to be normal. To prevent the effect of seasonal fluctuations in 25(OH)D levels on, the patients were enrolled into the study only during the autumn and winter months.
Randomization was performed using a simple randomization method in which red and blue sheets of paper were placed in pre-prepared envelopes. Eligible patients were asked to select an envelope and were randomly allocated to one of the two study groups based on the color of the sheet present within envelope. The injection solutions were prepared by a diabetes nurse who was unblinded to the study groups. However, the injections were administered by an outpatient nurse who was blinded to the study groups. Medical records of the study patients were entered into a database by data entry personnel who were blinded to the study groups. The allocation of the patients to the respective groups was revealed to the data entry personnel by the study nurse after the completion of patient assessments.
Results
The study included 57 patients (34 [59.6%] women and 23 [40.4%] men). The demographic characteristics of the study patients are shown in Table 1, and their laboratory results are shown in Table 2.
 |
Table 1 Demographic Characteristics |
 |
Table 2 Laboratory Results |
Table 3 shows the DN4 neuropathic pain scale scores of the study patients. All the patients had neuropathic pain at baseline (total DN4 score >4). Neuropathic pain scores significantly reduced from the baseline to the study endpoint in the patients in the treatment group (p = 0.008). However, no significant change in neuropathic pain scores was observed in the patients in the placebo group (p = 0.500; Figure 2).
 |
Table 3 DN4 Neuropathic Pain Scale Scores |
 |
Figure 2 DN4 scores in the treatment and control groups. |
The patients in the treatment group showed a significant increase in Berg balance test scores from the baseline to the study endpoint (p = 0.001). However, no significant change was observed in Berg balance test scores of the patients in the placebo group (p = 0.223; Figure 3, Table 4).
 |
Table 4 Berg Balance Test Scores |
 |
Figure 3 The change in Berg balance scores in treatment and control groups. |
In the evaluation of DN4 subgroups (Table 3), the patients in the treatment group showed a significant decrease in burning scores from baseline to the study endpoint (p = 0.006). The patients in the treatment group showed a significant decrease in electric shock scores from the baseline to the study endpoint (p = 0.001). However, the patients in the placebo group did not show a significant change in burning and electric shock scores from the baseline to the study endpoint (p>0.999 for all).
Although the patients in the treatment group showed a decrease in tingling from the baseline to the study endpoint, the decrease was statistically insignificant (p = 0.065). The patients in the treatment group showed decreases in the following presentations from the baseline to the study endpoint, the decreases were statistically insignificant (p> 0.05 for all): numbness, itching, hypoesthesia to touch, hypoesthesia to pinprick. Painful cold, pins and needles, and brushing did not show any decrease from the baseline to the study endpoint in the treatment group (p>0.999 for all). However, no significant changes were observed in the following presentations from the baseline to the study endpoint in the placebo group: painful cold, tingling, pins and needles, numbness, itching, hypoesthesia to touch, hypoesthesia to pinprick, and brushing (p> 0.999 for all).
Discussion
The present study compared neuropathic pain and balance before and after vitamin D replacement therapy in patients with DN. Although previous studies have evaluated only DN-related pain, our study is the first to evaluate both neuropathic pain and balance.12–15,21 Our results showed a significant improvement in the total DN4 questionnaire scores that indicates the presence of neuropathic pain and Berg balance test scores that determines balance after vitamin D replacement therapy.
Various considerations are available regarding the dose, administration route, and duration of vitamin D replacement therapy. A meta-analysis by Kearns et al22 indicated that the application of a single large vitamin D dose is more effective than in supplementing vitamin D deficiency and that single vitamin D3 doses of ≥300,000 IU are the most effective in improving vitamin D status and in suppressing PTH levels for up to 3 months. In the present study, we used a single intramuscular vitamin D dose of 300,000 IU in the vitamin D replacement therapy.
Different studies have used different therapeutic approaches for examining the association between vitamin D deficiency and neuropathic pain. One study reported that a topical agent containing vitamin D (QR-333) decreased neuropathic pain.23 Another study reported a significant reduction in pain scores of patients with type 2 DM and neuropathic pain symptoms who completed a 3-month course of vitamin D3 tablets.24 Basit et al administered a single large dose (600,000 IU) of vitamin D IM in patients with DN and neuropathic pain and observed a significant alleviation in pain symptoms after approximately 10 weeks.25 In the present study, we applied vitamin D replacement therapy as a single intramuscular vitamin D dose of 300,000 IU and this application significantly improved the DN4 questionnaire scores of the patients with DN. Because the placebo effect on pain scores is generally expected to occur within the first 4–6 weeks25,26 and because pain assessments in the present study were performed at 12 weeks, we believe that the placebo effect in the present study was negligible. In conclusion, our study showed that improvement in neuropathic pain with vitamin D replacement incompatible with these studies.
However, some studies have reported no significant decrease in neuropathic pain scores after vitamin D administration.27 This may be because these studies may have assessed neuropathic pain and its subcomponents based on all or no principles instead of assessing them quantitatively, which may have resulted in the failure of observing a decrease in pain scores.
In the present study, although the improvements in the burning and electric shock subscores of the DN4 questionnaire indicated an improvement in the small fibers, the significant changes in the Berg balance test scores suggested an improvement in the large fibers. The latter observation is consistent with the results of previous electrophysiology studies involving patients with DN who received vitamin D treatment.28,29 One study reported that vitamin D supplementation exerted a positive effect on the balance score of stroke patients.30 Because impaired sensations are associated with balance disorder in stroke patients, our results are coherent with the available literature.
Electrophysiological data suggest that the time needed to document the changes in Berg balance test scores is >8 weeks, which corresponds to axonal regeneration time.21 In the present study, the follow-up examinations were performed after 12 weeks, which allowed for the completion of axonal regeneration. This may explain the discrepancy observed between the results of the present study and those of some previous studies.
As a result of this study, not only it can be said that vitamin D treatment was decreased total DN4 questionnaire scores, but also in the subgroup analysis of DN4 questionnaire, vitamin D treatment was decreased electric shock scores and burning scores. We observed that only painful cold sensation, pins and needles and brushing in DN4 subgroup scores did not decrease after vitamin D treatment. One plausible explanation for this finding may be variable sensory profiles on patients with diabetic neuropathic pain that previously defined by Bouhassira et al.31 Comorbidities such as peripheral vascular disease or heart disease might be a confounder for painful cold item.
Limitations
Major limitations of the present study include the small sample size, assessment of neuropathic pain level based only on its symptoms, absence of confirmatory neurophysiological examinations and no exclusion of comorbidities that might confound painful cold. In addition, there was no consensus on the optimum protocol of vitamin D replacement therapy. Limited studies have examined vitamin D replacement therapy and the optimum dose and duration of this therapy to minimize side effects.
Conclusion
In conclusion, vitamin D replacement therapy reduced neuropathic pain and improved balance in patients with DN. However, further studies examining the effects of vitamin D on nerve cells at molecular and electrophysiological levels and prospective studies focusing on the long-term effects of vitamin D replacement therapy on DN are warranted. However, the results of the present study suggest that a vitamin D replacement schedule might be planned in addition to anti-diabetic treatment to address vitamin D deficiency in patients with diabetes in order to resolve neuropathic pain symptoms and to alleviate balance impairment associated with DN.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Callaghan BC, Cheng HT, Stables CL, Smith AL, Feldman EL. Diabetic neuropathy: clinical manifestations and current treatments. Lancet Neurol. 2012;11(6):521–534. doi:10.1016/S1474-4422(12)70065-0
2. Edwards JL, Vincent AM, Cheng HT, Feldman EL. Diabetic neuropathy: mechanisms to management. Pharmacol Ther. 2008;120:1–34. doi:10.1016/j.pharmthera.2008.05.005
3. Zeng L, Alongkronrusmee D, vanRijn RM. An integrated perspective on diabetic, alcoholic, and drug-induced neuropathy, etiology, and treatment in the US. J Pain Res. 2017;10:219–228. doi:10.2147/JPR
4. Skalli S, Muller M, Pradines S, Halimi S, Wion-Barbot N. Vitamin D deficiency and peripheral diabetic neuropathy. Eur J Intern Med. 2012;23(2):e67–8. doi:10.1016/j.ejim.2011.11.008
5. Ahmadieh H, Aza ST, Lakkis N, Arabi A. Hypovitaminosis d in patients with type 2 diabetes mellitus: a relation to disease control and complications. ISRN Endocrinol. 2013;2013:641098. doi:10.1155/2013/641098
6. Taniura H, Ito M, Sanada N, et al. Chronic vitamin D3 treatment protects against neurotoxicity by glutamate in association with upregulation of vitamin D receptor mRNA expression in cultured rat cortical neurons. J Neurosci Res. 2006;83(7):1179–1189. doi:10.1002/(ISSN)1097-4547
7. Chabas DS, Marqueste T, Garcia S, et al. Cholecalciferol (Vitamin D3) improves myelination and recovery after nerve injury. PLoS ONE. 2013;8:e65034. doi:10.1371/journal.pone.0065034
8. Chabas OA, Rao G, Garcia S, et al. Vitamin D2 potentiates axon regeneration. J Neurotrauma. 2008;25:1247–1256. doi:10.1089/neu.2008.0593
9. Celikbilek A, Gocmen AY, Tanik N, et al. Decreased serum vitamin D levels are associated with diabetic peripheral neuropathy in a rural area of Turkey. Acta Neurol Belg. 2015;115(1):47–52. doi:10.1007/s13760-014-0304-0
10. Anand P, Terenghi G, Warner G, Kopelman P, Williams-Chestnut RE, Sinicropi DV. The role of endogenous nerve growth factor in human diabetic neuropathy. Nat Med. 1196;2:703–707. doi:10.1038/nm0696-703
11. Fukuoka M, Sakurai K, Ohta T, Kiyoki M, Katayama I. Tacalcitol, an active vitamin D3, induces nerve growth factor production in human epidermal keratinocytes. Skin Pharmacol Appl Skin Physiol. 2001;14:226–233. doi:10.1159/000056351
12. Shehab D, Al-Jarallah K, Abdella N, Mojiminiyi OA, Al Mohamedy H. Prospective evaluation of the effect of short-term oral vitamin D supplementation on peripheral neuropathy in type 2 diabetes mellitus. Med Princ Pract. 2015;24:250–256. doi:10.1159/000375304
13. Pinelli NR, Jaber LA, Brown MB, Herman WH. Serum 25-hydroxy vitamin d and insulin resistance, metabolic syndrome, and glucose intolerance among Arab Americans. Diabetes Care. 2010;33(6):1373–1375.
14. Alam U, Nelson AJ, Cuthbertson DJ, Malik RA. An update on vitamin D and B deficiency in the pathogenesis and treatment of diabetic neuropathy: a narrative review. Future Neurol. 2018;13(3):135–142. doi:10.2217/fnl-2017-0034
15. Putz Z, Martos T, Németh N, et al. Is there an association between diabetic neuropathy and low vitamin D levels? Curr Diab Rep. 2014;14:537. doi:10.1007/s11892-014-0537-6
16. Timar B, Timar R, Gaiță L, Oancea C, Levai C, Lungeanu D. The impact of diabetic neuropathy on balance and on the risk of falls in patients with type 2 diabetes mellitus: a cross-sectional study. PLoS ONE. 2016;11.4:e0154654. doi:10.1371/journal.pone.0154654
17. Akbari M, Jafari H, Moshashaee A, Forugh B. Do diabetic neuropathy patients benefit from balance training? J Rehabil Res Dev. 2012;49:2.
18. Muir SW, Montero‐Odasso M. Effect of vitamin D supplementation on muscle strength, gait and balance in older adults: a systematic review and meta‐analysis. J Am Geriatr Soc. 2011;59(12):2291–2300. doi:10.1111/j.1532-5415.2011.03733.x
19. Akdeniz S, Hepguler S, Öztürk C, Atamaz FC. The relation between vitamin D and postural balance according to clinical tests and tetrax posturography. J Phys Ther Sci. 2016;28(4):1272–1277. doi:10.1589/jpts.28.1272
20. Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911–1930. doi:10.1210/jc.2011-0385
21. Lv WS, Zhao WJ, Gong SL, et al. Serum 25-hydroxyvitamin D levels and peripheral neuropathy in patients with type 2 diabetes: a systematic review and meta-analysis. J Endocrinol Invest. 2015;38(5):513–518. doi:10.1007/s40618-014-0210-6
22. Kearns MD, Alvarez JA, Tangpricha V. Large, single-dose, oral vitamin D supplementation in adult populations: a systematic review. Endocr Pract. 2014;20(4):341–351. doi:10.4158/EP13265.RA
23. Valensi P, Le Devehat C, Richard JL, et al. A multicenter, double-blind, safety study of QR-333 for the treatment of symptomatic diabetic peripheral neuropathy: a preliminary report. J Diabetes Complications. 2005;19(5):247–253. doi:10.1016/j.jdiacomp.2005.05.011
24. Lee P, Chen R. Vitamin D as an analgesic for patients with type 2 diabetes and neuropathic pain. Arch Intern Med. 2008;168(7):771–772. doi:10.1001/archinte.168.7.771
25. Basit A, Basit KA, Fawwad A, et al. Vitamin D for the treatment of painful diabetic neuropathy. BMJ Open Diabetes Res Care. 2016;4(1):000148. doi:10.1136/bmjdrc-2015-000148
26. Tavakoli M, Asghar O, Alam U, et al. Novel insights on diagnosis, diabetic neuropathy: focus on painful diabetic neuropathy. Ther Adv Endocrinol Metab. 2010;1:69–88. doi:10.1177/2042018810370954
27. Alam U, Fawwad A, Shaheen F, Tahir B, Basit A, Malik RA. Improvement in neuropathy specific quality of life in patients with diabetes after Vitamin D supplementation. J Diabetes Res. 2017;7928083.
28. Bilir B, Tulubas F, Bilir BE, et al. The association of vitamin D with inflammatory cytokines in diabetic peripheral neuropathy. J PhysTher Sci. 2016;28(7):2159–2163.
29. Alamdari A, Mozafari R, Tafakhori A, et al. An inverse association between serum vitamin D levels with the presence and severity of impaired nerve conduction velocity and large fiber peripheral neuropathy in diabetic subjects. Neurol Sci. 2015;36:1121–1126. doi:10.1007/s10072-015-2207-0
30. Sari A, Durmus B, Karaman CA, Ogut E, Aktas I. A randomized, double-blind study to assess if vitamin D treatment affects the outcomes of rehabilitation and balance in hemiplegic patients. J Phys Ther Sci. 2018;30(6):874–878. doi:10.1589/jpts.30.874
31. Bouhassira D, Wilhelm S, Schacht A, et al. Neuropathic pain phenotyping as a predictor of treatment response in painful diabetic neuropathy: data from the randomized, double-blind, COMBO-DN study. Pain. 2014;155(10):2171–2179. doi:10.1016/j.pain.2014.08.020
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
